Abstract
Prevailing theory and research suggests the psychological and physiological discomfort associated with tobacco withdrawal may play a formative role in the risk of cessation failure. Yet, research elucidating cognitive-affective vulnerability characteristics that contribute to increased tobacco withdrawal severity during periods of planned abstinence is highly limited. In the current study, we explored whether smokers with greater reductions of Anxiety Sensitivity (AS) and dysphoria during a smoking cessation intervention would experience less severe post-quit tobacco withdrawal. Specifically, the interactive effect of change (from pre-intervention baseline to quit-day) in AS and dysphoria in relation to post-quit withdrawal severity (quit-day through 12-weeks post-quit) was examined among treatment seeking adult smokers enrolled in a smoking cessation trial (N = 198; 55.3% female; 86.8% Caucasian; Mage = 38.8, SD =14.0). Results indicated that the interactive effect of change in AS and dysphoria was related to linear change in post-quit withdrawal symptoms. Specifically, larger reductions in AS were associated with a faster decline in the severity of withdrawal symptoms across the 12-week post-quit period only for individuals with lower (but not higher) reductions in dysphoria. Additionally, the findings indicated that reducing levels of AS and dysphoria pre-quit is broadly related to the degree of change in post-quit withdrawal symptoms. Collectively, these data suggest there is apt to be clinical merit to employing strategies to address AS and/or dysphoria to more effectively manage emergent withdrawal symptoms following smoking cessation treatment. Keywords: Anxiety sensitivity, dysphoria, smoking cessation, tobacco withdrawal
Tobacco withdrawal represents a constellation of affective, cognitive, and somatic features that onset upon the discontinuation of chronic smoking because of the expression of nicotine-induced neuroadaptations and loss of the smoking ritual (Baker et al., 2007). Withdrawal is a key marker of tobacco dependence and is likely a major contributor to smoking relapse (Baker et al., 2007). Empirical data supports a robust association between withdrawal symptom severity and quit success (Piasecki et al., 2000; Piasecki, 2006).
A wide range of cognitive-affective factors (e.g., emotional states, clinical syndromes, or cognitive styles) have been implicated in tobacco withdrawal (Abrantes, et al., 2008; Leventhal, et al., 2007a; Leventhal, & Zvolensky, 2015). This work broadly suggests individual differences in cognitive-affective vulnerabilities can increase the risk of more severe withdrawal (Covey, 1999). As one illustrative example, non-clinical anhedonia symptoms are associated with more severe tobacco withdrawal (Langdon, et al., 2013). Such work suggests identifying distinct (cognitive-affective) processes that govern withdrawal is a key, clinically-relevant, question to address for smokers in general, and those with cognitive-affective vulnerabilities specifically.
One promising cognitive-affective construct to better understand tobacco withdrawal is Anxiety Sensitivity (AS). AS reflects the tendency to fear of anxiety-related sensations arising from beliefs that these sensations have harmful psychological, social, and somatic consequences (Reiss & McNally, 1985). AS is a relatively stable, but malleable, construct involved in the etiology and maintenance of anxiety and depressive disorders (Otto et al., 2016). Smokers with increased AS perceive quitting as more difficult (Johnson, Farris, Schmidt, Smits, & Zvolensky, 2013) and are more motivated to smoke to reduce negative affect (Battista et al., 2008). Furthermore, AS explains the relation of emotional disorders with numerous clinical indicators of smoking severity, such as cigarette dependence (Zvolensky, Farris, Leventhal, & Schmidt, 2014). Past work suggests smokers with greater AS experience more severe tobacco withdrawal during periods of smoking abstinence (Zvolensky, Farris, Guillot, & Leventhal, 2014) as well as greater risk of early lapse (Brown, Kahler, Zvolensky, Lejuez, & Ramsey, 2001) and relapse (Assayag, Bernstein, Zvolensky, Steeves, & Stewart, 2012).
As a malleable construct, AS can change in response to life events (e.g., stress exposure; Marshall, Miles, & Stewart, 2010), and be reduced via specialized (i.e., AS-specific) and non-specialized interventions (i.e., cognitive-behavioral stress management; Ablon, Levy, & Katzenstein, 2006; McHugh, et al., 2014: Schmidt, et al., 2007). Moreover, AS can be reduced via targeted, brief (e.g., less than 60-minute) interventions (Keough, & Schmidt, 2012; Smits, Berry, Tart, & Powers, 2008) and such change in AS mediates improvement in anxiety/depressive symptoms (Schmidt, Capron, Raines, & Allan, 2014; Timpano, Raines, Shaw, Keough, & Schmidt, 2016). Furthermore, smoking treatments that focus on reducing AS prior to quitting yield better rates of smoking abstinence (Zvolensky, Bogiaizian, Salazar, Farris, & Bakhshaie, 2014). Specifically, pre-quit reductions in AS decrease the odds of early cessation failure (Smits et al., 2016). Yet, a central gap in the AS-smoking literature is whether change in AS prior to a quit attempt is related to distinct tobacco withdrawal trajectories. Because AS serves as an affect amplifier (Zvolensky et al., 2014a), it would be expected that decreased AS prior to quitting would be related to less post-quit severe tobacco withdrawal (Leventhal & Zvolensky, 2015).
Theoretical models of smoking-affect associations predict that AS is likely to interplay with other affect processes in predicting smoking behavior generally, and tobacco withdrawal specifically (Leventhal & Zvolensky, 2015). Dysphoria, representing depressive symptoms characterized by intense unhappiness and discontent, may be one such common candidate (Cella, Cooper, Dymond, & Reed, 2008; Starcevic, 2007). Past work has found that dysphoria is associated with numerous aspects of smoking behavior, including greater dependence, higher smoking rates, and more negative affect reduction smoking motives (Leventhal, Zvolensky, & Schmidt, 2011), as well as more perceived barriers to cessation (Buckner et al., 2015). Moreover, greater levels of dysphoria are associated with more severe and protracted withdrawal symptoms (Covey, Glassman, & Stetner, 1990; Hall, Muñoz, Reus, & Sees, 1993). Dysphoria also can increase in response to life stress (Beevers, & Carver, 2003) and be reduced via specialized (Dobkin, Panzarella, Fernandez, Alloy, & Cascardi, 2004; Seligman, Steen, Park, & Peterson, 2005) and non-specialized (Reinecke, Ryan, & DuBois, 1998) interventions. Other work suggests reductions in dysphoria are related to better psychological and addictive behavior outcomes (Ginsberg, Hall, Reus, & Muñoz, 1995). Thus, while dysphoria would be expected to be related to more severe tobacco withdrawal, it has not been frequently studied within the context of other variables, such as AS.
One key clinically-relevant question that has thus far been unexamined is whether change in AS or dysphoria singularly or interactively is related to distinct tobacco withdrawal trajectories. Theoretically, AS and dysphoria may act on tobacco withdrawal in distinct ways, although there is limited work to date on this topic. One study found that the interaction of AS and anhedonia (a facet of dysphoria) at study entry predicted more severe post-quit withdrawal symptoms during smoking cessation treatment (Langdon et al., 2013). The nature of this association was such that the pre-quit levels of elevated AS and anhedonia predicted greater withdrawal. Other work has identified an interactive effect for AS and dysphoria in terms of smoking-related emotion regulatory cognition (Garey et al., 2016). Interestingly, the nature of the interactive effect suggested dysphoria may mitigate the effects of AS for smoking processes. Specifically, the effect of AS for smoking variables may be ‘blunted’ when dysphoria is high (Garey et al., 2016). Theoretically, these data suggest the emotional inertia (commonly associated with dysphoria; Brose, Schmiedek, Koval, & Kuppens, 2015) may diminish the role of AS in terms of (certain) smoking processes. Specifically, dysphoric symptoms may be such a robust predictor of smoking that concomitant AS does not further impact such outcomes. At lower levels of dysphoria, however, the effects of AS on smoking processes are more apt to be evident (Garey et al., 2016).
