Abstract
Background
The purpose of this retrospective study was to evaluate the feasibility and efficacy of definitive concurrent chemoradiotherapy (CCRT) with capecitabine and cisplatin for elderly patients with locally advanced esophageal squamous cell carcinoma.
Methods
A total of 90 patients were included from two different centers. Forty‐nine patients were treated with CCRT consisting of capecitabine (850 mg/m2, oral, twice a day for 1–14 days) and cisplatin (20 mg/m2) weekly during radiotherapy (RT). The remaining 41 patients were treated with RT alone. The overall response, overall survival, progression‐free survival, and toxicity rates were recorded.
Results
Compared to the RT group (51.2%; P = 0.029), the overall response rate in the CCRT group (73.5%) was obviously higher. A complete response was achieved in 34.7% and 14.6% of patients in the CCRT and RT groups, respectively (P = 0.030). Median progression‐free and median overall survival rates were significantly higher in the CCRT group (24.7 and 30.6 months; P < 0.05) compared to the RT group (16.6 and 18.7 months). Acute toxic effects were more severe in the CCRT group, but no significant difference in grade 3 or 4 acute toxicities was observed between the groups.
Conclusion
Both CCRT with capecitabine and cisplatin and RT alone are feasible to treat elderly patients and yield a good performance status with locally advanced esophageal squamous cell carcinoma. CCRT improved the tumor response without increasing the side effects compared to RT alone. CCRT is recommended for patients over 65 with good performance status.
Keywords: Concurrent chemoradiotherapy, elderly patient, esophageal squamous cell carcinoma, radiotherapy
Introduction
Esophageal cancer is the eighth most common cancer worldwide and the overall prognosis for patients is still poor.1 While esophageal adenocarcinoma has emerged as the major histological subtype in Western countries, squamous cell carcinoma is the more common histology in certain geographic areas, including Brazil, northern China, Iran, Russia, and South Africa.2 Esophageal squamous cell carcinoma (ESCC) occurs at a median age of ≥ 65 years.3 Therefore, it is important to obtain a valid and better‐tolerated therapeutic choice for elderly patients with ESCC.
The treatment modality for esophageal cancer includes surgery, radiotherapy (RT), chemotherapy, or combination therapy. For elderly patients with unresectable tumors or those who are medically unfit for surgery, RT and chemotherapy are two treatment options that can achieve both symptomatic relief and survival prolongation. In general, elderly patients have not been adequately considered in randomized clinical studies, and this age group is often considered ineligible for curative intended concurrent chemoradiotherapy (CCRT) as a result of age and the presence of comorbid illnesses.4 More importantly, the CCRT treatment dose is usually based on the results of clinical studies, which have commonly concentrated on non‐elderly patients.5, 6
The most commonly used chemotherapy agents for definitive chemoradiotherapy are 5‐fluorouracil (5‐FU) and cisplatin. Unfortunately, studies have shown that the elderly have relatively poor compliance of combined modality therapy, resulting in decreased survival and serious bone marrow and gastrointestinal toxicities.7, 8 To improve clinical outcomes, several combinations of chemotherapy with RT have been evaluated. Studies have shown that thymidine phosphorylase, the key enzyme involved in the final activation step for capecitabine, is active in several tumor tissues compared to normal tissues.9, 10 The convenience of oral administration and safe and manageable toxicity has made capecitabine concurrent RT a fascinating treatment modality for esophageal cancer.11, 12, 13 To date, however, information on the clinical activity of capecitabine and cisplatin concurrent with RT in elderly patients with esophageal cancer is limited.4
With this in mind, we conducted this retrospective study to evaluate the efficacy of CCRT with capecitabine and cisplatin for elderly patients with locally advanced ESCC compared to RT alone.
