Abstract
Pulmonary pleomorphic carcinoma has been shown to respond remarkably to PD‐1 inhibitors; however, the biomarkers for this therapy have not been fully proven. We report a case of pulmonary pleomorphic carcinoma with overexpressed PD‐L1, in which a complete response to nivolumab was sustained for >14 months. Immunohistochemical analysis revealed few PD‐1+ immune cells and regulatory T cells in the tumor, in addition to predominant infiltration of CD8+ cells and macrophages. Our findings suggest that the presence of a small number of PD‐1+ immune cells and regulatory T cells should be investigated as candidate therapeutic biomarkers.
Keywords: Immunotherapy, programmed death‐1, regulatory T cells
Introduction
Pulmonary pleomorphic carcinoma (PPC), an aggressive subtype of lung cancer, has been shown to respond remarkably to PD‐1 inhibitors.1, 2, 3, 4 PPC frequently has favorable response factors to PD‐1 inhibitors, such as high tumor mutational burden, high PD‐L1 expression on tumor cells, and a large number of tumor‐infiltrating immune cells.5, 6, 7 However, the therapeutic biomarkers of PD‐1 inhibitors have not been fully proven. In some patients with PPC, the disease progresses during nivolumab treatment despite high PD‐L1 expression.1 The immunologic profiles of PPC may provide clues to the underlying mechanisms of response to PD‐1 inhibitors in lung cancer. Herein, we report a case of PPC that exhibited a complete response to nivolumab treatment and immunohistochemically examine the profiles of tumor‐infiltrating immune cells.
Case report
A 75‐year‐old male current smoker was referred to our department for evaluation of a lung nodule on chest radiography. Chest computed tomography showed an 18 mm solid nodule in the left upper lobe. He underwent surgery for suspected lung cancer. During the operation, dissemination of cancer cells was found on the left pleura. The patient was eventually diagnosed as stage IV (pT2aN0M1a) PPC with pleural metastases. No EGFR mutations or ALK arrangement was observed.
The patient received five courses of carboplatin and paclitaxel chemotherapy, but a new metastatic lesion in the left adrenal gland appeared five months after the commencement of treatment (Fig 1a,b) and did not respond to three subsequent courses of docetaxel (Fig 1c). Ten months after the initiation of chemotherapy, the treatment regimen was revised to nivolumab as third‐line. The tumor rapidly regressed and a complete response was achieved on the third administration (Fig 1d). Nivolumab treatment was discontinued after the sixth administration because of severe myalgia of unknown cause. The patient has not been administered any further anti‐cancer drugs. Positron emission tomography at 12 months after the last administration of nivolumab showed no 18F–fluorodeoxyglucose uptake in any part of the body. His lung cancer has remained in complete remission for 14 months.
Figure 1.
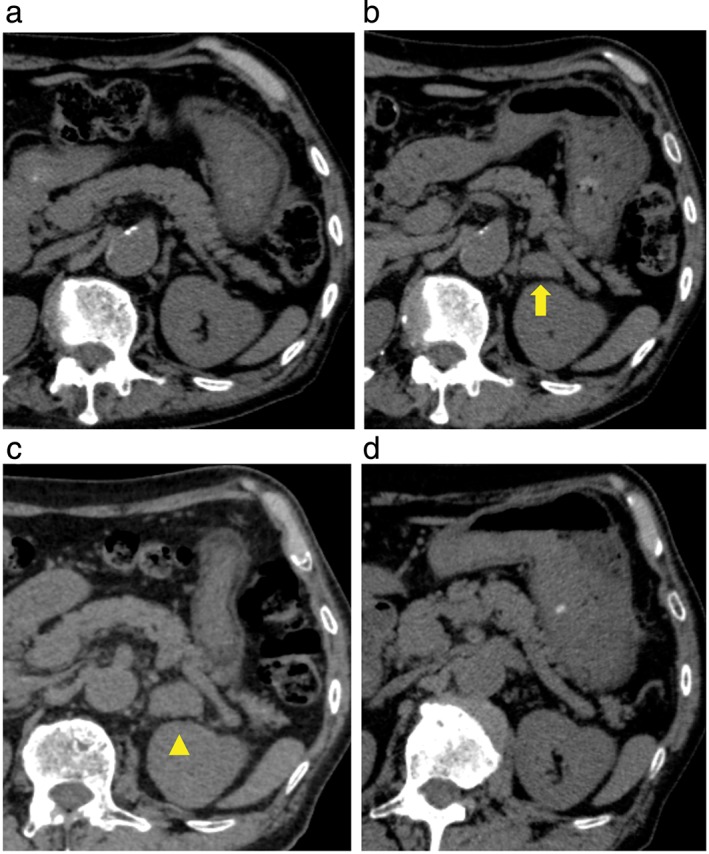
Chest computed tomography scans in a patient with pulmonary pleomorphic carcinoma. (a) Before treatment with carboplatin/paclitaxel, no tumor is detected in the left adrenal gland. (b) After five courses of carboplatin/paclitaxel therapy, a new 15 mm tumor (arrow) is seen in the left adrenal gland. (c) Before treatment with nivolumab, after three cycles of docetaxel, the size of the adrenal tumor increased to 25 mm in diameter (arrowhead). (d) After six courses of nivolumab therapy, the adrenal tumor was eliminated.