Although there has been promise thus far in exploring how AS and dysphoria interplay in certain aspects of smoking behavior, past work has yet to explore the nature of pre-quit change in these constructs in relation to tobacco withdrawal. It is important to elucidate the interactive nature of change in these constructs in terms of withdrawal because such work will directly inform therapeutic processes during smoking cessation treatment (Chua, et al., 2011; Leventhal, & Zvolensky, 2015). An important next research step therefore is to examine how pre-quit changes in AS and dysphoria as well as their interaction influences withdrawal symptom severity. Specifically, such work will enhance understanding of the malleability of AS and dysphoria and their role as targetable risk factors in the context of smoking cessation treatment. Consistent with research documenting that dysphoric symptoms may be risk factor for emotionally laden tobacco withdrawal (Breslau, Kilbey, & Andreski, 1992; Garey et al., 2016; Pomerleau, Marks, & Pomerleau, 2000), the effects of change in AS would be expected to be evident in instances wherein change in dysphoria is minimal.
Together, the current study examined the effects of change (from pre-intervention baseline to quit-day) in AS and dysphoria in relation to post-quit withdrawal severity among adult smokers enrolled in a smoking cessation trial (Schmidt, Raines, Allan, & Zvolensky, 2016). Drawing from past work (Garey et al., 2016), it was hypothesized that change in dysphoria prior to quit attempt would moderate the effects of the pre-quit change in AS for withdrawal symptoms. Specific ally, smokers would benefit the most from the reduction in AS when they experience lower levels of dysphoria.
Method
Participants
Participants were smokers enrolled in a clinical trial evaluating the efficacy of a transdiagnostic smoking cessation treatment relative to a standard smoking cessation treatment (Schmidt, et al., 2016). Eligibility criteria for the trial included: (1) 18–65 years of age; (2) being a daily smoker for at least 1 year; (3) currently smoking a minimum of 8 cigarettes per day; and (4) motivation to quit smoking. Exclusion criteria for the trial included: (1) current use of pharmacotherapy for smoking cessation (except the nicotine patch, which was provided by the study); (2) limited mental competency and inability to provide informed, voluntary, written consent; (3) endorsement of current or past psychotic-spectrum symptoms; (4) current suicidality or homicidality; (5) history of significant medical condition; and (6) planning to relocate within the next 6 months.
Measures
Structured Clinical Interview-Non-Patient Version for DSM-IV (SCID-N/P;First, Spitzer, Gibbon, & Williams, 1994) is a semi-structured interview guide used to diagnose psychopathology. The interviews were administered by trained staff and supervised by independent doctoral-level psychologists. All interviews were audio-taped. Approximately 12.5% of interviews were randomly selected to verify accuracy of the diagnostic coding process; no discrepancies in diagnostic coding were noted.
The Medical Screening Form (MSF; Scheftner & Endicott, 1984) is a structured instrument administered by trained interviewers to assess lifetime medical history. This interview has excellent psychometric properties and has been extensively and successfully used in previous work for screening physical health problems and medication usage (e.g., Hays, Kallich, Mapes, Coons, & Carter, 1994). In the present study, a composite variable was computed as an index of tobacco-related medical illnesses, which was used as a covariate in all models. Items in which participants indicated that they had ever been diagnosed (i.e., respiratory disease, asthma, heart problems, and hypertension, all coded 0 = no, 1 = yes) were summed to create a total score (observed range from 0 to 4), with greater scores reflecting the presence of multiple markers of tobacco-related medical illnesses. The medical history form has been used as an indicator of medical problems among cigarette smokers in other work (e.g., Brandt, et al., 2015).
Smoking History Questionnaire (SHQ; Brown, Lejuez, Kahler, & Strong, 2002) is a self-report questionnaire used to assess smoking history and pattern (e.g. smoking rate, age of onset of initiation). It has been successfully used in previous studies as a measure of smoking history (Zvolensky, Lejuez, Kahler, & Brown, 2004). The present study used the following variables from the SHQ: average number of cigarettes smoked per day, age of onset of first cigarette, and age at onset of regular (daily) cigarette smoking.
Fagerström Test for Cigarette Dependence (FTCD; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) is a well-established 6-item scale designed to assess gradations in tobacco dependence. The measure exhibits good internal consistency reliability, high degree of test-retest reliability (Pomerleau, Carton, Lutzke, Flessland, & Pomerleau, 1994), and positive relations with key smoking variables (e.g., salivary cotinine; Heatherton et al., 1991; Payne, Smith, McCracken, McSherry, & Anthony, 1994). This variable was used for descriptive purposes.
Carbon Monoxide
Biochemical verification of smoking status was completed by carbon monoxide (CO) analysis of breath samples using a CMD/CO Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc.). Baseline level of CO and a time-varying variable representing the fluctuations in CO from quit-day to week 12 were used as covariates.
Nicotine Replacement Therapy Monitoring Form
A researcher-generated form was employed to measure use of nicotine patch during the cessation period. This measure has been employed in past work to monitory nicotine replacement usage (Zvolensky et al., in press). At the end of each quit week, participants responded to a question about whether they had used any nicotine replacement therapy during the past week.
Anxiety Sensitivity Index-III (ASI-III; Taylor, et al., 2007) is an 18-item self-report measure of the sensitivity to and fear of the potential negative consequences of anxiety-related symptoms and sensations. Respondents were asked to indicate on a 5-point Likert-type scale (0 = “very little” to 4 = “very much”) the degree to which they were concerned about each possible negative consequence. The ASI-III has been validated among smokers (Farris, et al., 2015). In the present study, we evaluated the ASI-III at baseline and quit-day sessions (Cronbach’s alpha = .91 and .90, respectively).
Inventory of Depression and Anxiety Symptoms – Dysphoria Subscale (IDAS; (Watson et al., 2007) is a 64-item self-report instrument that assesses distinct affect symptom dimensions within the past two weeks. Items are answered on a 5-point Likert scale ranging from “not at all” to “extremely.” The IDAS subscales show strong internal consistency, convergent and discriminant validity with psychiatric diagnoses and self-report measures (Watson, et al., 2007), and short-term retest reliability (r = 0.79) with both community and psychiatric patient samples (Watson et al., 2007). The IDAS has been successfully employed among smoking research (Mahaffey, et al., 2016). In the present study, we employed the 10-item Dysphoria Subscale (e.g., “I felt depressed”) evaluated at baseline and quit-day sessions (Cronbach’s alpha = .89 and .91, respectively).
Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1998) was used to assess the experience of tobacco withdrawal symptoms over the last 24 hours. Utilizing a 5-point Likert-type scale (0 = none to 4 = severe), respondents rated the degree to which symptoms (including craving, irritability/frustration or anger, anxiety, difficulty concentrating, restlessness, increased appetite, depressed or sad mood, and insomnia) were experienced. The MNWS has shown good validity and reliability in previous studies (Allen, Hatsukami, Christianson, & Nelson, 1996; Hughes, Gust, Skoog, Kennan, & Fenwick, 1991). Internal consistency for MNWS was good in the present sample (Cronbach’s alphas = .80, .82, .80, .80, and .81 for quit-day, week 1, week 2, week 4, and week 12 post-quit, respectively).