Methods
Patient selection
Between January 2009 and August 2011, all patients with ESCC were treated with RT alone or with the combination capecitabine and cisplatin chemotherapy regimen. The following eligibility criteria were used to assess patients: histologically confirmed squamous cell carcinoma; age ≥ 65 years; stage IIb or III (American Joint Committee on Cancer Staging Manual, 7th edition); Eastern Cooperative Oncology Group (ECOG) performance status 0–2; no previous therapy for esophageal cancer; adequate hepatic (total bilirubin ≤ 1.5 times the upper limit of normal, aspartate transaminase and alanine transaminase ≤ 2 times the upper limit of normal) and renal function (calculated creatinine clearance ≥ 60 mL/min, or serum creatinine < 1.5 mg/dL); and signed informed consent. Patients were deemed ineligible for surgery if they met the following criteria: refused to undergo surgery; had cervical esophageal cancer that required laryngopharyngeal esophagectomy, which is usually associated with disruption of speech and swallowing and compromises a patient's quality of life; and elderly patients and reduced performance status as determined by the presence of chronic medical illnesses.
Patients were considered ineligible for the study under the following conditions: other malignancy; previously received chemotherapy or RT; or medical comorbidities that would prevent the patient from completing the planned therapy. The independent ethics committees at Qianfoshan Hospital Affiliated with Shandong University and Shandong Cancer Hospital approved the study.
Pretreatment evaluation
Routine pre‐treatment evaluation included a complete history and physical examination; barium X‐ray esophagography; endoscopy of the upper gastrointestinal tract; biopsy of the primary tumor; and computed tomography (CT) scanning of the neck, chest, and abdomen with intravenous contrast. Endoscopic ultrasonography (EUS) and 18F–fluorodeoxyglucose (FDG) positron emission tomography‐CT (PET‐CT) were performed if necessary. A complete blood cell count and biochemistry evaluation was routinely performed.
Treatment schedule and dose modification
For advanced esophageal cancer, infusional fluorouracil and capecitabine may be used interchangeably without compromising efficacy (except as indicated). Under these circumstances, cisplatin and oxaliplatin may also be used interchangeably (National Comprehensive Cancer Network Guidelines Version 1, 2015).
According to the recommendations of previous clinical trials, the chemotherapy regimen was capecitabine (850 mg/m2, oral, twice daily) administered at days 1–14 every three weeks and cisplatin (20 mg/m2) implemented through a one hour intravenous infusion on day 1 per week.
Radiation was administered on the first day of chemotherapy. All patients were treated with three‐dimensional conformal radiotherapy (3D–CRT). In general, patients simulated under CT lay in the supineposition on an immobilization device. The gross tumor volume was determined from chest CT and PET‐CT results, and included the primary tumor and the involved lymph nodes. The clinical target volume contained contours of the gross tumor volume along with a 1.5 cm circumferential and 4 cm superior/inferior expansion. For planning target volume, a 0.5 cm expansion beyond the clinical target volume was used. A total dose of 56.0–59.4 Gy was administered in 30–33 fractions of 1.8–2.0 Gy/fraction with five fractions each week.
Evaluation of efficacy and toxicities
For the first two years, follow‐up was performed every three months; thereafter, every six months. Patients were evaluated by CT scans, barium X‐ray, or endoscopy every three months and whenever clinically indicated. Tumor response was evaluated by barium X‐ray, CT scanning, 18F–FDG PET‐CT or EUS. Efficacy was determined according to Response Evaluation Criteria in Solid Tumors, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).14 CR on PET was defined as FDG uptake on the PET scan after treatments so that tumor tissue was indistinguishable from surrounding normal tissue, according to PET Response Criteria in Solid Tumors version 1.0.15 The persistence or recurrence of the primary tumor and regional lymph nodes was defined as local/regional failure, while distant failure included metastasis to any site.
Safety and toxicity assessments were performed by regular patient interviews, laboratory tests, and physical examinations. Potential dose‐limiting toxicities were graded according to National Cancer Institute Common Toxicity Criteria version 3.0. Chemotherapy and RT were withheld if the granulocyte count was < 1000 cells/μL or if the platelet count was < 50 000 cells/μL, and neither treatment was resumed until both measures recovered to 1500 and 75 000 cells/μL, respectively.