Histopathologic review of the surgical specimen at the time of diagnosis showed proliferation of cancer cells with intratumoral infiltration of mononuclear cells (Fig 2a). Immunohistochemical examination indicated that > 90% of the tumor cells expressed PD‐L1 (Fig 2b). Prominent CD3+ T lymphocytes and CD68+ macrophages were found in the tumor (Fig 3a,b). CD56+ natural killer cells were not detected (data not shown). Infiltration of CD8+ cells was more predominant than that of CD4+ cells (Fig 3c,d). A small number of PD‐1+ small‐sized mononuclear cells and FOXP3+ regulatory T cells (Tregs) were scattered in the tumor (Fig 3e,f). The ratios of PD‐1+/CD8+ and FOXP3+/CD4+ cells were 1–5%. There was no difference in PD‐L1, PD‐1, and other lymphocyte marker expression between carcinoma and sarcomatoid components within the tumor (data not shown). The antibody clones used were as follows: PD‐1 (SP269) and PD‐L1 (SP142, Spring Bioscience, Pleasanton, CA, USA); CD3 (F7.2.38), CD68 (KP‐1), and CD56 (123C3 Dako, Santa Clara, CA, USA); CD8 (4B11) and CD4 (4B12, Leica Biosytems, Nussloch, Germany); and FOXP3 (236A/E7, Abcam, Cambridge, UK).
Figure 2.
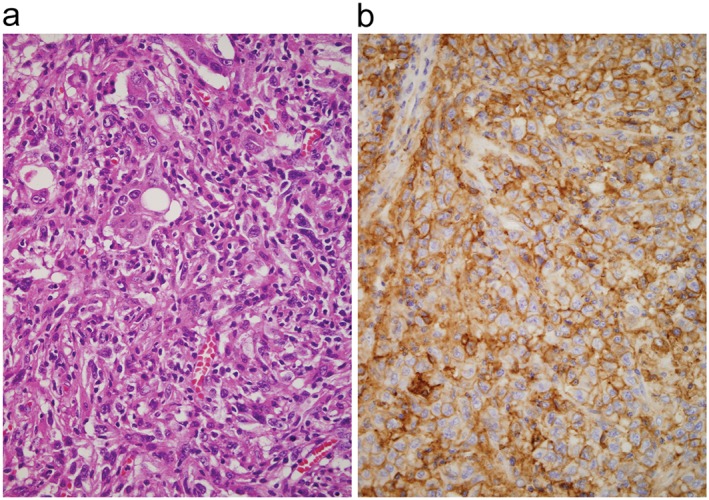
Photomicrographs of the transbronchial biopsy specimen taken from a patient with pulmonary pleomorphic carcinoma. (a) Cancer cells with large‐sized nuclei are seen (hematoxylin & eosin stain, original magnification 400×). (b) Immunohistochemical examination showed that > 90% of the tumor cells expressed PD‐L1 at a high intensity (SP142 clone stain, original magnification 400×).
Figure 3.
