Abstract
Background
Elevated plasma fibrinogen (Fbg) levels contribute to tumor progression and metastasis; however, limited research on Fbg in small cell lung cancer (SCLC) has been conducted. This study evaluated the prognostic value of Fbg levels in patients with SCLC.
Methods
Data on plasma Fbg level, clinical features, and overall survival were retrospectively collected. Kaplan–Meier estimates and log‐rank tests were used to analyze the relationship between Fbg level and survival. Multivariate analyses were performed to determine independent prognostic factors. Subgroup analyses were performed based on extensive/limited disease and Eastern Cooperative Oncology Group status.
Results
A total of 120 patients with SCLC were included. The one, three, and five‐year survival rates for the entire cohort were 48.3%, 9.2%, and 1.7%, respectively. Univariate analyses revealed that age, alcohol use, clinical stage, pleural effusion, Eastern Cooperative Oncology Group grade, and Fbg and lactate dehydrogenase levels were associated with survival (P < 0.05). The median survival time for patients with high Fbg levels (> 400 mg/dL) was shorter than for those with low Fbg levels (8 vs. 14 months; P = 0.013). Furthermore, multivariate analysis revealed that Fbg was negatively and independently associated with SCLC prognosis (hazard ratio 1.505, 95% confidence interval 1.018–2.226; P = 0.041). Higher Fbg levels were associated with shorter survival in the extensive disease subgroup (7 vs. 12 months; P = 0.004).
Conclusions
Elevated plasma Fbg was an independent factor associated with poor outcomes in SCLC patients and could serve as a prognostic biomarker.
Keywords: Fibrinogen, prognosis, small‐cell lung carcinoma, survival
Introduction
Lung cancer remains the leading cause of cancer‐related death worldwide.1 Small cell lung cancer (SCLC), the most aggressive type of lung cancer, has a five‐year survival rate of only 1–3%, and approximately two‐thirds of patients with SCLC have advanced disease at diagnosis.2, 3 Treatment for SCLC patients includes chemotherapy, radiotherapy, and, in rare cases, surgery. As prognosis varies widely, prognostic biomarkers for patients with SCLC are urgently needed.
Many studies have reported an association between blood coagulation abnormalities and several malignancies, and hypercoagulation is associated with a poor prognosis.4, 5, 6, 7, 8 Indeed, activation of the coagulation system and procoagulant changes have been associated with angiogenesis and tumor cell invasion, cancer progression, and metastasis.9
Fibrinogen (Fbg), a plasma coagulation factor synthesized by the hepatocytes, is an acute‐phase reactant protein and a proinflammatory marker that plays an important role in platelet aggregation, increasing plasma viscosity, vasoconstriction, growth factor release, and fibrin deposition.10 Elevated Fbg levels are associated with distant tumor metastasis and poor outcomes in various malignancies.11, 12, 13 In addition, Fbg is reported to have prognostic significance in non‐small‐cell lung cancer (NSCLC);14, 15 however, reports of Fbg levels in SCLC are rare.
Considering that SCLC does not share the same biological characteristics and natural history of NSCLC, the aims of this study were to evaluate the pretreatment plasma Fbg levels and clinical parameters associated with overall survival (OS), and to analyze the prognostic value of Fbg in patients with SCLC.16
Methods
Study design and patients
This cohort study retrospectively reviewed the records of 120 SCLC patients who underwent transbronchial needle aspiration, endobronchial ultrasound‐guided biopsy, supraclavicular lymph node biopsy, or surgical confirmation of disease at Beijing Chao‐Yang Hospital, Capital Medical University, China, between January 2011 and December 2015. The inclusion criteria were: (i) pathological diagnosis of SCLC; (ii) no history of preoperative adjuvant therapy; (iii) preoperative plasma Fbg level measurement within one week of biopsy or surgery; (iv) Eastern Cooperative Oncology Group (ECOG) grade 0–2 and administration of first‐line chemotherapy or a postoperative chemotherapy regimen of etoposide (VP‐16) + cisplatin or VP‐16 + carboplatin, or ECOG grade 3–4 and no administration of chemotherapy; and (v) availability of complete patient records. Patients who received regular anticoagulation therapy or had other malignancies were excluded.
