Abstract
Background
The objective of this study was to evaluate the effects of gene polymorphisms, including UGT1A1*7, *27, and *29, on the safety of irinotecan therapy.
Methods
The eligibility criteria were: lung cancer patients scheduled to undergo irinotecan therapy, aged ≥ 20 years, with a performance status of 0–2. Thirty‐one patients were enrolled and their blood was collected and used to examine the frequency of UGT1A1*6, *7, *27, *28, and *29 polymorphisms and the concentrations of irinotecan, SN‐38, and SN‐38G after irinotecan therapy.
Results
The patients’ characteristics were as follows: male/female 25/6, median age 71 years (range 55–84), stage IIB/IIIA/IIIB/IV 2/6/11/12, and adenocarcinoma/squamous cell carcinoma/small cell carcinoma/other 14/10/3/4, respectively. The −/−, *6/−, *7/−, *27/−, *28/−, and *29/− UGT1A1 gene polymorphisms were observed in 10 (32%), 10 (32%), 2 (6%), 2 (6%), 7 (23%), and 0 (0%) cases, respectively. The UGT1A1*27 polymorphism occurred separately from the UGT1A1*28 polymorphism. The lowest leukocyte counts of the patients with the UGT1A1*27 and UGT1A1*6 gene polymorphisms were lower than those observed in the wild‐type patients. SN‐38 tended to remain in the blood for a prolonged period after the infusion of irinotecan in patients with UGT1A1*27 or UGT1A1*28 polymorphisms. No severe myelotoxicity was seen in the patients with UGT1A1*7.
Conclusion
UGT1A1*27 can occur separately from UGT1A1*28 and is related to leukopenia during irinotecan treatment. UGT1A1*7 is less relevant to irinotecan‐induced toxicities, and UGT1A1*29 seems to have little clinical impact.
Keywords: Polymorphism, UGT1A1*27, UGT1A1*7
Introduction
Irinotecan, a semisynthetic water‐soluble prodrug that is metabolized to the active metabolite SN‐38, which inhibits the function of DNA topoisomerase I by stabilizing a reversible enzyme‐DNA cleavable complex, is widely used against solid tumors including lung, colorectal, gastric, gynecological, and other types of cancer.1, 2, 3, 4, 5 However, it causes adverse events, such as severe neutropenia and diarrhea, in 13–25% of patients.6, 7 Genetic polymorphisms of the UGT1A1 gene can alter the glucuronidation of SN‐38 and have a major influence on its pharmacokinetics and toxicity.8 The genetic polymorphisms UGT1A1*28 and UGT1A1*6 have already been shown to be associated with decreased UGT1A1 activity, increased SN‐38 levels, and a greater risk of adverse events during irinotecan chemotherapy.9, 10, 11, 12
The UGT1A1*27 polymorphism is in linkage disequilibrium with UGT1A1*28, and a previous study reported that they always co‐occur (i.e. they represent a haplotype).13 In addition, the UGT1A1*27 polymorphism has been demonstrated to reduce the enzyme activity of UGT in basic research.14 However, the impact of UGT1A1*27 on the toxicity of irinotecan is poorly understood because it exhibits a low frequency of 0.5–1%. UGT1A1*7 and UGT1A1*29 affect the common exons of UGT1A1 isoforms, producing substantial reductions in their functional activities, and the UGT1A1*7 polymorphism is prevalent among patients that suffer irinotecan treatment‐related hospitalization or severe irinotecan‐related toxicities.15, 16
Hence, we conducted a study of polymorphisms of the UGT1A1 gene. The main objectives of this study were to determine the clinical impact and pharmacokinetic effects of UGT1A1*27 and other gene polymorphisms in patients who receive irinotecan‐based chemotherapy.
Methods
The study protocol was reviewed and approved by the ethics committee of Nagasaki Municipal Hospital (Nagasaki Harbor Medical Center City Hospital). Written informed consent was obtained from all study participants. This study was an independent collaborative (unsponsored) group study.
Patients and treatment
The patient eligibility criteria were as follows: a histologically and/or cytologically confirmed diagnosis of lung cancer, scheduled irinotecan therapy, age ≥ 20 years, a performance status of 0–2, and written informed consent. Treatment commenced within a week of enrollment. Irinotecan was administered as a single agent or as part of combination chemotherapy. Irinotecan dissolved in 250 mL of 5% dextrose or normal saline was infused intravenously (i.v.) over 60 minutes. Irinotecan treatment was postponed if the patient's leukocyte count fell to < 3000/μL, their platelet count dropped to < 10 × 104/μL, or diarrhea occurred on the day of the planned infusion or the previous day; treatment was abandoned if it could be re‐started within a week.