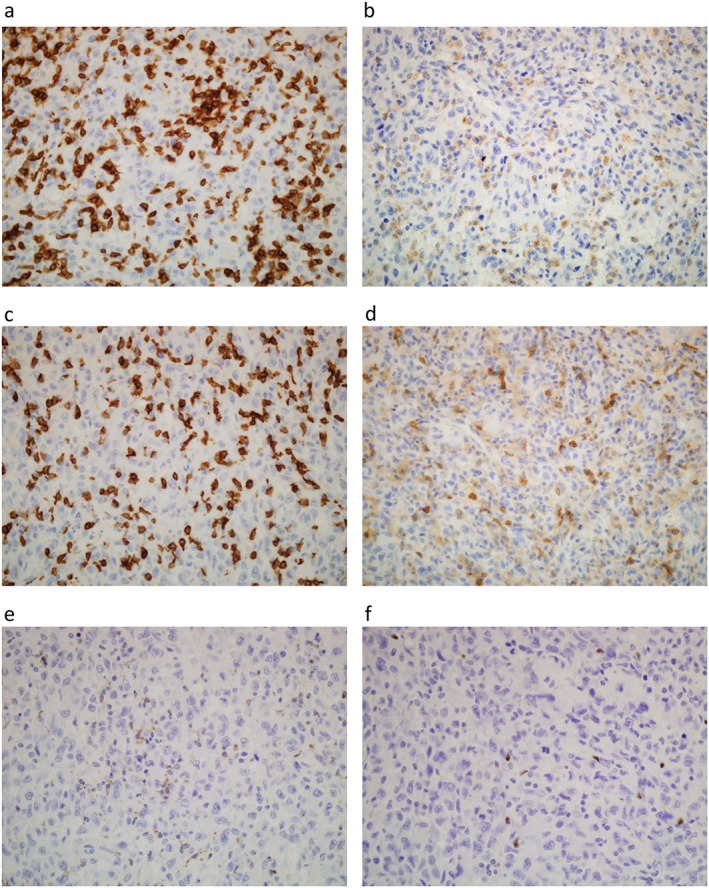
Immunohistochemical examination of tumor‐infiltrating immune cells in a patient with pulmonary pleomorphic carcinoma. (a) CD3+ lymphocytes, (b) CD68+ macrophages, (c) CD8+ cells, (d) CD4+ cells, (e) PD‐1+ cells, and (f) FOXP3+ regulatory T cells are seen within the tumor (original magnification 100×).
Written informed consent for the publication of this case report was obtained from the patient.
Discussion
Immunohistochemical analysis revealed that few CD8+ cells exhibited PD‐1. Although PD‐1 inhibitors, such as nivolumab, target PD‐1 molecules on T cells, the role of PD‐1 expression as a therapeutic biomarker remains elusive. In melanoma and anal cancer, high numbers of PD‐1+ tumor‐infiltrating lymphocytes reportedly correlate with the response to PD‐1 inhibitors.8, 9 However, a recent exploratory study reported an 86% response rate to nivolumab in selected lung cancer patients with tumor cells overexpressing PD‐L1+ and low levels of PD‐1+ tumor‐infiltrating lymphocytes.10 In a mouse cancer model, the antitumor activity of an anti‐PD‐1 antibody was associated with an increase in intratumoral CD8+ cells weakly positive for PD‐1.11 Several in vitro studies using flow cytometry have shown that CD8+ cells that highly express PD‐1 release less cytokines and display less cytotoxic activity than cells that weakly express PD‐1.11, 12 Nivolumab restores the release of cytokines in CD8+ cells that are weakly positive for PD‐1, but not in cells that are highly positive for PD‐1.13 High levels of PD‐1 expressing CD8+ cells are considered irreversibly dysfunctional, even when exposed to PD‐1 inhibitor therapy.
PD‐1+ macrophages are reported to increase with tumor progression, but in this case, most macrophages did not express PD‐1.14 Macrophages dynamically switch between M1 and M2 polarization in response to microenvironmental signals. M2 macrophages frequently express high levels of PD‐1 and suppress antitumor immunity, whereas M1 macrophages express low levels of PD‐1 and have immunostimulatory effects.14, 15 In a mouse cancer model, a PD‐1 inhibitor was shown to reduce tumor growth, at least in part, through the activity of macrophages.14 These results suggest that a small number of PD‐1+ immune cells in the tumor, including CD8+ cells and macrophages, may be a favorable predictor of response to PD‐1 inhibitors.
Another important finding in this case was the small number of FOXP3+ Tregs in the tumor. Tregs have been shown to inhibit antitumor immunity and enhance tumor progression. The ratio of Treg/CD4+ cells ranges from 5% to 20% in lung cancer, whereas the ratio in our case was 1–5%.16, 17 In patients with resected lung cancer, low infiltration of Tregs in the tumor is correlated with favorable recurrence‐free and overall survival rates.18 In cancer patients, lower counts of circulating Tregs in the blood are associated with response to nivolumab.19 We recently reported that nivolumab did not show antitumor activity in a lung cancer patient with tumor cells overexpressing PD‐L1, accompanied by predominantly infiltrating CD8+ cells and Tregs.20 The in vitro coexistence of Tregs suppresses nivolumab‐induced release of interferon‐γ from effector T cells.21 Therefore, Tregs may interfere with the immunostimulatory effects of PD‐1 inhibitors.
Our findings suggest that a small number of PD‐1+ immune cells and Tregs in the tumor, as well as PD‐L1 overexpression and predominant infiltration of CD8+ cells, may be candidate predictive biomarkers of PD‐1 inhibitors for lung cancer. Further studies using a large cohort are required to elucidate whether the presence of few PD‐1+ immune cells and Tregs may contribute to the survival of lung cancer patients and whether there is a threshold number of cells for survival benefit.