Procedure
Data for the present study were collected during a multi-site randomized controlled clinical trial examining the efficacy of two smoking cessation interventions described in detail elsewhere (Schmidt et al., 2016). Interested persons responding to community-based advertisements (e.g., flyers, newspaper ads, radio announcements) contacted the research team and were provided with a detailed description of the study via phone. Participants were then screened for initial eligibility, and if eligible, scheduled for a baseline appointment. At the baseline appointment, participants provided written informed consent, were interviewed using the SCID-I/NP, and completed a computerized self-report assessment battery as well as biochemical verification of smoking status to evaluate eligibility criteria.
Participants deemed eligible for the larger trial were randomly assigned to active or control treatment. The two treatment conditions included: a 4-session cognitive-behavioral smoking cessation program with an added AS reduction component (active; i.e., Panic-Smoking Program), or a standard cognitive-behavioral smoking cessation program (control). Both treatments took place over four, 90-minute sessions occurring once per week. The Panic-Smoking Program (active) integrates interoceptive exposure, cognitive restructuring, and psychoeducation exercises developed for panic prevention and treatment programs with standard smoking cessation counseling. The Standard Cessation Program (control) includes only the smoking-related components of the Panic-Smoking Program as well as a review of general health information not specific to anxiety or smoking (to equilibrate contact time across the two conditions). Both treatment groups received nicotine replacement therapy via the transdermal nicotine patch that was initiated at treatment Session 4 (quit-day). Participants were offered the nicotine replacement therapy for up to 12 weeks post-quit. Therapy sessions were supervised by principal investigators (MJZ and NBS) and checked for treatment fidelity by independent reviewers. Participants completed post-treatment assessment at 1-, 2-, 4-, and 12-weeks post quit. Participants were compensated $12.50 for completing the baseline visit, an additional $25 if they completed all treatment sessions, and $15 for each follow-up assessment they completed. We did not exclude individuals who smoked during the post quit period from the current study because past work suggests excluding these individuals can create a potentially under-representative sample (Cook et al., 2015; Hughes, 2007). The study protocol was approved by the Institutional Review Boards at the University of Vermont and Florida State University (clinicaltrials.gov # NCT01753141).
Analytic Strategy
The present study examined the main and interactive effects of change in AS and dysphoria as predictors of change in post-quit withdrawal symptoms over time assessed at quit day, 1-, 2-, 4-, and 12-weeks post-quit. Change in AS and dysphoria was calculated from baseline (pre-intervention) to quit day. Specifically, change for these predictors was derived using a two-step approach: (1) the difference score between quit-day and baseline was computed, with a positive score indicating a greater reduction in AS and dysphoria and, (2) a residualized change score was derived by regressing the difference score on a baseline score. This residualized score was derived to represent a “pure” change score after variance associated with the baseline score was removed (Tucker, Damarin, Messick, 1966) and was used in all following analyses. Tobacco withdrawal symptoms were modeled using data collected at quit-day and over the post-quit follow-up assessments (1-, 2-, 4-, and 12-weeks post-quit).
Five growth curve models were used to examine post-quit change in withdrawal symptoms from quit-day to post-quit assessments. Data were analyzed using multilevel linear modeling (MLM) in PROC MIXED procedure in SAS 9.4 software (SAS, Inc., Cary, NC). The estimation of unknown parameters in the models was based on the restricted maximum-likelihood estimation. The models included two levels, where repeated assessments across time (level-1; variables included time and time-varying breath CO;) were nested within participants (level-2; variables included change in AS, change in dysphoria, gender, baseline levels of CO, treatment condition, tobacco-related medical illness, and any use of nicotine replacement therapy). To allow for the time irregularity in the assessments, a time-structured predictor was included. Thus, the time values corresponded with the actual time-spaces (in weeks) between each follow-up assessment (Singer & Willett, 2003).
A fully unconditional model (Model 1) was used to estimate the intraclass correlation and design effect (Muthén, & Satorra, 1995). The magnitude of intraclass correlation and the size of the design effect (i.e., design effect = 1 + (Average cluster size −1)*Interclass correlation) were used to ensure that the growth curve models were the best data analytic approach in the context of the current study. Second, an unconditional linear growth model (Model 2) including examined quit-day levels (intercept) and a linear change in withdrawal symptoms (slopes) was used to examine whether change of withdrawal symptoms is adequately determined by a straight line. Third, an unconditional quadratic growth model (Model 3) was used to examine whether change of withdrawal symptoms is better determined by a curved line. Specifically, Model 3 examined quit-day levels (intercept) and both linear and quadratic changes in withdrawal symptoms (slopes). Fourth, a conditional model with level-2 predictors (Model 4) was used to predict quit-day levels (intercept) and change in withdrawal symptoms (slopes) with change in dysphoria, change in AS, and their interaction.
A two-step process was used to fit model 4: (a) the main effects model where predictors were simultaneously entered in the model and the main effects of each predictor were examined, and (b) the interactive effects model where the interaction terms of the two predictors were added into the main effects model. Finally, a conditional model with level-2 predictors and level-2 covariates (Model 5) was used to examine whether individual characteristics from model 4 significantly predict growth over and above the effects of covariates. Consistent with theoretical and empirical work, covariates included: gender (Leventhal, et al., 2007b), baseline levels of CO (Vogt, Selvin, & Billings, 1979), treatment condition (Hooten, et al., 2014), tobacco-related medical illness (McLeish, Farris, Johnson, Bernstein, & Zvolensky, 2016), use of nicotine replacement therapy (0 = no use and 1 = any use during the first two weeks following cessation; Silagy, Lancaster, Stead, Mant, & Fowler, 2004), and time-varying breath CO (Hughes, 2007).1
In all models, continuous variables were grand mean centered. Intercept and slopes in the models were considered as random to account for intraindividual variability of quit-day level and post-quit change in withdrawal symptoms. Although the study was concerned with the fixed effects included in the models, the random effects were modeled to account for the variability of the observed effects across individuals (statistics not presented). The unstructured covariance matrix was used for the Random statement to allow for separate estimation of variances and covariances with no expected pattern (Raudenbush & Bryk, 2002). The first-order autoregressive covariance matrix was used for the Repeated statement to allow for correlation among residuals as they were closer in time (i.e., the first assessments generally have higher correlations; Gibbons et al., 1993). Satterthwaite approximation was used to calculate the degrees of freedom (Satterthwaite, 1946).
Finally, using prototypical values (1 SD above and below mean of each predictor), we ran a post-hoc slope analysis of model 5. These analyses explored the interactive effects of change in dysphoria, change in AS on quit-day (initial) status, and the rate of change in withdrawal symptoms over time using 4 prototypical subgroups (Raudenbush, Brenna, & Barnett, 1995; Singer & Willett, 2003): (a) high AS reduction/high dysphoria reduction (1 SD above both means), (b) high AS reduction/limited dysphoria reduction (1 SD above and below mean respectively), (c) limited AS reduction/high dysphoria reduction (1 SD below and above means respectively), and (d) limited AS reduction/limited dysphoria reduction (1 SD below both means).
Results
Sample Descriptive Statistics
Of 529 smokers who were assigned to either the active or the control treatment, 265 completed the quit-day assessment and 198 (55.3% female; 86.8% Caucasian; Mage = 38.8, SD =14.0) completed at least one of the post-quit follow-up assessment. Only those participants who completed at least one post-quit follow-up assessment were included in the current analyses (active treatment: n = 114; control treatment: n = 84).