The doses of the chemotherapy agents were as follows.13, 14, 17, 18, 19, 20 Small cell lung cancer: (i) irinotecan single agent, 100 mg/m2 weekly; (ii) irinotecan combined with cisplatin, 60 mg/m2 on days 1, 8, and 15 and 60 mg/m2 on day 1 every four weeks, respectively; (iii) irinotecan/cisplatin with concurrent radiotherapy, 40 mg/m2 on days 1, 8, and 15 and 60 mg/m2 on day 1 repeated every four weeks, respectively; and (iv) irinotecan combined with carboplatin, 50 mg/m2 on days 1, 8, and 15 and an area under the curve (AUC) value of 5 on day 1 repeated every four weeks, respectively. Non‐small cell lung cancer: (i) irinotecan single agent, 100 mg/m2 weekly; (ii) irinotecan combined with cisplatin, 60 mg/m2 on days 1, 8, and 15 and 80 mg/m2 on day 1 repeated every four weeks, respectively; (iii) irinotecan/cisplatin with concurrent radiotherapy, 50 mg/m2 on days 1, 8, and 15 and 60 mg/m2 on day 1 repeated every four weeks, respectively; and (iv) irinotecan combined with carboplatin, 50 mg/m2 on days 1, 8, and 15 and an AUC value of 5 on day 1 repeated every four weeks, respectively.
Genotyping assay, drug concentration, and response evaluation
Genomic DNA was extracted from 2 mL of peripheral blood in each patient. The UGT1A1*6, UGT1A1*7, UGT1A1*27, and UGT1A1*29 gene polymorphisms were detected via the PCR direct DNA sequencing method. The UGT1A1*28 gene polymorphism was detected using the polyacrylamide gel electrophoresis method. The drug concentrations of irinotecan, SN‐38, and SN‐38G were evaluated at 0, 1, 3, and 24 hours after the administration of irinotecan therapy by analyzing 3 mL blood samples. Venous blood samples were collected on day 1 in 5 mL glass tubes containing heparin before the administration of irinotecan, as well as at 0 and 1, 3, and 24 hours after the first infusion of irinotecan, for pharmacokinetic analysis. The plasma concentrations of irinotecan, SN‐38, and SN‐38G were determined via high‐performance liquid chromatography, and AUC values were calculated using the trapezoidal method in WinNonlin version 4.01 (Pharsight Corporation, Mountain View, CA, USA). Drug toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.21 Before the first cycle, a blood cell count, urinalysis, and biochemistry tests were performed to assess renal and hepatic function and electrolyte levels. These tests were repeated during treatment, while other examinations of marker levels were repeated as necessary.
Statistical analysis
Toxicities were compared between the groups of patients with each UGT1A1 gene polymorphism using the chi‐squared test. The lowest levels of various parameters were compared using the unpaired t‐test between the groups of patients with each UGT1A1 gene polymorphism. Data are expressed as mean ± standard deviation values. Progression‐free survival (PFS) was defined as the period from the start of irinotecan therapy to the determination of treatment failure (death or the documentation of disease progression) or the date of censoring at the last follow‐up examination. Overall survival (OS) was defined as the period from the start of irinotecan therapy until death from any cause or the date of censoring at the last follow‐up examination. Survival was evaluated using the Kaplan–Meier method, and differences in survival were analyzed using the log‐rank test. The t‐test was used to determine the significance of any differences between the groups, and Fisher's exact test was used to compare count data. All P values were two‐sided, and statistical significance was accepted at P < 0.05. SPSS version 23.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
Thirty‐one patients were enrolled in this study between February 2009 and September 2010, and all received chemotherapy including irinotecan. UGT1A1 gene polymorphisms, toxicities, and drug concentrations were evaluated in all patients. The baseline patient characteristics are shown in Table 1. The median age of the patients was 71, and the frequencies of male gender, stage IV disease, and adenocarcinoma were relatively high. The *6/−, *7/−, *27/−, or *28/− UGT1A1 polymorphism was observed in 21 (68%) patients (Table 2). There were no homozygous or complex heterozygous polymorphisms, nor was the *29/− polymorphism detected. The UGT1A1*27 polymorphism was detected in two patients who did not possess the UGT1A1*28 polymorphism (Table 3). The subjects lived in Nagasaki City; however, none of them were affected by the atomic bombing of the city.