Statistical analysis
Overall survival (OS) was calculated from the initiation of treatment to the date of death or the last follow‐up. Progression‐free survival (PFS) was measured from the initiation of treatment to the date of radiographic evidence of tumor progression or death from any cause. Survival rates were estimated using the Kaplan–Meier method. Differences between the survival curves were compared with the log‐rank test. Univariate and multivariate analyses were determined by Cox regression analysis. Statistical tests were based on a two‐sided significance level, and P values of < 0.05 were considered significant. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Patient and treatment characteristics
Ninety‐six patients were retrospectively included from January 2009 to August 2011, and 90 of these patients were evaluable. The patient characteristics are provided in Table 1. There were 52 men and 38 women with a median age of 71 years (range 65–84); 64.4% of the patients were < 70. Forty‐nine patients received CCRT, while the remaining 41 patients who refused chemotherapy were treated with RT alone. All patients had a performance status of 0–2. The median age of patients receiving CCRT (68.0 years) was slightly younger than those who received RT alone (71.3 years). The ratio of tumor length ≥ 5 cm in CCRT group (19/49) was higher than in the RT alone group (10/41); however, no statistically significant difference was observed in patients or tumor characteristics between the two arms.
Table 1.
Patient and disease characteristics
| Characteristics | Total (n = 90) | CCRT (n = 49) | RT alone (n = 41) | P |
|---|---|---|---|---|
| Gender | 0.771 | |||
| Male | 52 | 29 | 23 | |
| Female | 38 | 20 | 18 | |
| Age (years) | 0.535 | |||
| ≤ 70 | 58 | 33 | 25 | |
| > 70 | 32 | 16 | 16 | |
| ECOG performance status | 0.988 | |||
| 0–1 | 57 | 31 | 26 | |
| 2 | 33 | 18 | 15 | |
| Tumor location | 0.872 | |||
| Cervical | 32 | 17 | 15 | |
| Upper thoracic | 25 | 14 | 11 | |
| Middle thoracic | 19 | 10 | 9 | |
| Low thoracic | 14 | 8 | 6 | |
| Pathology differentiation | 0.856 | |||
| Well | 21 | 11 | 10 | |
| Moderate | 32 | 19 | 13 | |
| Poor | 22 | 11 | 11 | |
| Unknown | 15 | 8 | 7 | |
| Clinical stage | 0.768 | |||
| II | 60 | 32 | 28 | |
| III | 30 | 17 | 13 | |
| Locoregional lymph node metastases | 0.922 | |||
| < 3 | 51 | 28 | 23 | |
| ≥ 3 | 39 | 21 | 18 | |
| Tumor diameter | 0.149 | |||
| < 5 cm3 | 63 | 30 | 31 | |
| ≥ 5 cm3 | 27 | 19 | 10 | |
| Weight loss (over3 months) | 0.460 | |||
| < 5% | 60 | 31 | 29 | |
| ≥ 5% | 30 | 18 | 12 | |
| Comorbidity | ||||
| Yes | 54 | 26 | 28 | 0.142 |
| No | 36 | 23 | 13 |
CCRT, concurrent chemoradiotherapy; ECOG, PS Eastern Cooperative Oncology Group; RT, radiotherapy.
Treatment response and survival
All 90 patients were evaluated for treatment efficacy. Seventeen patients (34.7%) in the CCRT group and 6 (14.6%) in the RT group achieved CR (P = 0.03). The overall response rate (ORR) was also significantly higher in the CCRT group (73.5%) than in the RT group (51.2%) (P = 0.029). The response rates of CCRT (n = 49) and RT (n = 41) groups are presented in Table 2. The median OS for the entire patient cohort was 24.6 months. Compared to the RT group (16.6 months), the median PFS was obviously longer in the CCRT group (24.7 months) (P = 0.026) (Fig 1). As shown in Figure 2, the median OS was obviously longer in the CCRT group (30.6 months) than in the RT alone group (18.7 months) (P = 0.01). PD occurred in 14/90 (15.6%) patients, with no significant difference in treatment failures between the groups (P = 0.126). Local and distant failure rates were similar in both groups.