Participants reported smoking an average of 15.4 cigarettes per day (SD = 7.4), smoking their first cigarette at 14.7 years of age (SD = 3.6), and initiating regular (daily) smoking at 17.8 years of age (SD = 3.9). The average level of baseline breath CO was 19.7 (SD = 11.2) parts per million. The average score on the FTCD (Heatherton, et al., 1991) was 4.9 (SD = 2.2), indicating moderate levels of cigarette dependence. As determined by the baseline SCID-I/NP (First, et al., 1994), 31.4% of the sample met criteria for current (past year) Axis I psychopathology. The most common primary diagnoses included social anxiety disorder (10.0%), generalized anxiety disorder (5.7%), and major depressive disorder (4.1%).
Smoking and psychiatric characteristics of the sample, as well as all study variables are reported in Table 1.
Table 1.
| Demographic Variables | Number | Mean(SD)/(n)% | Range |
|---|---|---|---|
| Gender (female) | 198 | 109 (55.31%) | - |
| Age | 198 | 38.82 (14.04) | 18–64 |
| Ethnicity | 198 | ||
| Caucasian | 172 (86.83%) | - | |
| African American | 14 (6.84%) | - | |
| Hispanic | 8 (4.25%) | - | |
| Other | 4 (2.12%) | - | |
|
| |||
| Other Variables | |||
|
| |||
| Treatment condition | 198 | 115 (58.10%) | - |
| Use of Nicotine Replacement Therapy | 198 | 154 (77.77%) | - |
| Tobacco-related medical illnesses | 198 | 0.38 (0.49) | 0–3 |
| Baseline Breath CO | 198 | 19.68 (11.1) | 4–71 |
| Quit Day Breath CO | 198 | 9.30 (8.91) | 1–56 |
| Week 1 Breath CO | 190 | 6.22 (6.11) | 1–61 |
| Week 2 Breath CO | 173 | 6.29 (7.10) | 1–59 |
| Week 4 Breath CO | 143 | 7.71 (7.35) | 1–49 |
| Week 12 Breath CO | 103 | 11.38 (11.33) | 1–56 |
| Baseline ASI-III | 198 | 13.42 (11.20) | 1–54 |
| Quit-day ASI-III | 198 | 10.25 (9.71) | 0–49 |
| Baseline IDAS-DYS | 198 | 17.53 (7.06) | 10–42 |
| Quit-day IDAS-DYS | 198 | 17.27 (6.41) | 10–39 |
| Quit Day Withdrawal Symptoms | 198 | 14.64(4.24) | 8–27 |
| Week 1 Withdrawal Symptoms | 198 | 15.91(4.39) | 8–25 |
| Week 2 Withdrawal Symptoms | 167 | 14.94(4.29) | 8–26 |
| Week 4 Withdrawal Symptoms | 143 | 14.11(4.57) | 8–27 |
| Week 12 Withdrawal Symptoms | 100 | 13.73(4.28) | 8–26 |
| Week 1 7-day point prevalence abstinence | 181 | 67 (36.91%) | - |
| Week 2 7-day point prevalence abstinence | 159 | 26 (16.72%) | - |
| Week 4 7-day point prevalence abstinence | 133 | 17 (12.54%) | - |
| Week 12 7-day point prevalence abstinence | 102 | 10 (9.60%) | - |
Note. Tobacco-related medical illnesses as per the Medical Screening Form (MSF; Scheftner & Endicott, 1984); CO = Carbon Monoxide; ASI-3 = Anxiety Sensitivity Index–III (Taylor et. al., 2007); IDAS-DYS = Inventory of Depression and Anxiety Symptoms – Dysphoria Subscale (Watson et. al., 2007); Withdrawal Symptoms as per the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1998).
Growth Curve Models
Preliminary analyses
The intraclass correlation and the design effect for Model 1 were 0.56 and 2.8, respectively. Thus, about 56% of the total variance in withdrawal symptoms was due to inter-individual differences and 44% was due to intra-individual differences. The magnitude of intraclass correlation and the size of the design effect indicated significant variability at both within and between individual levels, which supports the use of multilevel modeling.
Fixed effects
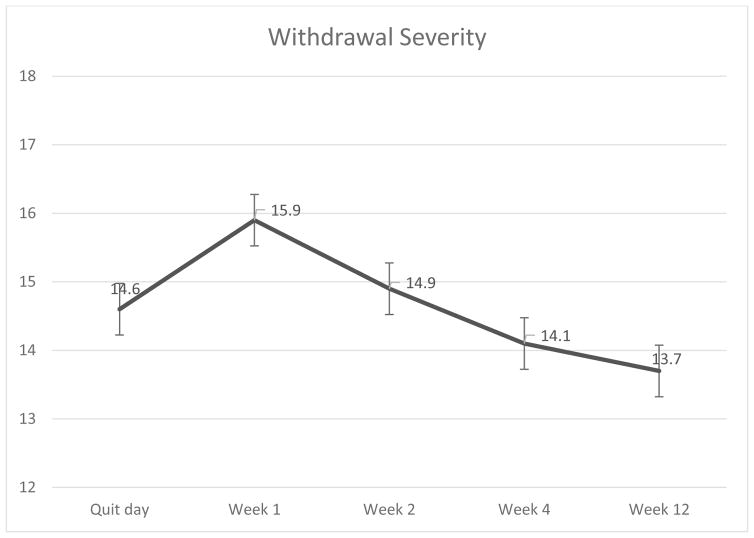
Table 2 presents the fixed effects of predictors for all models. For model 2, the results suggested a significant linear decrease in mean withdrawal symptoms over time. For Model 3, the results suggested that the rate of growth decelerated over time as evinced by a significant negative quadratic term (Table 2 and Figure 1).
Table 2.
Fixed effects of time and covariates across models.