Table 1.
Patient characteristics
| Characteristics | No. of patients |
|---|---|
| Gender | |
| Male | 25 |
| Female | 6 |
| Age (years) | |
| Median | 71 |
| Range | 55–84 |
| Stage | |
| IIB | 2 |
| IIIA | 6 |
| IIIB | 11 |
| IV | 12 |
| Histology | |
| Adenocarcinoma | 14 |
| Squamous cell carcinoma | 10 |
| Small cell carcinoma | 3 |
| Non‐small cell carcinoma | 2 |
| Adenosquamous cell carcinoma | 1 |
| LCNEC | 1 |
LCNEC, large cell neuroendocrine carcinoma.
Table 2.
Patients with UGT1A1 gene polymorphisms
| Total | −/− | *6/− | *7/− | *27/− | *28/− | *29/− | |
|---|---|---|---|---|---|---|---|
| N | 31 | 10 | 10 | 2 | 2 | 7 | 0 |
| (%) | (100) | (32) | (32) | (6) | (6) | (23) | (0) |
There were no homozygous or complex heterozygous polymorphisms. The frequency of UGT1A1 gene polymorphisms was 68%.
Table 3.
Patients with UGT1A1*27 gene polymorphisms
| Gender | Age | Histology | Stage | *6 | *7 | *27 | *28 | *29 |
|---|---|---|---|---|---|---|---|---|
| M | 55 | Small | IIIB | Wild | Wild | Hetero | Wild | Wild |
| F | 67 | Adeno | IIIB | Wild | Wild | Hetero | Wild | Wild |
Adeno, adenosquamous cell carcinoma; hetero, heterozygous; small, small cell carcinoma.
Treatment administration
Irinotecan was administered as part of combination chemotherapy in 23 (74%) patients, and was combined with cisplatin in 12 (39%), gemcitabine in 10 (32%), and carboplatin in 1 (3%) patient. The remaining eight patients (26%) were administered irinotecan monotherapy. Radiotherapy was administered concurrently in 20 (65%) patients: 60 Gy in 17 (85%) and 50 Gy in 3 (15%). The treatment dose of irinotecan was 100 mg/m2 in 11 (35%) patients, 60 mg/m2 in 3 (10%), 55 mg/m2 in 1 (3%), 50 mg/m2 in 13 (42%), 40 mg/m2 in 2 (6%), and 30 mg/m2 in 1 (3%) patient.
Toxicity
Grade 3 or worse toxicities experienced according to the types of UGT1A1 gene polymorphisms possessed by the patients are listed in Table 4. Fourteen (45%) of the 31 patients experienced grade 3 or worse toxicities, and 5 (16%) suffered grade 4 toxicities. There was one treatment‐related death, which occurred in a patient with the UGT1A1*28 polymorphism who developed radiation pneumonitis. One of the two patients with UGT1A1*27 suffered febrile neutropenia and grade 3 leukoneutropenia. The frequencies of leukopenia and neutropenia (≥ grade 3) were higher (43–50%) in patients with the *6, *27, and *28 polymorphisms than in the wild‐type (10–20%) patients. Diarrhea (≥ grade 3) was only observed in patients with the *6 polymorphism. There were no significant differences in the toxicities experienced by the patients with each UGT1A1 gene polymorphism.
Table 4.
UGT1A1 gene polymorphisms and toxicities (≥grade 3)
| −/− | *6/− | *7/− | *27/− | *28/− | |
|---|---|---|---|---|---|
| Febrile neutropenia | 0 | 1 | 0 | 1 | 0 |
| (%) | (0) | (10) | (0) | (50) | (0) |
| Leukopenia | 1 | 5 | 0 | 1 | 3 |
| (%) | (10) | (50) | (0) | (50) | (43) |
| Neutropenia | 2 | 5 | 0 | 1 | 3 |
| (%) | (20) | (50) | (0) | (50) | (43) |
| Anemia | 2 | 2 | 0 | 0 | 0 |
| (%) | (20) | (20) | (0) | (0) | (0) |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 1 |
| (%) | (0) | (0) | (0) | (0) | (14) |
| Diarrhea | 0 | 3 | 0 | 0 | 0 |
| (%) | (0) | (30) | (0) | (0) | (0) |
| Pneumonitis | 0 | 0 | 1 | 0 | 1† |
| (%) | (0) | (0) | (50) | (0) | (14) |
Grade 5.