Table 2.
Tumor response rates in the CCRT and RT alone groups (n [%])
| Response | CCRT | RT alone | P |
|---|---|---|---|
| CR | 17 (34.7%) | 6 (14.6%) | 0.030 |
| PR | 19 (38.8%) | 15 (36.6%) | 0.831 |
| SD | 8 (16.3%) | 11 (26.8%) | 0.224 |
| PD | 5 (10.2%) | 9 (22.0%) | 0.126 |
| ORR | 36 (73.5%) | 21 (51.2%) | 0.029 |
| DCR | 44 (89.8%) | 32 (78.0%) | 0.126 |
CCRT, concurrent chemoradiotherapy CR, complete response; DCR, disease control rate; ER, effective rate; ORR, overall response rate; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease.
Figure 1.
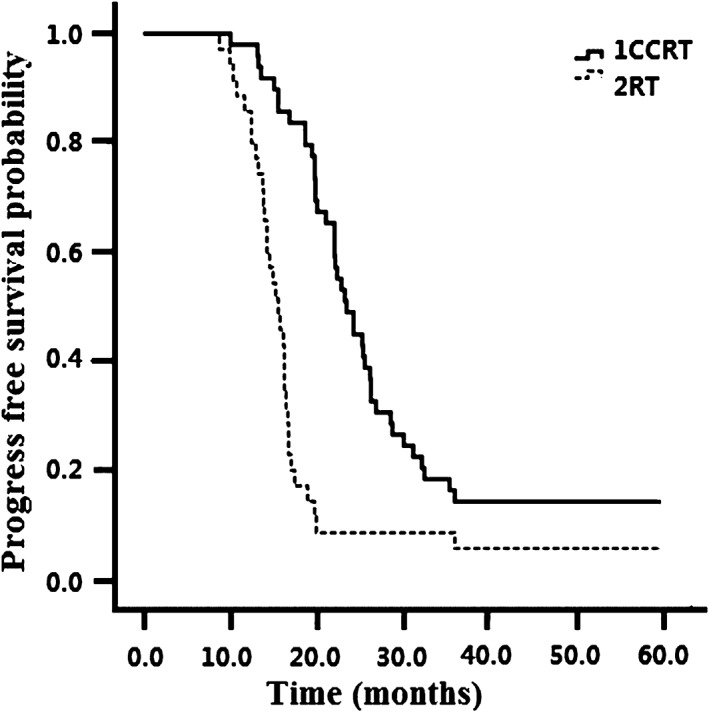
Progression‐free survival in the concurrent chemoradiotherapy (CCRT) and radiotherapy (RT) alone groups.
Figure 2.
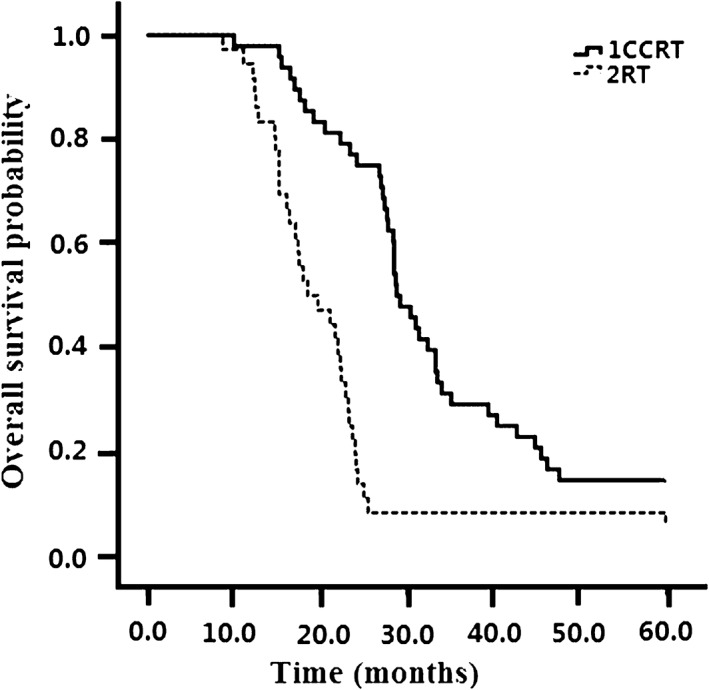
Overall survival in the concurrent chemoradiotherapy (CCRT) and radiotherapy (RT) alone groups.
