Abstract
Promising outcomes of salvage chemotherapy after nivolumab therapy have been reported; however, little is known about the detailed clinical and immunologic features in lung cancer patients in whom nivolumab is unsuccessful. We report two cases of nivolumab‐refractory lung cancer, in which chemotherapy resulted in rapid regression of the lung cancer. Upon initial diagnosis, the biopsy specimens showed PD‐ligand 1 (PD‐L1)‐expressing cancer cells, accompanied by tumor‐infiltrating lymphocytes with a favorable CD8/CD4 ratio. Immunosuppressive regulatory T cells and cells positive for TIM‐3 were also observed. Physicians should take caution in treating lung cancer patients after progression on nivolumab. Further studies with a large cohort are warranted to identify the patients that may benefit from salvage chemotherapy.
Keywords: Chemotherapy, immunotherapy, non‐small cell lung cancer
Introduction
Recently, salvage chemotherapy for lung cancer has shown synergistic anti‐tumor activity after previous therapy with nivolumab, a programmed death‐1 (PD‐1) inhibitor.1, 2, 3 However, little is known about the detailed clinical and immunologic features in patients in whom nivolumab treatment is unsuccessful and who thus receive subsequent chemotherapy. Herein, we reported an unexpected chemotherapy outcome of rapid regression of nivolumab‐refractory lung cancer in two patients. We hypothesized that chemotherapy improved the balance between immunostimulator and immunosuppressive cells to synergize with the immunostimulatory effects of the previous nivolumab treatment, despite disease progression under nivolumab treatment.
Case reports
Case 1
A 66‐year‐old female current smoker was diagnosed with stage IV undifferentiated non‐small cell lung cancer (NSCLC) with bone metastasis. There was no EGFR mutation or ALK rearrangement. She received cisplatin/gemcitabine followed by docetaxel. The best response after each regimen was a partial response and stable disease, respectively. However, 18 months after the initiation of chemotherapy, the lesions progressed (Fig 1a), and she was administered nivolumab as third‐line therapy. Although the tumor further enlarged after a total of six cycles of nivolumab, the possibility of pseudoprogression was considered and treatment was continued.
Figure 1.
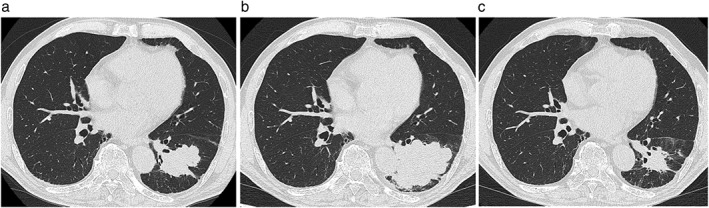
Chest computed tomography scans of a patient with undifferentiated non‐small cell lung cancer. (a) Before treatment with nivolumab, a 28 mm tumor is seen in the left lower lobe of the lung. (b) After nine courses of nivolumab therapy, the diameter of the lung tumor increased to 55 mm. (c) After treatment with two courses of S−1, the lung tumor decreased to 20 mm in diameter.
Despite nine cycles of nivolumab, disease progression and an increasing cough were evident (Fig 1b). Three weeks after the last administration of nivolumab, the treatment regimen was changed to S−1 at a dose of 60 mg twice daily for 28 consecutive days, followed by a two‐week rest period. S−1 has been reported to show efficacy and safety in previously treated NSCLC patients.4 The tumor rapidly regressed, resulting in a partial response six weeks later (Fig 1c). The patient’s lung cancer has remained progression‐free for five months.
Histopathologic review of the transbronchial biopsy specimen at the time of diagnosis showed large, undifferentiated cancer cells (Fig 2a). Immunohistochemical examination indicated that > 90% of the tumor cells expressed PD‐ligand 1 (PD‐L1) (Fig 2b). CD3+ T‐lymphocytes were found in the tumor stroma (Fig 3a). Infiltration of CD8+ cells was more predominant than CD4+ cells (Fig 3b,c). FOXP3+ regulatory T‐cells and cells positive for TIM‐3+ were included in the tumor stroma (Fig 3d,e).
Figure 2.