The lowest leukocyte counts of the wild‐type, *6/−, *7/−, *27/−, and *28/− patients were 3140 ± 1290/μL, 2050 ± 1119/μL, 5250 ± 2616/μL, 1650 ± 495/μL, and 2371 ± 1291/μL, respectively (Fig 1a). Patients with the UGT1A1*27 and UGT1A1*6 gene polymorphisms demonstrated significantly lower leukocyte counts than the wild‐type patients (P < 0.05, non‐parametric test). The lowest neutrophil counts of the wild‐type, *6/−, *7/−, *27/−, and *28/− patients were 1927 ± 1369/μL, 1125 ± 885/μL, 3993 ± 2185/μL, 1043 ± 335/μL, and 1196 ± 816/μL, respectively. The lowest hemoglobin levels of these patients were 10.5 ± 1.9 g/dL, 9.5 ± 2.1 g/dL, 10.5 ± 0.9 g/dL, 10.6 ± 0.7 g/dL, and 10.7 ± 2.1 g/dL, respectively. The lowest platelet counts of the patients were 15.0 ± 2.4 × 104/μL, 12.3 ± 4.4 × 104/μL, 24.6 ± 12.9 × 104/μL, 14.7 ± 2.5 × 104/μL, and 11.8 ± 4.7 × 104/μL, respectively. There were no differences in the lowest neutrophil counts, hemoglobin levels, or platelet counts between the wild‐type patients and those with gene polymorphisms (Fig 1b–d).
Figure 1.
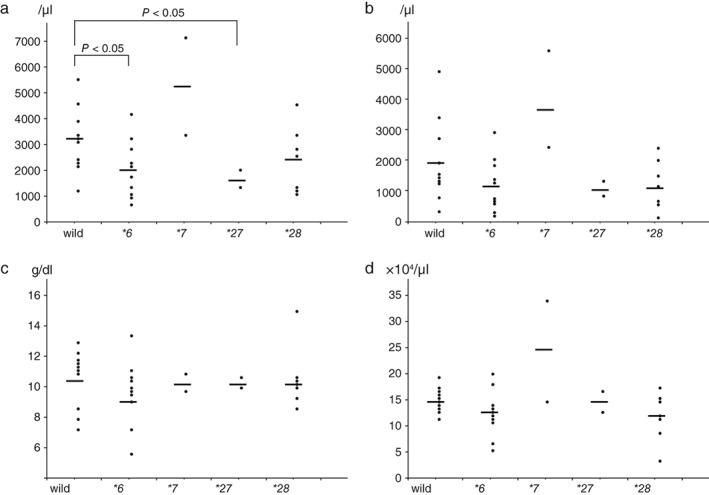
The lowest (a) leukocyte counts, (b) neutrophil counts, (c) hemoglobin levels, and (d) platelet counts observed in wild‐type patients and those with UGT1A1 gene polymorphisms.
Efficacy
The treatment response was assessable in 28 of the 31 patients. The overall response rate was 71% (complete response 7%, partial response 64%). Two patients with the UGT1A1*6 polymorphism achieved complete responses. In detail, 7 of 10 wild‐type patients (70%), 9 of 10 *6/− (90%) patients, 1 of 1 *7/− (100%) patients, 2 of 2 *27/− (100%) patients, and 1 of 5 *28/− (20%) patients exhibited responses to treatment. At the time of the survival assessment performed in September 2016, three patients were still alive, while 28 had died. The median PFS of the 31 patients was 6.3 months, and their one and two‐year survival rates were 34.2% and 21.4%, respectively. The median PFS of the different UGT1A1 polymorphism groups were as follows: heterozygous UGT1A1*6, 9.6 months; heterozygous UGT1A1*7, 6.0 months; heterozygous UGT1A1*27, 5.9 months; and heterozygous UGT1A1*28, 6.3 months. The median OS of the 31 patients was 11.1 months, and their one and two‐year survival rates were 45.2% and 25.8%, respectively. The median OS of the various UGT1A1 polymorphism groups were as follows: heterozygous UGT1A1*6, 19.1 months; heterozygous UGT1A1*7, 9.0 months; heterozygous UGT1A1*27, 9.8 months; and heterozygous UGT1A1*28, 10.6 months. Kaplan–Meier plots and log‐rank tests did not detect any differences in PFS or OS between the four groups.