Multivariate analyses for prognostic factors
The prognostic factors of all patients are provided in Table 3. Five risk factors, including tumor node metastasis (TNM) stage (stage II vs. stage III), ECOG performance status (≥ 2 vs.< 2), tumor length (≥ 5 cm vs.< 5 cm), weight loss (≥ 5 kg vs.< 5 kg), and chemotherapy treatment (yes vs.no) were characterized as significant independent prognostic factors. Patients at early TNM stage, good ECOG performance status, tumor length < 5 cm, and those receiving chemotherapy were likely to have improved prognosis. Further analysis also revealed that patients in the CCRT group with locoregional lymph node metastases had a better survival rate than those in the RT group.
Table 3.
Multivariate analyses of prognostic factors
| Risk factors | SE | 95% CI | P | t |
|---|---|---|---|---|
| TNM stage (Stage II vs. Ш) |
2.799 | 0.36–11.487 | 0.037 | 2.116 |
| ECOG performance status (≥ 2 vs.< 2) |
2.770 | 0.728–11.741 | 0.027 | 2.251 |
| Locoregional lymph node metastases (≥ 3 vs.< 3) |
2.628 | 1.63–12.078 | 0.011 | 2.608 |
| GTV of radiation (≥ 5 cm3 vs. < 5 cm3) |
2.661 | 1.281–11.857 | 0.015 | 2.469 |
| Weight loss (≥ 5 kg3 vs.< 5 kg3) |
2.774 | 0.973–12.002 | 0.022 | 2.338 |
| Chemotherapy (Yes vs. no) |
2.700 | 4.715–15.446 | < 0.001 | 3.734 |
CI, confidence interval; ECOG, PS Eastern Cooperative Oncology Group; GTV, gross tumor volume; SE, standard error; TNM, tumor node metastasis.
Treatment toxicity
All 90 patients were assessable for acute toxicities (Table 4). No treatment‐related death occurred. Commonly reported adverse events related to the treatment were leukopenia, anemia, thrombocytopenia, nausea, vomiting, and pulmonary toxicity. Acute toxicities were more common with CCRT than RT. It is worthwhile noting that the occurrence of grade 1 and 2 toxicities was significantly higher in the combined arm rather than in the RT alone arm, especially for leukopenia (grade 1: 53.1 vs. 36.6%, grade 2: 24.5 vs. 14.6%; P = 0.017). The rate of grade 3 or 4 radiation esophagitis in the CCRT group (26.5%) was higher than in the RT alone group (20.6%), but the difference was not statistically significant (P = 0.168). Radiation pneumonitis was more common in the CCRT group, but the rate of clinically significant pneumonitis (≥ grade 3) was similar in both groups (P = 0.522).
Table 4.