The Beijing Chao‐Yang Hospital ethics committee approved the study protocol and waived the requirement for informed consent, as the study was retrospective.
Data collection
Demographic (gender and age); habitus (smoking and alcohol); clinical (stage, pleural effusion, and ECOG); pathological (vascular invasion); and biochemical data were extracted from medical charts. Clinical tumor staging was reviewed according to the fourth version of the National Comprehensive Cancer Network guidelines (2016). The functional status of each patient was evaluated using the ECOG performance scale.17
Outcomes
The patients were routinely followed up by outpatient visits or hospitalization, depending upon their condition. OS was defined as the duration from treatment initiation to death from any cause. Follow‐up was censored at the time of death or on December 31, 2016.
Fibrinogen levels
Blood samples were taken at admission and before any treatment using 4 mL vacutainer tubes containing 3.2% sodium citrate. Tubes were centrifuged at 3000 rpm at 4°C for 10 min. Fbg levels were routinely determined by immunoturbidimetry (Sysmex CA‐7000/CS‐50100, Sysmex Corp., Kobe, Japan) using the clotting method. The normal range for the test is 170–400 mg/dL.18, 19
Statistical analysis
Continuous data were expressed as mean ± standard deviation (SD) if normally distributed. A Student's t‐test was used to screen for differences between the groups. Categorical data were expressed as percentages. A chi‐square test was used to examine categorical variables. Survival curves were analyzed using the Kaplan–Meier method, and clinical parameters were compared using the log‐rank test. Multivariate analysis of potential prognostic factors was performed using a Cox regression model. All statistical tests were conducted using SPSS version 19.0 (IBM, Armonk, NY, USA). P values < 0.05 were considered statistically significant.
Results
Patient characteristics
During the study period, a total of 214 patients were diagnosed with SCLC. Among these, 22 patients had no Fbg data within one week of biopsy or surgery, 19 received radiotherapy before Fbg measurement, 12 received anti‐tumor traditional Chinese medicine, 19 patients were lost to follow‐up (no survival data), and 22 patients received anticoagulation therapy before Fbg measurement. Therefore, 120 patients met the eligibility criteria and were included in the study. The clinical characteristics of the 120 patients with SCLC are summarized in Table 1. The complete study cohort consisted of 86 men (71.7%) and 34 women (28.3%), with a mean age of 63.2 ± 10.7 years. The median preoperative plasma Fbg level of the entire cohort was 384.3 ± 121.6 mg/dL.
Table 1.