Pharmacokinetics
A pharmacokinetic analysis of irinotecan involving all 31 patients was performed. The plasma concentration time curves of irinotecan, SN‐38, and SN‐38G are shown according to the UGT1A1 gene polymorphisms possessed by the patients in Figure 2. The patients with UGT1A1*27 and UGT1A1*28 polymorphisms exhibited small increases in their plasma SN‐38G levels, and retained clinically sufficient plasma SN‐38 concentrations for longer periods after the infusion of irinotecan compared to the wild‐type patients. In contrast, clinically sufficient plasma concentrations of SN‐38 were observed for prolonged periods in the patients with UGT1A1*7. The area under the plasma concentration‐time curve values of irinotecan, SN‐38, and SN‐38G were 2620.0 ± 1820.6 ng/h/mL, 137.8 ± 161.0 ng/h/mL, and 3796.3 ± 2512.8 ng/h/mL in the wild‐type patients; 2801.1 ± 1461.2 ng/h/mL, 172.0 ± 116.8 ng/h/mL, and 2131.7 ± 1399.2 ng/h/mL in the heterozygous UGT1A1*6 patients; 2231.3 ± 1360.2 ng/h/mL, 238.6 ± 138.0 ng/h/mL, and 773.9 ± 280.2 ng/h/mL in the heterozygous UGT1A1*7 patients; 1856.2 ± 583.8 ng/h/mL, 392.3 ± 491.1 ng/h/mL, and 2484.4 ± 339.5 ng/h/mL in the heterozygous UGT1A1*27 patients; and 5536.6 ± 3369.5 ng/h/mL, 463.9 ± 386.3 ng/h/mL, and 3231.3 ± 1906.4 ng/h/mL in the heterozygous UGT1A1*28 patients, respectively. The associations between each genotype and the AUC ratio (AUC of SN‐38G/AUC of SN‐38) were evaluated in 31 patients and are shown in Figure 3. The AUC ratios of the patients with UGT1A1 gene polymorphisms did not differ significantly but tended to be lower than those of the wild‐type patients.
Figure 2.
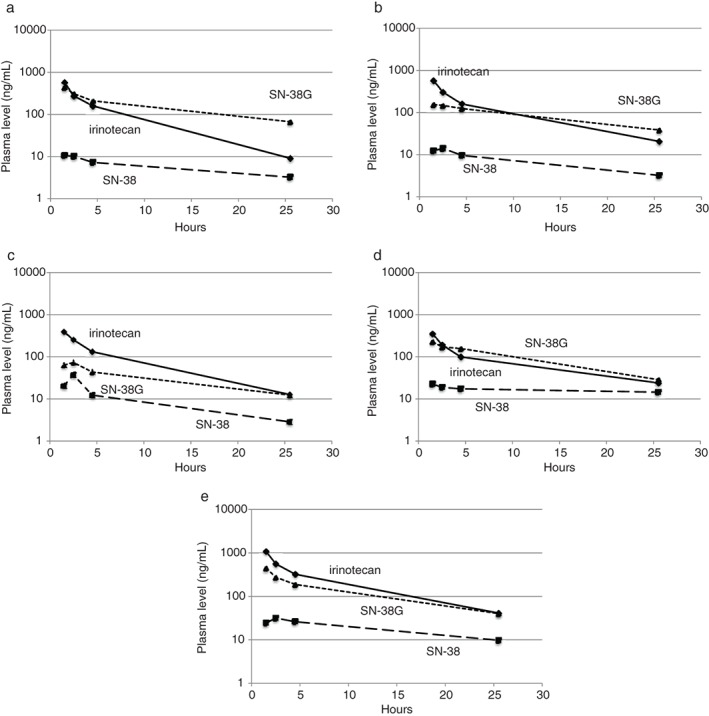
Blood concentration time curves of irinotecan, SN‐38 and SN‐38G (a) wild‐type, (b) UGT1A1*6, (c) UGT1A1*7, (d) UGT1A1*27, and (e) UGT1A1*28 heterozygous gene polymorphisms.
Figure 3.
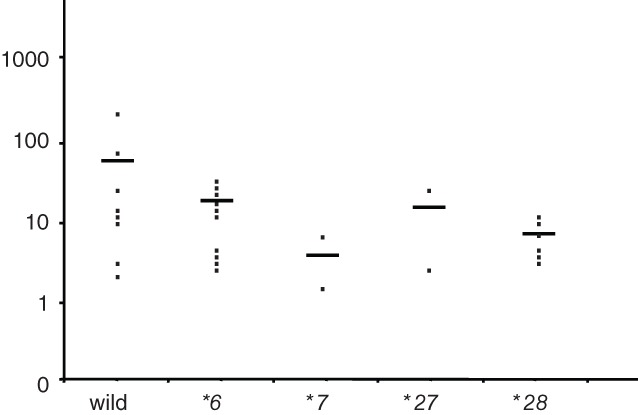
Area under the curve ratios of SN‐38G/SN‐38.