Treatment‐related toxicity
| Toxicity | CCRT | RT alone | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Leukocytopenia | 26 | 12 | 5 | 2 | 16 | 6 | 2 | 2 |
| Febrile neutropenia | 5 | 2 | 0 | 0 | 2 | 1 | 0 | 0 |
| Anemia | 22 | 8 | 4 | 2 | 10 | 7 | 2 | 2 |
| Thrombocytopenia | 18 | 9 | 7 | 4 | 14 | 6 | 2 | 2 |
| Nausea | 21 | 11 | 9 | 5 | 13 | 5 | 2 | 1 |
| Vomiting | 20 | 16 | 6 | 2 | 14 | 9 | 1 | 1 |
| Diarrhea | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Radiation esophagitis | 25 | 11 | 7 | 6 | 19 | 4 | 4 | 2 |
| Radiation pneumonitis | 6 | 2 | 2 | 1 | 4 | 3 | 3 | 1 |
| Hand/foot/skin syndrome | 11 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
CCRT, concurrent chemoradiotherapy; RT, radiotherapy.
Discussion
The standard treatment for elderly esophageal cancer patients (aged > 65) remains a subject of on‐going debate given the aggressiveness of the disease and the relatively high toxicity associated with systemic and locoregional treatments. In the present study, a retrospective analysis of a small number of elderly patients with esophageal cancer showed that ORRs in the combined group (73.5%) were much higher than in the RT alone (51.2%). Our data also confirmed that CCRT improved OS. These findings suggest that combined modality using a chemotherapy regimen of capecitabine and cisplatin with RT is an appropriate treatment for select elderly patients.
The optimal chemotherapy regimen for locally advanced ESCC has not yet been standardized. When deciding which regimen to use, it is important to consider clinical efficacy as well as safety and tolerability for patients with advanced ESCC.16, 17, 18, 19, 20 The combination of 5‐FU and cisplatin with RT is one of the most widely used chemotherapy regimens for advanced metastatic esophageal cancer, given its clinical efficacy and the radiosensitizing effect of each chemotherapy agent.21 In the landmark RTOG 85–01 trial, the five‐year OS for combined modality therapy was 26% (95% confidence interval 15–37%) compared to 0% following RT alone.22 Tougeron et al. also conducted a large clinical trial with 109 patients aged > 70. Cisplatin/5‐FU or cisplatin/irinotecan and 50–55 Gy RT was administered, yielding a two‐year survival rate of 35.5% and median OS of 15.2 ± 2.8 months. However, the incidence of ≥ grade 3 adverse events was relatively high at 23.8%.23
New chemotherapeutic agents are currently under investigation to improve chemoradiotherapy outcomes. A combination of capecitabine plus cisplatin has antitumor and radiosensitizing activities similar to those of 5‐FU and cisplatin.24 Lee et al. reported that the capecitabine/cisplatin regimen showed promising activity in metastatic ESCC with an ORR of 57.8% and median OS of 11.2 months.25 A study of 18 patients treated with definitive chemoradiotherapy with capecitabine and cisplatin recorded an ORR of 100% and two‐year OS of 70.7%.12 Other clinical studies of different tumor types using concurrent capecitabine and RT have been conducted. Ahn et al. conducted a study of 31 patients with stage III/IV resectable laryngeal‐hypopharyngeal squamous cell carcinoma treated with CCRT using the capecitabine/cisplatin regimen.26 Twenty‐three patients achieved CR in the primary site and 18 in the lymph nodes. During the 36‐month follow‐up period, anatomical laryngeal preservation was feasible in 27 patients. Gupta et al. conducted a study of 150 patients with locally advanced squamous cell cancer of the head and neck, and reported that patients receiving CCRT with capecitabine/cisplatin had a significantly higher ORR compared to those receiving cisplatin and 5‐FU.27
Treatment‐related toxicity remains a significant problem associated with multimodality therapy for elderly patients with locally advanced ESCC. In our retrospective research, low‐dose cisplatin per week and oral capecitabine with concurrent RT was tolerable. All 49 patients completed the six week chemotherapy regimen with concurrent thoracic RT as per the protocol. The number of patients who experienced grade 3 esophagitis and pneumonitis in our study was lower than that observed in patients treated with a conventional 5‐FU and cisplatin regimen. Overall, esophagitis was the most common non‐hematologic toxicity in studies using capecitabine/cisplatin CCRT. The rate of grade 4 esophagitis in the CCRT group (12.2%) was higher than in the RT alone group (5%). Although there was no statistically significant difference between the groups, the development of esophagitis is an important concern in this patient population. Aggressive supportive care with symptom management should always be provided to elderly patients undergoing combined modality therapy.