| Model 1 (Fully unconditional Model) | |||||
|
| |||||
| Effect | Estimate | SE | Pr > |t| | Low CI | High CI |
| Intercept | 14.66 | 0.22 | <.001 | 14.14 | 15.18 |
|
| |||||
| Model 2 (Unconditional linear growth model) | |||||
|
| |||||
| Effect | Estimate | SE | Pr > |t| | Low CI 5.75 | High CI |
| Intercept | 14.68 | 0.23 | <.001 | 14.17 | 15.19 |
| time | −.40 | .07 | <.001 | −.55 | −.24 |
|
| |||||
| Model 3 (Unconditional quadratic growth model) | |||||
|
| |||||
| Effect | Estimate | SE | Pr > |t| | Low CI | High CI |
| Intercept | 14.71 | 0.25 | <.001 | 14.20 | 15.22 |
| time | 1.26 | 0.20 | <.001 | 0.85 | 1.67 |
| time2 | −0.45 | 0.05 | <.001 | −0.55 | −0.35 |
|
| |||||
| Model 4 (Conditional main effect model) | |||||
|
| |||||
| Effect | Estimate | SE | Pr > |t| | Low CI | High CI |
| Δ Anxiety Sensitivity | −0.14 | 0.03 | <.001 | − 0.16 | −0.05 |
| Δ Anxiety Sensitivity*time | −0.04 | 0.03 | 0.15 | −0.09 | 0.02 |
| Δ Anxiety Sensitivity*time2 | .0.01 | 0.01 | 0.13 | −0.003 | 0.02 |
| Δ Dysphoria | −0.38 | 0.04 | <.001 | −0.46 | −0.32 |
| Δ Dysphoria*time | −0.04 | 0.04 | 0.27 | −0.03 | 0.11 |
| Δ Dysphoria*time2 | 0.001 | 0.001 | 0.95 | −0.02 | 0.01 |
|
| |||||
| Model 4 (Conditional interactive effect model) | |||||
|
| |||||
| Effect | Estimate | SE | Pr > |t| | Low CI | High CI |
| Δ Anxiety Sensitivity * Δ Dysphoria | 0.007 | 0.006 | 0.68 | −0.005 | 0.009 |
| Δ Anxiety Sensitivity * Δ Dysphoria*time | 0.01 | 0.006 | <.001 | 0.003 | 0.005 |
| Δ Anxiety Sensitivity * Δ Dysphoria*time2 | −0.006 | 0.001 | <.001 | −0.001 | −0.0004 |
|
| |||||
| Model 5 (Conditional main effect model with covariates)3 | |||||
|
| |||||
| Effect | Estimat | SE | Pr > |t| | Low CI | High CI |
| Intercept | 14.43 | 0.62 | <.001 | 13.21 | 15.66 |
| time | 0.71 | 0.54 | 0.18 | −0.34 | 1.78 |
| time2 | −0.31 | 0.12 | 0.01 | −0.57 | −0.06 |
| Time-varying CO 4 | 0.003 | 0.01 | 0.80 | −0.02 | 0.03 |
| Condition 5 | −0.37 | 0.48 | 0.44 | −1.33 | 0.58 |
| Condition*time | −0.21 | 0.16 | 0.20 | −0.55 | 0.11 |
| Gender | −0.02 | 0.47 | 0.95 | −0.96 | 0.91 |
| Gender*time | 0.03 | 0.16 | 0.83 | −0.28 | 0.35 |
| Tobacco-related illness | −0.17 | 0.72 | 0.80 | −1.61 | 1.25 |
| Tobacco-related illness*time | −0.24 | 0.25 | 0.35 | −0.74 | 0.26 |
| Baseline CO | 0.01 | 0.02 | 0.43 | −0.02 | 0.06 |
| Baseline CO*time | 0.01 | 0.02 | 0.59 | −0.03 | 0.05 |
| Baseline CO*time2 | −0.004 | 0.01 | 0.43 | −0.01 | 0.01 |
| Use of NRT | 0.06 | 0.59 | 0.91 | −1.11 | 1.23 |
| Use of NRT*time | 1.31 | 0.60 | 0.03 | 0.12 | 2.50 |
| Use of NRT*time2 | −0.29 | 0.14 | 0.04 | −0.58 | −0.001 |
| Δ Anxiety Sensitivity | −0.13 | 0.03 | 0.001 | −0.20 | −0.06 |
| Δ Anxiety Sensitivity*time | 0.003 | 0.03 | 0.91 | −0.06 | 0.07 |
| Δ Anxiety Sensitivity*time2 | −0.001 | 0.01 | 0.86 | −0.01 | 0.01 |
| Δ Dysphoria | −0.34 | 0.04 | <.001 | −0.44 | −0.25 |
| Δ Dysphoria*time | 0.001 | 0.04 | 0.97 | −0.09 | 0.09 |
| Δ Dysphoria*time2 | 0.01 | 0.01 | 0.49 | −0.01 | 0.03 |
|
| |||||
| Model 5 (Conditional interactive effect model with covariates) | |||||
|
| |||||
| Effect | Estimat | SE | Pr >|t| | Low CI | High CI |
| Δ Anxiety Sensitivity * Δ Dysphoria | 0.003 | 0.004 | 0.94 | −0.009 | 0.009 |
| Δ Anxiety Sensitivity * Δ Dysphoria*time | 0.002 e | 0.001 | 0.002 | 0.001 | 0.004 |
| Δ Anxiety Sensitivity * Δ Dysphoria*time2 | −0.002 | 0.001 | 0.06 | −0.005 | 0.0003 |
Note. N=198. Low CI & High CI= 95% confidence interval of the estimated beta coefficient; Gender:1 0=male and 1=female; CO = Carbon Monoxide; NRT = Nicotine Replacement Therapy; CPD = Cigarettes Per Day; Δ Anxiety Sensitivity = change in Anxiety Sensitivity (residualized change score); Δ Dysphoria = change in Dysphoria (residualized change score).
Figure 1.
Mean withdrawal symptom severity during the 12 weeks following a quit attempt (N = 198).
For the main effects of Model 4, the results suggested that “higher” change scores (reduction) of AS and dysphoria predicted lower levels of quit-day withdrawal symptoms, but not of linear and quadratic trajectories of withdrawal symptoms.
For the interactive effects of Model 4, results suggested that the interaction term was a statistically significant predictor of the linear and quadratic trajectories of withdrawal symptoms across the 12-week post-quit period, but not of quit-day withdrawal symptoms. Finally, after introducing the covariates in Model 5, the general pattern of the results remained consistent except for the quadratic trajectory, such that the interaction term became a marginally significant predictor (Table 2).
Post-hoc pairwise comparisons of differences between linear change in withdrawal symptoms showed a significant difference in slope for the high AS reduction/limited dysphoria reduction group compared with the limited AS reduction/limited dysphoria reduction group (Table 3). No significant difference was found in the other pair-wise comparisons. Inspection of the linear trajectories of the withdrawal symptom severity for the four prototypical groups revealed that the groups with higher levels of reduction in either AS or dysphoria (i.e., high AS reduction/high dysphoria reduction, high AS reduction/limited dysphoria reduction, and limited AS reduction/high dysphoria reduction) had lower levels of quit-day and week 12 withdrawal symptoms compared with the limited AS reduction/limited dysphoria reduction group (Figure 2). Moreover, the high AS reduction/limited dysphoria reduction group showed a fast reduction in their withdrawal symptoms, such that despite the higher initial levels, this group showed comparable levels of withdrawal symptoms at week 12 compared with high AS reduction/high dysphoria reduction and limited AS reduction/high dysphoria reduction groups (Figure 2). In other words, the linear trajectory of withdrawal symptoms appears to diverge across different degrees of AS reductions among “limited dysphoria reduction” (but not “high dysphoria reduction”) groups.
Table 3.
Post-hoc pairwise comparisons of the linear changes in withdrawal symptoms across the four prototypical groups.
| Pair of slopes | t-value for slope difference | p-value for slope difference |
|---|---|---|
| High AS reduction/High Dysphoria reduction Vs High AS reduction/Limited dysphoria reduction | 1.361 | 0.206 |
| High AS reduction/High Dysphoria reduction Vs Limited AS reduction/High Dysphoria reduction | −1.342 | 0.212 |
| High AS reduction/High Dysphoria reduction Vs Limited AS reduction/Limited dysphoria reduction | −1.538 | 0.160 |
| High AS reduction/Limited Dysphoria reduction Vs Limited AS reduction/High Dysphoria reduction | −1.062 | 0.316 |
| High AS reduction/Limited Dysphoria reduction Vs Limited AS reduction/Limited dysphoria reduction | −2.951 | 0.016 |
| Limited AS reduction/High Dysphoria reduction Vs Limited AS reduction/Limited dysphoria reduction | −0.734 | 0.483 |
Figure 2.
Differential linear change in withdrawal severity across four prototypical groups.
Variance explained
The unexplained variability (residual variance) in withdrawal symptoms reduced from 8.5% in Model 1 to 7.5% in Model 2, suggesting that about 12.0% of the within-individual variation in withdrawal symptoms was due to linear rate of change. This residual variance further changed from 7.5% in Model 2 to 7.4% in Model 3, suggesting that an additional 1.0% of the within-individual variation in withdrawal symptoms was due to the quadratic rate of change. Compared with Model 3, the unexplained variability substantially reduced in Model 4 from 7.4% to 5.8%, suggesting that the predictors accounted for 21.0% of the overall within-individual variability in withdrawal symptoms. Addition of covariates in Model 5 again reduced the unexplained variability to 5.5%, which suggests these covariates explain approximately 5.0% of the unexplained variance over and above the effects of the predictors in the model.