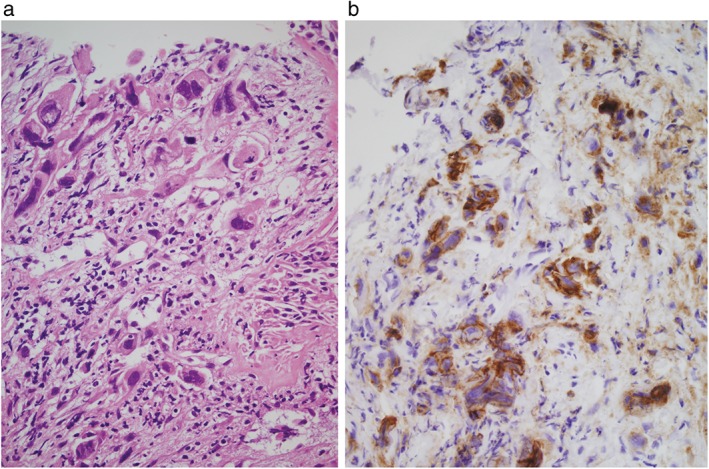
Photomicrographs of a transbronchial biopsy specimen of a patient with undifferentiated non‐small cell lung cancer. (a) Large, undifferentiated cancer cells are seen in the fibrous tissue (hematoxylin & eosin stain, original magnification 400×). (b) Immunohistochemical examination shows that > 90% of the tumor cells expressed programmed death ligand‐1 at a high intensity (original magnification 400×).
Figure 3.
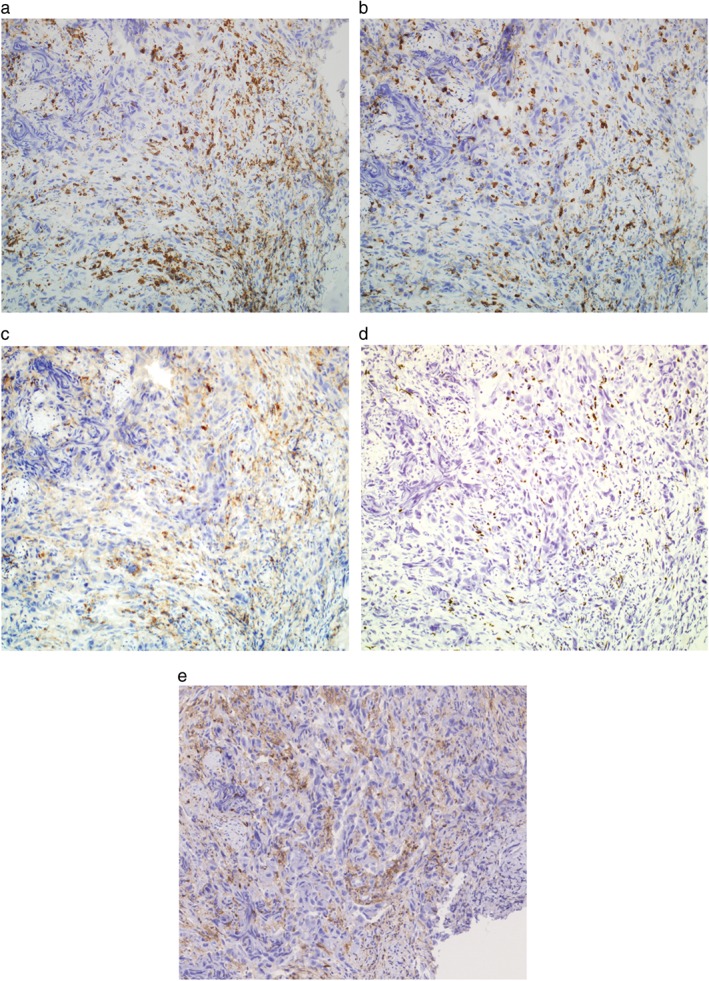
Immunohistochemical profiles of the tumor‐infiltrating lymphocytes in a patient with undifferentiated non‐small cell lung cancer. (a) CD3+ lymphocytes, (b) CD8+ cells, (c) CD4+ cells, (d) FOXP3+ regulatory T‐cells, and (e) TIM‐3+ cells are seen in the tumor stroma (original magnification 100×). The antibody clones used are as follows: CD3 (F7.2.38), CD8 (4B11), CD4 (4B12), FOXP3 (236A/E7), and TIM‐3 (D5D5R).