This study has some limitations. First, this is a retrospective study with a relatively small patient size, and it was limited by the heterogeneity of the patient population. Although the baseline characteristics for both patient groups were similar, patients receiving CCRT tended to be younger and their primary tumor size was > 5 cm. An additional issue is that long‐term toxicity was difficult to evaluate. Future clinical prospective trials should be conducted with a focus on identifying the optimum chemotherapy regimen, which would maximize clinical efficacy, minimize treatment‐related toxicities, and improve overall quality of life, as well as attempt to identify the subset of elderly patients most likely to benefit from this treatment approach.
In summary, our study provides compelling evidence that CCRT with capecitabine and cisplatin and RT alone are both feasible and safe treatment options for elderly patients with locally advanced ESCC and good performance status. CCRT improved ORR and OS with manageable safety profiles compared to RT alone. For patients aged > 65 with good performance status, CCRT is superior to RT alone. Further studies investigating the CCRT approach with a capecitabine/cisplatin chemotherapy regimen are clearly warranted.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This work was supported by a grant from the National Natural Science Foundation of China [No. 81672974].
References
- 1. Yanasoot A, Yolsuriyanwong K, Ruangsin S, Laohawiriyakamol S, Sunpaweravong S. Costs and benefits of different methods of esophagectomy for esophageal cancer. Asian Cardiovasc Thorac Ann 2017. https://doi.org/10.1177/0218492317731389. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 3. Daly JM, Fry WA, Little AG et al Esophageal cancer: Results of an American College of Surgeons patient care evaluation study. J Am Coll Surg 2000; 190: 562–72. [DOI] [PubMed] [Google Scholar]
- 4. Xing L, Liang Y, Zhang J et al Definitive chemoradiotherapy with capecitabine and cisplatin for elder patients with locally advanced squamous cell esophageal cancer. J Cancer Res Clin Oncol 2014; 140: 867–72. [DOI] [PubMed] [Google Scholar]
- 5. Stahl M, Stuschke M, Lehmann N et al Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. (Published erratum appears in J Clin Oncol 2006;24:531.). J Clin Oncol 2005; 23: 2310–7. [DOI] [PubMed] [Google Scholar]
- 6. Bedenne L, Michel P, Bouché O et al Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007; 25: 1160–8. [DOI] [PubMed] [Google Scholar]
- 7. Takeuchi S, Ohtsu A, Doi T et al A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol 2007; 30: 607–11. [DOI] [PubMed] [Google Scholar]
- 8. al‐Sarraf M, Martz K, Herskovic A et al Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: An intergroup study. (Published erratum appears in J Clin Oncol 1997;15:866.). J Clin Oncol 1997; 15: 277–84. [DOI] [PubMed] [Google Scholar]
- 9. Schüller J, Cassidy J, Dumont E et al Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 2000; 45: 291–7. [DOI] [PubMed] [Google Scholar]
- 10. Ishikawa T, Utoh M, Sawada N et al Tumor selective delivery of 5‐fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol 1998; 55 (7): 1091. [DOI] [PubMed] [Google Scholar]
- 11. Koo DH, Park SI, Kim YH et al Phase II study of use of a single cycle of induction chemotherapy and concurrent chemoradiotherapy containing capecitabine/cisplatin followed by surgery for patients with resectable esophageal squamous cell carcinoma: Long‐term follow‐up data. Cancer Chemother Pharmacol 2012; 69: 655–63. [DOI] [PubMed] [Google Scholar]