Discussion
As hypothesized, the interactive effects of change in AS and dysphoria were associated with linear change in post-quit withdrawal symptoms. Specifically, the smokers that benefitted most from a reduction in AS were those that had lower levels of change in dysphoria. Thus, a reduction in dysphoric symptoms moderated the effects of reduction in AS in relation to linear change of withdrawal symptoms; see Figure 2). The observed moderating role of change in dysphoria is consistent with past research demonstrating the mitigating role of dysphoria for the effects of AS on smoking-related outcomes (Garey et al., 2016), and extends it to dynamic models of AS and dysphoria. Specifically, the findings suggest smokers who do not experience a reduction in dysphoria following an intervention could benefit most from reducing AS. Notably, smokers who did not experience significant reductions in AS or dysphoria (i.e., limited AS reduction/limited dysphoria reduction) experienced the slowest reduction rate in tobacco withdrawal compared with the other three groups (see Figure 2). These novel findings broadly highlight importance of better understanding the interplay of change in cognitive-affective vulnerability characteristics in terms of trajectories of tobacco withdrawal. It also suggests there is a need to assess and employ decision rules to identify and more effectively target the population of smokers with distinct cognitive-affective vulnerability profiles (Bierut, Johnson, & Saccone, 2014).
Another clinically important finding was that change in AS and change in dysphoria each individually predicted quit-day withdrawal symptom severity. Specifically, smokers with greater decreases in either AS and/or dysphoria experienced less severe quit-day withdrawal symptoms. These observations are in line with past research which highlights the role of AS and dysphoria in terms of understanding the expression of tobacco withdrawal during acute periods of abstinence (Langdon et al., 2013; Zvolensky et al., 2014b). Thus, regardless of any interactive effects, these data suggest that pre-quit reduction of AS and dysphoria could benefit smokers by reducing tobacco withdrawal (Zvolensky et al., 2014a).
Overall, the present findings suggest that addressing AS and dysphoria prior to engaging in a quit attempt may (a) lessen the intensity of withdrawal symptoms experienced on quit-day; and (b) promote a faster decline in withdrawal symptom severity across the quit period. Given the strong association documented between withdrawal symptoms and smoking lapse/relapse (Piasecki et al., 2000), pre-quit screening of AS and dysphoria followed by the delivery of the interventions that target these factors (i.e. with respect to the individual’s specific vulnerability profile), should help decrease the severity of tobacco withdrawal, and by extension, improve cessation outcome.
There are several study limitations. First, our sample consisted of community-recruited, treatment-seeking daily cigarette smokers with moderate levels of cigarette dependence. Future studies may benefit by sampling from lighter and heavier smoking populations to determine the generalizability of the results to the general smoking population. Second, the study focused on AS and dysphoria. These constructs represent only two of many possible cognitive-affective characteristics that may be related to tobacco withdrawal. Future work could usefully continue to build multi-risk factor models of withdrawal by incorporating other promising variables (e.g., distress intolerance, pain-related anxiety; Leventhal & Zvolensky, 2015). Third, the current study employed a composite withdrawal measure that amalgamates symptoms into a single index, including some symptoms which may not contribute to relapse risk (e.g., hunger, concentration problems; McCarthy, Piasecki, Fiore, & Baker, 2006; Piper et al., 2011), and omitting other symptoms that do appear to reliably predict relapse (e.g., diminished positive affect; Strong et al., 2009; Piper et al., 2011). It may therefore be useful for future research to explore the nature of specific withdrawal symptom clusters in the context of change in AS and dysphoria. Fourth, due to the focus of the one of the study arms on targeting AS, it is possible that some of the observed effects may be relatively more robust and not fully generalizable to the other smoking cessation treatments. Future work could therefore examine the present research questions within the context of other smoking cessation interventions. Fifth, the study design did not permit an examination of whether AS and dysphoria increased in anticipation of a quit attempt, or were reactive responses to smoking cessation events. Future work using cross-lagged designs could help answer these types of questions. Similarly, the role of change in AS and/or dysphoria in affecting one’s decision to make a quit attempt at a particular time could be examined in future research. Finally, no significant difference in pre-quit reduction in either AS or dysphoria were observed across the treatment groups. Although the two treatments may have improved mental health in general, future research could usefully explore how to target these constructs in a more efficient manner in the context of smoking cessation.
Together, the current data offer novel insight into the clinically important role of change in AS and dysphoria in relation to tobacco withdrawal. A key finding was that the benefits of a pre-quit reduction in AS in terms of the severity of withdrawal was most prominent for smokers with lower pre-quit reduction in dysphoria. Additionally, the findings indicated that reducing levels of AS and dysphoria pre-quit is broadly related to the degree of change in post-quit withdrawal symptoms. Collectively, these data suggest there is apt to be clinical merit to employing strategies to address AS and/or dysphoria to more effectively manage emergent withdrawal symptoms following smoking cessation treatment. Future theory-driven work is needed to continue to understand the role of change in AS and dysphoria for withdrawal and other smoking processes (e.g., craving) and use such information to refine more personalized approaches to smoking cessation treatment.
Acknowledgments
Funding information:
National Institute of Mental Health http://dx.doi.org/10.13039/100000025 R01MH076629-01A1
Footnotes
We also examined the demographic characteristics of the current study participants compared to those that were not included in the analyses. Results indicated that there was no difference in terms of gender and smoking variables between the two groups.
The mean change in AS and dysphoria were 3.2 (SD=2.9) and 0.7 (SD=0.6), respectively. These scores were significantly correlated (r = .38, p<.001).
We re-ran analyses with psychopathology status as an additional co-variate. The results did not change in terms of statistical significance or pattern of findings. We did not include psychopathology status as an additional co-variate, as it is related to both the AS and dysphoria and tobacco withdrawal symptoms, and may therefore, risk over modeling the data (Miller & Chapman, 2001).
We also re-ran all the models with the time-varying CO verified abstinence rate as the covariate. The pattern of the results stayed the same.
The active and control groups did not show significant differences in baseline, quit-day, or the rates of change in either AS or dysphoria. Furthermore, the “homogeneity of slopes” tests did not show significant interactions between treatment condition and either change in AS or dysphoria.