Fbg levels and clinical characteristics of 120 patients with SCLC analyzed using the Kaplan–Meier method (log‐rank test)
| Variable | No. of patients (%) | MST (months) | P |
|---|---|---|---|
| Gender | 0.871 | ||
| Male | 86 (71.7%) | 12 | |
| Female | 34 (28.3%) | 14 | |
| Age (year) | 0.003 | ||
| < 65 | 61 (50.8%) 54.8 ± 7.2 |
15 | |
| ≥ 65 | 59 (49.2%) 72.0 ± 5.2 |
8 | |
| Smoking status | 0.325 | ||
| Never | 33 (27.5%) | 15 | |
| Ever | 87 (72.5%) | 12 | |
| Alcohol use | 0.018 | ||
| Never | 88 (73.3%) | 10 | |
| Ever | 32 (26.7%) | 18 | |
| Clinical stage | < 0.001 | ||
| LD | 47 (39.2%) | 19 | |
| ED | 73 (60.8%) | 10 | |
| Pleural effusion | 0.002 | ||
| No | 70 (58.3%) | 15 | |
| Yes | 50 (41.7%) | 9 | |
| Vascular invasion | 0.803 | ||
| No | 92 (76.7%) | 12 | |
| Yes | 28 (23.3%) | 14 | |
| Brain metastases | 0.112 | ||
| No | 110 (91.7%) | 13 | |
| Yes | 10 (8.3%) | 10 | |
| ECOG grade | < 0.001 | ||
| 0–2 | 92 (76.7%) | 14 | |
| 3–4 | 28 (23.3%) | 8 | |
| Fbg (mg/dL) | 0.013 | ||
| ≤ 400 | 65 (54.2%) | 15 | |
| > 400 | 55 (45.833%) | 8 | |
| LDH (U/L) | 0.001 | ||
| 80–250 | 56 (46.7%) | 18 | |
| > 250 | 64 (53.3%) | 8 | |
| Albumin (g/L) | 0.058 | ||
| 40–55 | 58 (48.3%) | 11 | |
| < 40 | 62 (51.7%) | 13 |
ECOG, Eastern Cooperative Oncology Group; ED, extensive disease; Fbg, fibrinogen; LD, limited disease; LDH, lactate dehydrogenase; MST, median survival time; SCLC, small‐cell lung carcinoma.
Survival
The median survival time (MST) for all patients was 12.0 months. At the end of the study, 107 (89.2%) patients had died, and 13 (10.8%) were alive. The one, three, and five‐year estimated survival rates for the entire cohort were 48.3%, 9.2%, and 1.7%, respectively.
Univariate analyses
Univariate Cox regression analyses showed that age ≥ 65 years (P = 0.004), alcohol use (P = 0.023), extensive disease (P < 0.001), pleural effusion (P = 0.004), ECOG grade 3–4 (P = 0.001), Fbg level > 400 mg/dL (P = 0.017), and lactate dehydrogenase (LDH) level > 250 U/L (P = 0.002) were significantly associated with prognosis in SCLC patients (Table 2). Kaplan–Meier analyses and log‐rank testing showed that MST for patients with high Fbg levels was shorter (8 months, 95% confidence interval [CI] 5–13 months) than for those with low Fbg levels (14 months, 95% CI 11–18 months; P = 0.013) (Fig 1).
Table 2.
Univariate analyses of variables associated with OS in 120 patients with SCLC according to a Cox regression model
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Gender | 0.976 | 0.635–1.471 | 0.875 |
| Age | 1.752 | 1.190–2.579 | 0.004 |
| Smoking status | 1.232 | 0.803–1.890 | 0.340 |
| Alcohol use | 0.593 | 0.378–0.930 | 0.023 |
| Clinical stage | 2.356 | 1.555–3.569 | < 0.001 |
| Pleural effusion | 1.778 | 1.205–2.622 | 0.004 |
| Vascular invasion | 1.057 | 0.675–1.656 | 0.809 |
| Brain metastases | 1.710 | 0.859–3.401 | 0.127 |
| ECOG grade | 2.129 | 1.375–3.296 | 0.001 |
| Fbg | 1.591 | 1.085–2.332 | 0.017 |
| LDH | 1.861 | 1.261–2.745 | 0.002 |
| Albumin | 1.447 | 0.974–2.150 | 0.067 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Fbg, fibrinogen; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival; SCLC, small‐cell lung carcinoma.
Figure 1.