- 12. Lee SJ, Ahn BM, Kim JG et al Definitive chemoradiotherapy with capecitabine and cisplatin in patients with esophageal cancer: A pilot study. J Korean Med Sci 2009; 24: 120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li BS, Zhou T, Wang ZT et al Phase I study of concurrent selective lymph node late course accelerated hyper‐fractionated radiotherapy and capecitabine, cisplatin for locally advanced esophageal squamous cell carcinoma. Radiother Oncol 2009; 93: 458–61. [DOI] [PubMed] [Google Scholar]
- 14. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 15. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 (Suppl 1): 122S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Font A, Arellano A, Fernández‐Llamazares J et al Weekly docetaxel with concomitant radiotherapy in patients with inoperable oesophageal cancer. Clin Transl Oncol 2007; 9: 177–82. [DOI] [PubMed] [Google Scholar]
- 17. Higuchi K, Koizumi W, Tanabe S et al A phase I trial of definitive chemoradiotherapy with docetaxel, cisplatin, and 5‐fluorouracil (DCF‐R) for advanced esophageal carcinoma: Kitasato Digestive Disease & Oncology Group trial (KDOG 0501). Radiother Oncol 2008; 87: 398–404. [DOI] [PubMed] [Google Scholar]
- 18. Li QQ, Liu MZ, YH H, Liu H, He ZY, Lin HX. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esophageal carcinoma. Dis Esophagus 2010; 23: 253–9. [DOI] [PubMed] [Google Scholar]
- 19. Day FL, Leong T, Ngan S et al Phase I trial of docetaxel, cisplatin and concurrent radical radiotherapy in locally advanced oesophageal cancer. Br J Cancer 2011; 104: 265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruhstaller T, Widmer L, Schuller JC et al Multicenter phase II trial of preoperative induction chemotherapy followed by chemoradiation with docetaxel and cisplatin for locally advanced esophageal carcinoma (SAKK 75/02). Ann Oncol 2009; 20: 1522–8. [DOI] [PubMed] [Google Scholar]
- 21. Yamashita H, Nakagawa K, Tago M et al Radiation therapy combined with cis‐diammine‐glycolatoplatinum (nedaplatin) and 5‐fluorouracil for Japanese stage II‐IV esophageal cancer compared with cisplatin plus 5‐fluorouracil regimen: A retrospective study. Dis Esophagus 2006; 19: 15–9. [DOI] [PubMed] [Google Scholar]
- 22. Cooper JS, Guo MD, Herskovic A et al Chemoradiotherapy of locally advanced esophageal cancer: Long‐term follow‐up of a prospective randomized trial (RTOG 85‐01). Radiation Therapy Oncology Group. JAMA 1999; 281: 1623–7. [DOI] [PubMed] [Google Scholar]
- 23. Tougeron D, Di Fiore F, Thureau S et al Safety and outcome of definitive chemoradiotherapy in elderly patients with oesophageal cancer. Br J Cancer 2008; 99: 1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vaishampayan UN, Ben‐Josef E, Philip PA et al A single‐institution experience with concurrent capecitabine and radiation therapy in gastrointestinal malignancies. Int J Radiat Oncol Biol Phys 2002; 53: 675–9. [DOI] [PubMed] [Google Scholar]
- 25. Lee J, Im YH, Cho EY et al A phase II study of capecitabine and cisplatin (XP) as first‐line chemotherapy in patients with advanced esophageal squamous cell carcinoma. Cancer Chemother Pharmacol 2008; 62: 77–84. [DOI] [PubMed] [Google Scholar]
- 26. Ahn D, Kim JH, Sohn JH, Sin CM, Lee JE. Laryngeal preservation in stage III/IV resectable laryngo‐hypopharyngeal squamous cell carcinoma following concurrent chemoradiotherapy with capecitabine/cisplatin. Mol Clin Oncol 2013; 1: 685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta S, Khan H, Barik S, Negi MP. Clinical benefits of concurrent capecitabine and cisplatin versus concurrent cisplatin and 5‐flurouracil in locally advanced squamous cell head and neck cancer. Drug Discov Ther 2013; 7: 36–42. [DOI] [PubMed] [Google Scholar]