Disclosure
No authors report any conflict of interest.
References
- 1. Kanazu M, Uenami T, Yano Y et al Case series of pleomorphic carcinomas of the lung treated with nivolumab. Thorac Cancer 2017; 8: 724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ito K, Hataji O, Katsuta K et al "Pseudoprogression" of pulmonary pleomorphic carcinoma during nivolumab therapy. J Thorac Oncol 2016; 11: e117–9. [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto Y, Miura T, Horiuchi H, Usui K. The successful treatment of pulmonary pleomorphic carcinoma with pembrolizumab: A case report. Case Rep Oncol 2017; 10: 752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikematsu Y, Yoneshima Y, Ijichi K et al Marked response to pembrolizumab in a patient with pulmonary pleomorphic carcinoma highly positive for PD‐L1. Lung Cancer 2017. https://doi.org/10.1016/j.lungcan.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 5. Schrock AB, Li SD, Frampton GM et al Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thorac Oncol 2017; 12: 932–42. [DOI] [PubMed] [Google Scholar]
- 6. Vieira T, Antoine M, Hamard C et al Sarcomatoid lung carcinomas show high levels of programmed death ligand‐1 (PD‐L1) and strong immune‐cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016; 98: 51–8. [DOI] [PubMed] [Google Scholar]
- 7. Chang YL, Yang CY, Lin MW, Wu CT, Yang PC. High co‐expression of PD‐L1 and HIF‐1α correlates with tumour necrosis in pulmonary pleomorphic carcinoma. Eur J Cancer 2016; 60: 125–35. [DOI] [PubMed] [Google Scholar]
- 8. Tumeh PC, Harview CL, Yearley JH et al PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris VK, Salem ME, Nimeiri H et al Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single‐arm, phase 2 study. Lancet Oncol 2017; 18: 446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazzaschi G, Madeddu D, Bocchialini G et al Favorable clinical outcome and response to immunotherapy share a common PD‐L1/PD‐1 based NSCLC immune contexture. 2017 ELCC Annual Meeting Proceedings. Ann Oncol 2017; 28 (Suppl 2): ii1–5. [Google Scholar]
- 11. Kansy BA, Concha‐Benavente F, Srivastava RM et al PD‐1 status in CD8+ T cells associates with survival and anti‐PD‐1 therapeutic outcomes in head and neck cancer. Cancer Res 2017. https://doi.org/10.1158/0008-5472.CAN-16-3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei F, Zhong S, Ma Z et al Strength of PD‐1 signaling differentially affects T‐cell effector functions. Proc Natl Acad Sci U S A 2013; 110: e2480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thommen DS, Schreiner J, Müller P et al Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 2015; 3: 1344–55. [DOI] [PubMed] [Google Scholar]
- 14. Gordon SR, Maute RL, Dulken BW et al PD‐1 expression by tumour‐associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017; 545: 495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen W, Wang J, Jia L, Liu J, Tian Y. Attenuation of the programmed cell death‐1 pathway increases the M1 polarization of macrophages induced by zymosan. Cell Death Dis 2016; 7: e2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin H, Sun L, Tang L, Yu W, Li H. Expression of GARP is increased in tumor‐infiltrating regulatory T cells and is correlated to clinicopathology of lung cancer patients. Front Immunol 2017; 8: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurose K, Ohue Y, Sato E et al Increase in activated Treg in TIL in lung cancer and in vitro depletion of Treg by ADCC using an antihuman CCR4 mAb (KM2760). J Thorac Oncol 2015; 10: 74–83. [DOI] [PubMed] [Google Scholar]
- 18. Yan X, Jiao SC, Zhang GQ, Guan Y, Wang JL. Tumor‐associated immune factors are associated with recurrence and metastasis in non‐small cell lung cancer. Cancer Gene Ther 2017; 24: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferris RL, Blumenschein G, Harrington K et al Tumor‐associated immune cell PD‐L1 expression and peripheral immune profiling: Analyses from CheckMate 141. 2017 AACR Annual Meeting Proceedings. Cancer Res 2017; 77 (Suppl): Abstract CT021. [Google Scholar]
- 20. Ogawara D, Soda H, Iwasaki K et al Remarkable response of nivolumab‐refractory lung cancer to salvage chemotherapy. Thorac Cancer 2018; 9: 175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang C, Thudium KB, Han M et al In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res 2014; 2: 846–56. [DOI] [PubMed] [Google Scholar]