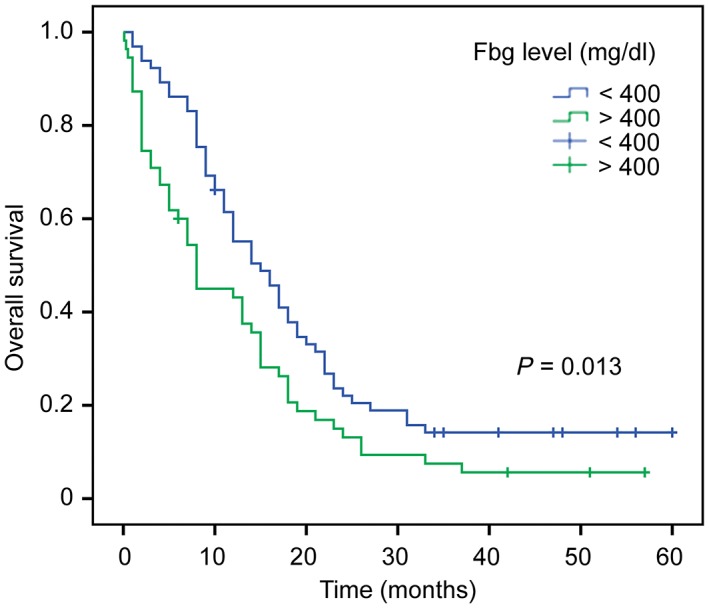
Kaplan–Meier curves of overall survival of patients stratified by fibrinogen level (P = 0.013).
Multivariate analysis
The aforementioned variables of statistical significance were included in a multivariate Cox regression model. The results revealed that clinical stage (hazard ratio [HR] 2.035, 95% CI 1.193–3.470; P = 0.009), ECOG grade (HR 1.850, 95% CI 1.064–3.216; P = 0.029), and Fbg level (HR 1.505, 95% CI 1.018–2.226; P = 0.041) were independently associated with OS (Table 3).
Table 3.
Multivariate analyses of variables associated with OS in 120 patients with SCLC according to a Cox regression model
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.212 | 0.776–1.893 | 0.399 |
| Alcohol use | 0.627 | 0.392–1.002 | 0.051 |
| Clinical stage | 2.035 | 1.193–3.470 | 0.009 |
| Pleural effusion | 0.936 | 0.575–1.524 | 0.791 |
| ECOG grade | 1.850 | 1.064–3.216 | 0.029 |
| Fbg | 1.505 | 1.018–2.226 | 0.041 |
| LDH | 1.197 | 0.762–1.880 | 0.436 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Fbg, fibrinogen; HR, hazard ratio; LDH, lactate dehydrogenase; OS, overall survival; SCLC, small‐cell lung carcinoma.
Subgroup analyses
The patients were stratified according to extensive versus limited disease and ECOG 0–2 versus 3–4. Higher Fbg levels were associated with shorter survival times in the extensive disease subgroup (P = 0.004) (Fig 2a), but no significant difference was observed for patients in the limited disease, ECOG 3–4, or ECOG 0–2 subgroups (all P > 0.05) (Fig 2b–d).
Figure 2.
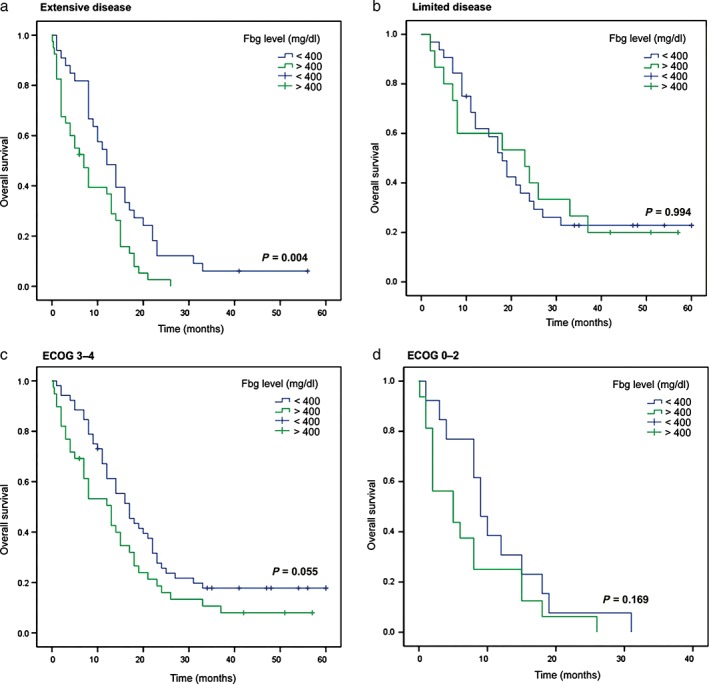
Kaplan–Meier curves of overall survival stratified by fibrinogen level. (a) Extensive disease (P = 0.004), (b) limited disease (P = 0.994), (c) Eastern Cooperative Oncology Group (ECOG) grade 3–4 (P = 0.055), and (d) ECOG grade 0–2 (P = 0.169) subgroups.