References
- Ablon JS, Levy RA, Katzenstein T. Beyond brand names of psychotherapy: Identifying empirically supported change processes. Psychotherapy: Theory, Research, Practice, Training. 2006;43(2):216–231. doi: 10.1037/0033-3204.43.2.216. [DOI] [PubMed] [Google Scholar]
- Abrantes AM, Strong DR, Lejuez CW, Kahler CW, Carpenter LL, Price LH, … Brown RA. The role of negative affect in risk for early lapse among low distress tolerance smokers. Addictive Behaviors. 2008;33(11):1394–1401. doi: 10.1016/j.addbeh.2008.06.018. doi.org/10.1016/j.addbeh.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Christianson D, Nelson D. Symptomatology and energy intake during the menstrual cycle in smoking women. Journal of Substance Abuse. 1996;8(3):303–319. doi: 10.1016/S0899-3289(96)90170-4. [DOI] [PubMed] [Google Scholar]
- Assayag Y, Bernstein A, Zvolensky MJ, Steeves D, Stewart SS. Nature and role of change in anxiety sensitivity during NRT-aided cognitive-behavioral smoking cessation treatment. Cognitive Behavior Therapy. 2012;41(1):51–62. doi: 10.1080/16506073.2011.632437. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, … Hyland A. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9(Suppl 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista SR, Stewart SH, Fulton HG, Steeves D, Darredeau C, Gavric D. A further investigation of the relations of anxiety sensitivity to smoking motives. Addictive Behaviors. 2008;33(11):1402–1408. doi: 10.1016/j.addbeh.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Carver CS. Attentional bias and mood persistence as prospective predictors of dysphoria. Cognitive Therapy and Research. 2003;27(6):619–637. doi: 10.1023/A:1026347610928. [DOI] [Google Scholar]
- Bierut LJ, Johnson EO, Saccone NL. A glimpse into the future–Personalized medicine for smoking cessation. Neuropharmacology. 2014;76:592–599. doi: 10.1016/j.neuropharm.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CP, Bakhshaie J, Garey L, Schmidt NB, Leventhal AM, Zvolensky MJ. The moderating role of smoking amount per day on the relations between anxiety sensitivity, smoking dependence, and cognitive–affective aspects of smoking among treatment seeking smokers. Addictive Behaviors Reports. 2015;1:26–33. doi: 10.1016/j.abrep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: Findings from an epidemiologic study of young adults. The American Journal of Psychiatry. 1992;149(4):464–9. doi: 10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- Brose A, Schmiedek F, Koval P, Kuppens P. Emotional inertia contributes to depressive symptoms beyond perseverative thinking. Cognition and Emotion. 2015;29(3):527–538. doi: 10.1080/02699931.2014.916252. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Zvolensky MJ, Lejuez CW, Ramsey SE. Anxiety sensitivity: Relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addictive Behaviors. 2001;26(6):887–899. doi: 10.1016/S0306-4603(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111(1):180–185. doi: 10.1037/0021-843X.111.1.180. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Farris SG, Zvolensky MJ, Shah SM, Leventhal AM, Minnix JA, Schmidt NB. Dysphoria and smoking among treatment seeking smokers: the role of smoking-related inflexibility/avoidance. American Journal of Drug & Alcohol Abuse. 2015;41(1):45–51. doi: 10.3109/00952990.2014.927472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Cooper A, Dymond SO, Reed P. The relationship between dysphoria and proneness to hallucination and delusions among young adults. Comprehensive Psychiatry. 2008;49(6):544–550. doi: 10.1016/j.comppsych.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ. Self-related neural response to tailored smoking-cessation messages predicts quitting. Nature Neuroscience. 2011;14(4):426–427. doi: 10.1038/nn.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. Journal of Abnormal Psychology. 2015;124(1):215–225. doi: 10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey LS. Nicotine dependence and its associations with psychiatric disorders: Research evidence and treatment implications. In: Seidman DF, Covey LS, editors. Helping the Hard-Core Smoker: A Clinician s Guide. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers; 1999. [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Comprehensive Psychiatry. 1990;31(4):350–354. doi: 10.1016/0010-440X(90)90042-Q. [DOI] [PubMed] [Google Scholar]
- Dobkin RD, Panzarella C, Fernandez J, Alloy LB, Cascardi M. Adaptive inferential feedback, depressogenic inferences, and depressed mood: A laboratory study of the expanded hopelessness theory of depression. Cognitive Therapy and Research. 2004;28(4):487–509. doi:1023/B:COTR.0000045560.71692.88. [Google Scholar]
- Farris SG, DiBello AM, Allan NP, Hogan J, Schmidt NB, Zvolensky MJ. Evaluation of the Anxiety Sensitivity Index-3 among treatment-seeking smokers. Psychological Assessment. 2015;27(3):1123–1128. doi: 10.1037/pas0000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for Axis I DSM-IV disorders. New York: Biometrics Research; 1994. [Google Scholar]
- Garey L, Bakhshaie J, Brandt CP, Langdon KJ, Kauffman BY, Schmidt NB, … Zvolensky MJ. Interplay of dysphoria and anxiety sensitivity in relation to emotion regulatory cognitions of smoking among treatment-seeking smokers. The American Journal on Addictions. 2016;25(4):267–274. doi: 10.1111/ajad.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, … Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: Application to the NIMH Treatment of Depression Collaborative Research Program dataset. Archives of General Psychiatry. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Ginsberg D, Hall SM, Reus VI, Muñoz RF. Mood and depression diagnosis in smoking cessation. Experimental and Clinical Psychopharmacology. 1995;3(4):389–395. doi: 10.1037/1064-1297.3.4.389. [DOI] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI, Sees KL. Nicotine, negative affect, and depression. Journal of Consulting and Clinical Psychology. 1993;61(5):761. doi: 10.1037/0022-006X.61.5.761. [DOI] [PubMed] [Google Scholar]
- Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOLTM) instrument. Quality of Life Research. 1994;3(5):329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hooten WM, Townsend CO, Hays JT, Ebnet KL, Gauvin TR, Gehin JM, … Warner DO. A cognitive behavioral smoking abstinence intervention for adults with chronic pain: a randomized controlled pilot trial. Addictive Behaviors. 2014;39(3):593–599. doi: 10.1016/j.addbeh.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Measurement of the effects of abstinence from tobacco: a qualitative review. Psychology of Addictive Behaviors. 2007;21(2):127–137. doi: 10.1037/0893-164X.21.2.127. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: a replication and extension. Archives of General Psychiatry. 1991;48(1):52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami D. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7(1):92. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Farris SG, Schmidt NB, Smits JA, Zvolensky MJ. Panic attack history and anxiety sensitivity in relation to cognitive-based smoking processes among treatment-seeking daily smokers. Nicotine & Tobacco Research. 2013;15(1):1–10. doi: 10.1093/ntr/ntr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough ME, Schmidt NB. Refinement of a brief anxiety sensitivity reduction intervention. Journal of Consulting and Clinical Psychology. 2012;80(5):766–772. doi: 10.1037/a0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KJ, Leventhal AM, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Anhedonia and anxiety sensitivity: Prospective relationships to nicotine withdrawal symptoms during smoking cessation. Journal of Studies on Alcohol and Drugs. 2013;74(3):469–478. doi: 10.15288/jsad.2013.74.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Heishman SJ, Lerman C, Pickworth WB. Associations between Cloninger’s temperament dimensions and acute tobacco withdrawal. Addictive Behaviors. 2007a;32(12):2976–2989. doi: 10.1016/j.addbeh.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Experimental and Clinical Psychopharmacology. 2007b;15(1):21. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. 2015;141(1):176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ, Schmidt NB. Smoking-related correlates of depressive symptom dimensions in treatment-seeking smokers. Nicotine & Tobacco Research. 2011;13(8):668–676. doi: 10.1093/ntr/ntr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey BL, Gonzalez A, Farris SG, Zvolensky MJ, Bromet EJ, Luft BJ, Kotov R. Smoking to Regulate Negative Affect: Disentangling the Relationship Between Posttraumatic Stress and Emotional Disorder Symptoms, Nicotine Dependence, and Cessation-Related Problems. Nicotine & Tobacco Research. 2016;18(6):1471–1478. doi: 10.1093/ntr/ntv175. [DOI] [PubMed] [Google Scholar]