Discussion
The present study attempted to determine the clinical impact of the UGT1A1*7, UGT1A1*27, and UGT1A1*29 gene polymorphisms in patients who receive irinotecan‐based chemotherapy. Our results indicated that UGT1A1*27 can occur separately from UGT1A1*28 and is related to irinotecan‐related leukopenia, UGT1A1*7 is less relevant to irinotecan‐related toxicities, and UGT1A1*29 does not seem to have a clinical impact.
Irinotecan is a semi‐synthetic prodrug that is metabolized by carboxylesterase to form the active metabolite SN‐38, which is subsequently metabolized in the liver by UGT1A1 to the inactive metabolite SN‐38 glucuronide (SN‐38G).21 UGT1A1 is mainly expressed in the liver and is considered to be responsible for the inactivation of SN‐38, and genetic polymorphisms in UGT1A1 result in reduced enzyme activity and increased irinotecan toxicity. The most frequently studied UGT1A1 gene polymorphism, UGT1A1*28, has been determined to be an extremely strong risk factor for severe irinotecan‐related toxicities, such as diarrhea and neutropenia, in Caucasians and Asians.9, 10, 22 Therefore, the UGT1A1*28 polymorphism was defined as a predictor of irinotecan toxicity by the United States Food and Drug Administration in 2005. In our prospective phase II study and meta‐analysis of Asian patients, the UGT1A1*6 polymorphism was found to be associated with irinotecan‐related neutropenia in both homozygotes and heterozygotes.23, 24 The maximum tolerated doses of irinotecan chemotherapy in cancer patients with UGT1A1*28 or UGT1A1*6 polymorphisms are 20–50% lower compared to those of wild type.25, 26
The UGT1A1*27 gene was believed to be in linkage disequilibrium with UGT1A1*28. Specifically, basic research suggested that these polymorphisms co‐occur (form a haplotype) and reduce UGT enzyme activity.12, 27 A previous study reported that out of three patients that were heterozygous for the UGT1A1*27 gene polymorphism, two were homozygous and one was heterozygous for the UGT1A1*28 gene polymorphism.10 We conducted a prospective study to determine the clinical impact of the UGT1A1*27 gene polymorphism in patients that were confirmed to possess the UGT1A1*28 haplotype and were receiving irinotecan‐based chemotherapy.28 However, we did not identify any patients that possessed both the UGT1A1*27 and *28 gene polymorphisms; therefore, the abovementioned hypothesis might be inaccurate. In the present study, we found two patients with the UGT1A1*27 polymorphism who also possessed the wild‐type UGT1A1*28 gene, and all seven of the patients with the UGT1A1*28 polymorphism also possessed the wild‐type UGT1A1*27 gene. The two patients with the UGT1A1*27 polymorphism lived in Nagasaki City; however, they were not affected by the atomic bombing of the city. The patients in our current study did not overlap in our previous two studies.26, 28 Therefore, it seems that UGT1A1*27 can occur separately from UGT1A1*28 in some circumstances.
Only seven patients with the heterozygous UGT1A1*27 gene polymorphisms treated with irinotecan have been reported in the literature (in 2 clinical studies). In our previous study of the UGT polymorphisms present in 77 patients, the heterozygous UGT1A1*27 polymorphism was found in four patients (5.2%), and three of the four patients experienced grade 4 neutropenia.23 Ando et al. examined UGT polymorphisms in 118 patients, and the heterozygous UGT1A1*27 polymorphism was found in three patients (2.5%), all three of which developed severe irinotecan‐related toxicities (grade 4 leukopenia and/or grade 3 or worse diarrhea).10 The present study examined the UGT polymorphisms possessed by 31 patients, and the heterozygous UGT1A1*27 polymorphism was found in two patients (6.5%). One of these patients suffered febrile neutropenia. Taking the findings of the three studies together, 226 patients were examined for UGT polymorphisms, and the heterozygous UGT1A1*27 polymorphism was found in nine patients, seven of whom experienced severe irinotecan‐related toxicities. Therefore, the UGT1A1*27 polymorphism was found at a frequency of 4.0% (9/226). All of these patients were heterozygous for the UGT1A1*27 polymorphism, and 77.8% (7/9) experienced irinotecan‐associated severe toxicities. Some of the patients possessed the UGT1A1*28 polymorphism, whereas others did not. In the patients with UGT1A1*27 and UGT1A1*28 polymorphisms, a clinically sufficient blood concentration of SN‐38 tended to be detected for up to 24 hours after the infusion of irinotecan. In patients with UGT1A1*6 polymorphisms, blood concentration of SN‐38 shows similar behavior with wild type, but irinotecan seems to retain comparatively high concentration and might be concerned with toxicities.