Discussion
High Fbg levels are associated with poor outcomes in many types of cancer,12, 13 including NSCLC,14, 15 but data are lacking for SCLC. The results of the present study strongly suggest that elevated pretreatment plasma Fbg levels are independently associated with poorer OS rates in patients with SCLC.
SCLC is an aggressive tumor. More than 90% of SCLC patients relapse after treatment, and the five‐year survival rate is only 1–3%.2 In the present study, the five‐year survival rate in patients with SCLC was 1.7%, consistent with results reported in the literature.2 Various baseline clinical characteristics (such as clinical stage, ECOG grade, and weight loss) are associated with patient outcomes.20 The multivariate analysis performed in this study revealed that advanced clinical stage and high ECOG grade were associated with poor outcomes, which was in line with previous findings.20 Moreover, the results of the present study indicated that elevated plasma Fbg levels were significantly associated with poor outcomes. These results are consistent with those observed in patients with NSCLC14, 15 and other types of cancer.11, 12, 13 Indeed, studies have indicated that high Fbg levels are associated with reduced survival in NSCLC, and renal cell and colon cancers.11, 21 In patients with advanced NSCLC, serum Fbg alterations are useful to identify those who will benefit from preoperative chemotherapy.6 It is well known that SCLC does not share the biological characteristics of NSCLC,16 hence the importance of verifying the eventual prognostic impact of Fbg in SCLC. The present study showed that plasma Fbg level and survival are associated, as in other cancers and NSCLC.
The results of the present study and numerous other studies have indicated that patients with lung cancer have an increased propensity toward clotting, as well as fibrinolytic system aberrations.22, 23, 24 Fbg is synthesized by the liver and converted into insoluble fibrin by activated thrombin. It plays a vital role in blood clotting, fibrinolysis, inflammatory responses, wound healing, and oncogenesis.25 Fbg might promote stable adhesion between tumor cells, platelets, and endothelial cells.26 Moreover, Fbg increases metastatic potential, in part by suppressing natural killer cell‐mediated apoptosis of tumor cells.27 Palumbo et al. showed that tumor cell spread and the establishment of micrometastasis was suppressed in Fbg‐deficient mice, confirming that Fbg is a determinant of spontaneous metastatic potential.26 Furthermore, Fbg also influences the levels of several growth factors, including VEGF and FGF2, promoting tumor cell adhesion, proliferation, and migration, as well as neoplastic angiogenesis.28, 29 Moreover, in the present study, abnormal activation of coagulation and fibrinolysis was reflected in increased plasma Fbg levels, thereby implying that increased Fbg levels were associated with a poor prognosis in SCLC patients.
Because patients with limited/extended disease and patients with different ECOG status have different survival rates,20 subgroup analyses of Fbg levels were performed in patients with extensive versus limited disease and in patients with ECOG grade 0–2 versus 3–4. Surprisingly, high Fbg levels were only associated with poor survival in patients with extensive disease. Therefore, it could be hypothesized that the impact of ECOG on survival is possibly independent from Fbg level, and that Fbg level has no impact in patients in which cancer has not yet disseminated. Nevertheless, additional studies are necessary to determine the mechanisms of Fbg leading to poorer survival.