- Marshall GN, Miles JN, Stewart SH. Anxiety sensitivity and PTSD symptom severity are reciprocally related: evidence from a longitudinal study of physical trauma survivors. Journal of Abnormal Psychology. 2010;119(1):143. doi: 10.1037/a0018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: an electronic diary study. Journal of Abnormal Psychology. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Kertz SJ, Weiss RB, Baskin-Sommers AR, Hearon BA, Björgvinsson T. Changes in distress intolerance and treatment outcome in a partial hospital setting. Behavior Therapy. 2014;45(2):232–240. doi: 10.1016/j.beth.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeish AC, Farris SG, Johnson AL, Bernstein JA, Zvolensky MJ. Evaluation of smokers with and without asthma in terms of smoking cessation outcome, nicotine withdrawal symptoms, and craving: Findings from a self-guided quit attempt. Addictive Behaviors. 2016;63:149–154. doi: 10.1016/j.addbeh.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding Analysis of Covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Satorra A. Complex sample data in structural equation modeling. Sociological Methodology. 1995;25:267–316. doi: 10.2307/271070. [DOI] [Google Scholar]
- Otto MW, Eastman A, Lo S, Hearon BA, Bickel WK, Zvolensky M, … Doan SN. Anxiety sensitivity and working memory capacity: Risk factors and targets for health behavior promotion. Clinical Psychology Review. 2016;49:67–78. doi: 10.1016/j.cpr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM. Assessing nicotine dependence: A comparison of the Fagerström Tolerance Questionnaire (FTQ) with the Fagerström Test for Nicotine Dependence (FTND) in a clinical sample. Addictive Behaviors. 1994;19(3):307–317. doi: 10.1016/0306-4603(94)900329. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26(2):196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology. 2000;109(1):74–86. doi: 10.1037/0021-843X.109.1.74. [DOI] [PubMed] [Google Scholar]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, … Baker TB. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology. 2011;216(4):569–578. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerstrom tolerance questionnaire and the Fagerstrom test for nicotine dependence. Addictive Behaviors. 1994;19(1):33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerleau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptomatology. Nicotine & Tobacco Research. 2000;2(3):275–280. doi: 10.1080/14622200050147547. doi.org/10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Brennan RT, Barnett RC. A multivariate hierarchical model for studying psychological change within married couples. Journal of Family Psychology. 1995;9(2):161–174. doi: 10.1037/0893-3200.9.2.161. [DOI] [Google Scholar]
- Raudenbush SW, Bryk AS. Advanced quantitative techniques in the social sciences. Newbury Park, CA: Sage; 2002. Hierarchical linear models: Applications and data analysis methods. [Google Scholar]
- Reinecke MA, Ryan NE, DuBois DL. Cognitive-behavioral therapy of depression and depressive symptoms during adolescence: a review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37(1):26–34. doi: 10.1097/00004583-199801000-00013. [DOI] [PubMed] [Google Scholar]
- Reiss S, McNally RJ. The expectancy model of fear. In: Reiss S, Bootzin RR, editors. Theoretical Issues in Behavior Therapy. New York: Academic Press; 1985. pp. 107–122. [Google Scholar]
- Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bulletin. 1946;2(6):110–114. [PubMed] [Google Scholar]
- Scheftner W, Endicott J. Medical history form II. Dr. Scheftener at Rush-Presbyterian-St. Luke’s Medical Center; 1753 West Congress Parkway, Chicago, IL 60612: 1984. [Google Scholar]
- Schmidt NB, Capron DW, Raines AM, Allan NP. Randomized clinical trial evaluating the efficacy of a brief intervention targeting anxiety sensitivity cognitive concerns. Journal of Consulting and Clinical Psychology. 2014;82(6):1023. doi: 10.1037/a0036651. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Eggleston AM, Woolaway-Bickel K, Fitzpatrick KK, Vasey MW, Richey JA. Anxiety Sensitivity Amelioration Training (ASAT): A longitudinal primary prevention program targeting cognitive vulnerability. Journal of Anxiety Disorders. 2007;21(3):302–319. doi: 10.1016/j.janxdis.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Raines AM, Allan NP, Zvolensky MJ. Anxiety sensitivity risk reduction in smokers: A randomized control trial examining effects on panic. Behaviour Research and Therapy. 2016;77:138–146. doi: 10.1016/j.brat.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. American Psychologist. 2005;60(5):410. doi: 10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. The Cochrane Database of Systematic Reviews. 2004;3:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford, NY: Oxford University Press; 2003. [Google Scholar]
- Smits JA, Berry AC, Tart CD, Powers MB. The efficacy of cognitive-behavioral interventions for reducing anxiety sensitivity: A meta-analytic review. Behaviour Research and Therapy. 2008;46(9):1047–1054. doi: 10.1016/j.brat.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Smits JAJ, Zvolensky MJ, Davis ML, Rosenfield D, Marcus BH, Church TS, Powers MB, Frierson GM, Otto MW, Hopkins LB, Brown RA, Baird SO. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with high anxiety sensitivity: A randomized controlled trial. Psychosomatic Medicine. 2016;78:354–364. doi: 10.1097/PSY.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic V. Dysphoric about dysphoria: towards a greater conceptual clarity of the term. Australasian Psychiatry. 2007;15(1):9–13. doi: 10.1080/10398560601083035. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, Brown RA. Impact of bupropion and cognitive–behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research. 2009;11(10):1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, … Coles M. Robust dimensions of anxiety sensitivity: development and initial validation of The Anxiety Sensitivity Index-3. Psychological Assessment. 2007;19(2):176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Timpano KR, Raines AM, Shaw AM, Keough ME, Schmidt NB. Effects of a brief anxiety sensitivity reduction intervention on obsessive compulsive spectrum symptoms in a young adult sample. Journal of Psychiatric Research. 2016;83:8–15. doi: 10.1016/j.jpsychires.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LR, Damarin F, Messick S. A base-free measure of change. Psychometrika. 1966;31(4):457–473. doi: 10.1007/BF02289517. [DOI] [PubMed] [Google Scholar]
- Vogt TM, Selvin S, Billings JH. Smoking cessation program: Baseline carbon monoxide and serum thiocyanate levels as predictors of outcome. American Journal of Public Health. 1979;69:1156–1159. doi: 10.2105/ajph.69.11.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Kotov R, Simms LJ, Chmielewski M, McDade-Montez EA, … Stuart S. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychological Assessment. 2007;19(3):253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bogiaizian D, Salazar PL, Farris SG, Bakhshaie J. An anxiety sensitivity reduction smoking-cessation program for Spanish-speaking smokers (Argentina) Cognitive and Behavioral Practice. 2014a;21(3):350–363. doi: 10.1016/j.cbpra.2013.10.005. [DOI] [Google Scholar]
- Zvolensky MJ, Farris SG, Guillot CR, Leventhal AM. Anxiety sensitivity as an amplifier of subjective and behavioral tobacco abstinence effects. Drug and Alcohol Dependence. 2014b;142:224–230. doi: 10.1016/j.drugalcdep.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Farris SG, Leventhal AM, Schmidt NB. Anxiety sensitivity mediates relations between emotional disorders and smoking. Psychology of Addictive Behaviors. 2014c;28(3):912–920. doi: 10.1037/a0037450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez CW, Kahler CW, Brown RA. Nonclinical panic attack history and smoking cessation: An initial examination. Addictive Behaviors. 2004;29(4):825–830. doi: 10.1016/j.addbeh.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Paulus DJ, Garey L, Raines AM, Businelle M, Shankman SA, Manning K, Goodwin RD, Schmidt NB. Anxiety sensitivity and nicotine replacement therapy side effects: Examining the role of emotion dysregulation among treatment-seeking smokers. Journal of Studies on Alcohol and Drugs. doi: 10.15288/jsad.2017.78.877. in press. [DOI] [PubMed] [Google Scholar]