The UGT1A1*7 gene polymorphism has only been detected in a few previous clinical studies.10 In the current study, we detected the UGT1A1*7 gene polymorphism in two patients, at a frequency of 6.5%. The toxicities associated with this polymorphism were generally mild, and no cases of grade 3 or worse myelotoxicity were experienced. In addition, the period during which a clinically sufficient blood concentration of SN‐38 was detected was not prolonged in these patients. One patient experienced pneumonitis after chemoradiotherapy, but recovered after being treated with corticosteroid therapy. Therefore, the UGT1A1*7 polymorphism was found at a frequency of 1.3% (2/149; all cases were heterozygous) and was not associated with severe irinotecan‐related toxicities. As for the UGT1A1*29 gene polymorphism, the findings of the present and a previous study suggest that it has little clinical impact.10
In conclusion, UGT1A1*27 and UGT1A1*7 are both rare polymorphisms. UGT1A1*27 can occur separately from UGT1A1*28 in some circumstances and is related to leukopenia during irinotecan treatment. On the other hand, UGT1A1*7 is less relevant to irinotecan‐induced toxicities, and UGT1A1*29 seems to have little clinical impact.
Disclosure
No authors report any conflict of interest.
References
- 1. Hsing YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein‐linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 1985; 260: 14873–8. [PubMed] [Google Scholar]
- 2. Noda K, Nishiwaki Y, Kawahara M et al Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small‐cell lung cancer. N Engl J Med 2002; 346: 85–91. [DOI] [PubMed] [Google Scholar]
- 3. Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med 2005; 352: 476–87. [DOI] [PubMed] [Google Scholar]
- 4. Boku N, Yamamoto S, Fukuda H et al Fluorouracil versus combination of irinotecan plus cisplatin versus S‐1 in metastatic gastric cancer: A randomized phase 3 study. Lancet Oncol 2009; 10: 1063–9. [DOI] [PubMed] [Google Scholar]
- 5. Sugiyama T, Yakushiji M, Kamura T et al Irinotecan (CPT‐11) and cisplatin as first‐line chemotherapy for advanced ovarian cancer. Oncology 2002; 63: 16–22. [DOI] [PubMed] [Google Scholar]
- 6. Fukuoka M, Niitani H, Suzuki A et al A phase II study of CPT‐11, a new derivative of camptothecin, for previously untreated non‐small‐cell lung cancer. J Clin Oncol 1992; 10: 16–20. [DOI] [PubMed] [Google Scholar]
- 7. Shimada Y, Yoshino M, Wakui A et al Phase II study of CPT‐11, a new camptothecin derivative, in metastatic colorectal cancer. CPT‐11 Gastrointestinal Cancer Study Group. J Clin Oncol 1993; 11: 909–13. [DOI] [PubMed] [Google Scholar]
- 8. Iyer L, King CD, Whitington PF et al Genetic predisposition to the metabolism of irinotecan (CPT‐11): Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN‐38) in human liver microsomes. J Clin Invest 1998; 101: 847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Innocenti F, Undevia SD, Iyer L et al Genetic variants in the UGT‐glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004; 22: 1382–8. [DOI] [PubMed] [Google Scholar]
- 10. Ando Y, Saka H, Ando M et al Polymorphisms of UGP‐glucuronosyltransferase gene and irinotecan toxicity: A pharmacogenetic analysis. Cancer Res 2000; 60: 6921–6. [PubMed] [Google Scholar]
- 11. Han JY, Lim HS, Shin ES et al Comprehensive analysis of UGT1A1 polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non‐small‐cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 2006; 24: 2237–44. [DOI] [PubMed] [Google Scholar]
- 12. Sai K, Saeki M, Saito Y et al UGT1A1 haplotypes associated with reduced glucuronidation and increased serum bilirubin in irinotecan‐administered Japanese patients with cancer. Clin Pharmacol Ther 2004; 75: 501–15. [DOI] [PubMed] [Google Scholar]