Previous studies have shown that LDH levels are associated with SCLC prognosis.30, 31 In the present study, LDH levels were associated with prognosis in univariate analysis, but not in multivariate analysis. It must be stressed that LDH is increased in cancer patients, but also in other conditions such as heart failure, hypothyroidism, anemia, some infections, and acute pancreatitis.32 Therefore, the lack of association could be a result of other factors influencing the LDH levels in our patients. Additional studies are necessary to address this point.
The present study has some limitations. Indeed, it was a retrospective, single‐center study with a small sample size. Data were limited to what was available in the medical charts and prevented in‐depth analysis of the coagulation pathways on survival. Multicenter studies should be performed to confirm the prognostic value of plasma Fbg level in patients with SCLC.
The results of the present study strongly suggest that higher Fbg levels were significantly and independently associated with poor prognosis in patients with SCLC. Fbg could serve as a simple and effective biomarker for assessing prognosis in SCLC patients.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by Beijing Chao‐Yang Hospital, Capital Medical University. The authors thank all the patients who participated in the study and the investigators for their assistance with research.
Contributor Information
Shanshan Fan, Email: fss_and_yx@163.com.
Guangyu An, Email: agy23104464@163.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Oh JR, Seo JH, Hong CM et al Extra‐thoracic tumor burden but not thoracic tumor burden on (18)F‐FDG PET/CT is an independent prognostic biomarker for extensive‐disease small cell lung cancer. Lung Cancer 2013; 81: 218–25. [DOI] [PubMed] [Google Scholar]
- 3. Mamdani H, Induru R, Jalal SI. Novel therapies in small cell lung cancer. Transl Lung Cancer Res 2015; 4: 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polterauer S, Seebacher V, Hefler‐Frischmuth K et al Fibrinogen plasma levels are an independent prognostic parameter in patients with cervical cancer. Am J Obstet Gynecol 2009; 200 (647): e1–7. [DOI] [PubMed] [Google Scholar]
- 5. Tsimafeyeu IV, Demidov LV, Madzhuga AV, Somonova OV, Yelizarova AL. Hypercoagulability as a prognostic factor for survival in patients with metastatic renal cell carcinoma. J Exp Clin Cancer Res 2009; 28: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao J, Zhao M, Jin B et al Tumor response and survival in patients with advanced non‐small‐cell lung cancer: The predictive value of chemotherapy‐induced changes in fibrinogen. BMC Cancer 2012; 12: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tas F, Kilic L, Serilmez M, Keskin S, Sen F, Duranyildiz D. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med 2013; 107: 451–7. [DOI] [PubMed] [Google Scholar]
- 8. Antoniou D, Pavlakou G, Stathopoulos GP et al Predictive value of D‐dimer plasma levels in response and progressive disease in patients with lung cancer. Lung Cancer 2006; 53: 205–10. [DOI] [PubMed] [Google Scholar]
- 9. Ay C, Dunkler D, Pirker R et al High D‐dimer levels are associated with poor prognosis in cancer patients. Haematologica 2012; 97: 1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Monraats PS, Rana JS, Zwinderman AH et al ‐455G/A polymorphism and preprocedural plasma levels of fibrinogen show no association with the risk of clinical restenosis in patients with coronary stent placement. Thromb Haemost 2005; 93: 564–9. [DOI] [PubMed] [Google Scholar]
- 11. Erdem S, Amasyali AS, Aytac O, Onem K, Issever H, Sanli O. Increased preoperative levels of plasma fibrinogen and D dimer in patients with renal cell carcinoma is associated with poor survival and adverse tumor characteristics. Urol Oncol 2014; 32: 1031–40. [DOI] [PubMed] [Google Scholar]
- 12. Pichler M, Hutterer GC, Stojakovic T, Mannweiler S, Pummer K, Zigeuner R. High plasma fibrinogen level represents an independent negative prognostic factor regarding cancer‐specific, metastasis‐free, as well as overall survival in a European cohort of non‐metastatic renal cell carcinoma patients. Br J Cancer 2013; 109: 1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takeuchi H, Ikeuchi S, Kitagawa Y et al Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol 2007; 22: 2222–7. [DOI] [PubMed] [Google Scholar]
- 14. Pavey SJ, Hawson GA, Marsh NA. Impact of the fibrinolytic enzyme system on prognosis and survival associated with non‐small cell lung carcinoma. Blood Coagul Fibrinolysis 2001; 12: 51–8. [DOI] [PubMed] [Google Scholar]
- 15. Zhu JF, Cai L, Zhang XW et al High plasma fibrinogen concentration and platelet count unfavorably impact survival in non‐small cell lung cancer patients with brain metastases. Chin J Cancer 2014; 33: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, Senior RM. Fishman's Pulmonary Diseases and Disorders, 5th edn. McGraw‐Hill Education, New York, NY: 2015. [Google Scholar]
- 17. Oken MM, Creech RH, Tormey DC et al Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–55. [PubMed] [Google Scholar]
- 18. Lu K, Zhu Y, Sheng L, Liu L, Shen L, Wei Q. Serum fibrinogen level predicts the therapeutic response and prognosis in patients with locally advanced rectal cancer. Hepatogastroenterology 2011; 58: 1507–10. [DOI] [PubMed] [Google Scholar]
- 19. Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res 2012; 38: 651–7. [DOI] [PubMed] [Google Scholar]
- 20. Buccheri G, Ferrigno D. Prognostic factors in lung cancer: Tables and comments. Eur Respir J 1994; 7: 1350–64. [DOI] [PubMed] [Google Scholar]
- 21. Wojtukiewicz MZ, Zacharski LR, Moritz TE, Hur K, Edwards RL, Rickles FR. Prognostic significance of blood coagulation tests in carcinoma of the lung and colon. Blood Coagul Fibrinolysis 1992; 3: 429–37. [PubMed] [Google Scholar]
- 22. Gabazza EC, Taguchi O, Yamakami T, Machishi M, Ibata H, Suzuki S. Evaluating prethrombotic state in lung cancer using molecular markers. Chest 1993; 103: 196–200. [DOI] [PubMed] [Google Scholar]
- 23. Buccheri G, Ferrigno D, Ginardi C, Zuliani C. Haemostatic abnormalities in lung cancer: Prognostic implications. Eur J Cancer 1997; 33: 50–5. [DOI] [PubMed] [Google Scholar]
- 24. Bick RL. Coagulation abnormalities in malignancy: A review. Semin Thromb Hemost 1992; 18: 353–72. [DOI] [PubMed] [Google Scholar]
- 25. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost 2005; 3: 1894–904. [DOI] [PubMed] [Google Scholar]
- 26. Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen‐deficient mice. Cancer Res 2002; 62: 6966–72. [PubMed] [Google Scholar]
- 27. Zheng S, Shen J, Jiao Y et al Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci 2009; 100: 859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood 2000; 96: 3772–8. [PubMed] [Google Scholar]
- 29. Sahni A, Simpson‐Haidaris PJ, Sahni SK, Vaday GG, Francis CW. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor‐2 (FGF‐2). J Thromb Haemost 2008; 6: 176–83. [DOI] [PubMed] [Google Scholar]
- 30. Hermes A, Gatzemeier U, Waschki B, Reck M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer ‐ A retrospective single institution analysis. Respir Med 2010; 104: 1937–42. [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Guo M, Fan J et al Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta‐analysis. Cancer Biomark 2016; 16: 415–23. [DOI] [PubMed] [Google Scholar]
- 32. Ackers C, Rustin GJ. Lactate dehydrogenase is not a useful marker for relapse in patients on surveillance for stage I germ cell tumours. Br J Cancer 2006; 94: 1231–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