- 13. Kudoh S, Fujiwara Y, Takada Y et al Phase II study of irinotecan combined with cisplatin in patients with previously untreated small‐cell lung cancer. West Japan Lung Cancer Group. J Clin Oncol 1998; 16: 1068–74. [DOI] [PubMed] [Google Scholar]
- 14. Masuda N, Fukuoka M, Takada M et al CPT‐11 in combination with cisplatin for advanced non‐small‐cell lung cancer. J Clin Oncol 1992; 10: 1775–80. [DOI] [PubMed] [Google Scholar]
- 15. Fakih MG, Ross ME, Starostik P. Increased frequency of uridine diphosphate glucuronosyltransferase 1A1 7/7 in patients experiencing severe irinotecan‐induced toxicities. Clin Colorectal Cancer 2007; 6: 583–7. [DOI] [PubMed] [Google Scholar]
- 16. Gupta B, LeVea C, Litwin A, Fakih MG. Reversible grade 4 hyperbilirubinemia in a patient with UGT1A1 7/7 genotype treated with irinotecan and cetuximab. Clin Colorectal Cancer 2007; 6: 447–9. [DOI] [PubMed] [Google Scholar]
- 17. Negoro S, Fukuoka M, Masuda N et al Phase I study of weekly intravenous infusions of CPT‐11, a new derivative of camptothecin, in the treatment of advanced non‐small‐cell lung cancer. J Natl Cancer Inst 1991; 83: 1164–8. [DOI] [PubMed] [Google Scholar]
- 18. Oka M, Fukuda M, Kuba M et al Phase I study of irinotecan and cisplatin with concurrent split‐course radiotherapy in limited‐disease small‐cell lung cancer. Eur J Cancer 2002; 38: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 19. Oka M, Fukuda M, Fukuda M et al Phase I study of irinotecan and cisplatin with concurrent split‐course radiotherapy in unresectable and locally advanced non‐small cell lung cancer. Eur J Cancer 2001; 37: 1359–65. [DOI] [PubMed] [Google Scholar]
- 20. Fukuda M, Oka M, Soda H et al Phase I study of irinotecan combined with carboplatin in previously untreated solid cancers. Clin Cancer Res 1999; 5: 3963–9. [PubMed] [Google Scholar]
- 21. Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN‐38, a metabolite of the camptothecin derivative CPT‐11, in the antitumor effect of CPT‐11. Cancer Res 1991; 51: 4187–91. [PubMed] [Google Scholar]
- 22. Iyer L, Das S, Janisch L et al UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2002; 2: 43–7. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura Y, Soda H, Oka M et al Randomized phase II trial of irinotecan with paclitaxel or gemcitabine for non‐small cell lung cancer: Association of UGT1A1*6 and UGT1A1*27 with severe neutropenia. J Thorac Oncol 2011; 6: 121–7. [DOI] [PubMed] [Google Scholar]
- 24. Han FF, Guo CL, Yu D et al Associations between UGT1A1*6 or UGT1A1*6/*28 polymorphisms and irinotecan‐induced neutropenia in Asian cancer patients. Cancer Chemother Pharmacol 2014; 73: 779–88. [DOI] [PubMed] [Google Scholar]
- 25. Innocenti F, Schilsky RL, Ramírez J et al Dose‐finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J Clin Oncol 2014; 32: 2328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fukuda M, Shimada M, Kitazaki T et al Phase I study of irinotecan for previously treated lung cancer patients with the UGT1A1*28 or *6 polymorphism: Results of the Lung Oncology Group in Kyushu (LOGIK1004A). Thorac Cancer 2017; 8: 40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jinno H, Tanaka‐Kagawa T, Hanioka N et al Glucuronidation of 7‐ethyl‐10‐hydroxycamptothecin (SN‐38), an active metabolite of irinotecan (CPT‐11), by human UGT1A1 variants, G71R, P229Q, and Y486D. Drug Metab Dispos 2003; 31: 108–13. [DOI] [PubMed] [Google Scholar]
- 28. Fukuda M, Suetsugu T, Shimada M et al Prospective study of the UGT1A1*27 gene polymorphism during irinotecan therapy in patients with lung cancer: Results of Lung Oncology Group in Kyusyu (LOGIK 1004B). Thorac Cancer 2016; 7: 467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