Case 2
A 75‐year‐old male former smoker was diagnosed with stage IIIA lung adenocarcinoma with pulmonary metastases. No EGFR mutation or ALK rearrangement was detected. The patient underwent treatment with cisplatin/pemetrexed, followed by docetaxel and S−1. The best response after each regimen was a partial response, stable disease, and progressive disease, respectively. Eighteen months after the initiation of chemotherapy, the lung tumor enlarged (Fig 4a) and the serum CYFRA 21‐1 level increased from 2.9 ng/mL to 4.5 ng/mL (reference value < 3.5 ng/mL).
Figure 4.
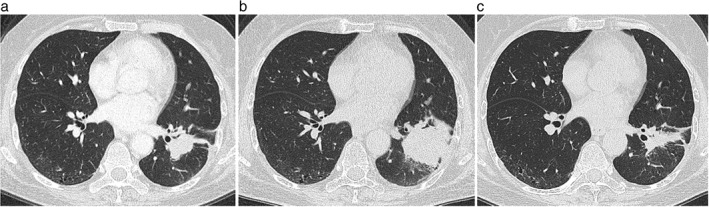
Chest computed tomography scans of a patient with lung adenocarcinoma. Before treatment with nivolumab, (a) a 45 mm primary tumor is observed in the left lower lobe of the lung. (b) After six courses of nivolumab therapy, the primary lung tumor increased to 75 mm in diameter. (c) After two courses of carboplatin/albumin‐bound paclitaxel therapy, the primary lung tumor decreased to a diameter of 25 mm.
The patient was administered nivolumab as fourth‐line therapy; however, after three cycles, the tumor increased in size. After six cycles of nivolumab, disease progression was evident (Fig 4b) and the CYFRA 21‐1 level further increased to 6.4 mg/mL. Three weeks after the last administration of nivolumab, his therapy was changed to carboplatin/ albumin‐bound paclitaxel, which was administered to target an area under the blood concentration‐time curve of 5 mg/mL/min on day 1, and a dose of 100 mg/m2 on days 1, 8, and 15. Carboplatin/albumin‐bound paclitaxel has been reported to show promising efficacy and tolerability in previously treated patients with NSCLC.5 One month later, the tumor rapidly regressed, leading to a decrease in the CYFRA 21‐1 level to 2.3 ng/mL. Two months later, a partial response was achieved (Fig 4c). The patient’s lung cancer has remained progression‐free for five months. The remaining transbronchial biopsy specimen taken for diagnosis was insufficient for retrospective evaluation of PD‐L1 expression.
Written informed consent for the publication of these case reports was obtained from the patients.
Discussion
In this case study, the administration of S−1 or carboplatin/albumin‐bound paclitaxel, even as fourth or higher‐line therapy, resulted in the rapid regression of nivolumab‐refractory lung cancer. Third‐line cytotoxic chemotherapy has been reported to have a low response rate of 3–9%.6, 7 Recently, three retrospective studies, including a case–control study, presented a promising response rate of 25–39% after salvage chemotherapy following exposure to PD‐1/PD‐L1 inhibitors (Table 1).1, 2, 3 The case–control study revealed an odds ratio of 0.30 (95% confidence interval 0.18–0.50) for achieving a partial response to salvage chemotherapy.1 In phase I/II trials, concurrent administration of the PD‐1 inhibitor with first‐line chemotherapy showed a high response rate of > 50%.8, 9 Chemotherapy has been suggested to synergize with PD‐1 inhibitors in some lung cancer patients.
Table 1.
Treatment outcomes of salvage chemotherapy following exposure to immune checkpoint inhibitors, as reported in the literature
| Study design | Number of patients | Number of lines of prior chemotherapy | Immune checkpoint inhibitors | Salvage chemotherapy | Efficacy of salvage chemotherapy | Reference |
|---|---|---|---|---|---|---|
| Retrospective case–control study | 67 | Mean: 2.4 (95% CI 2.1–2.6) | Nivolumab (84%) | DTX (62%) | ORR: 27% | Leger et al.1 |
| Pembrolizumab (10%) | PEM (20%) | Odds ratio: 0.30 (95%CI: 0.18–0.50) | ||||
| Atezolizumab (6%) | GEM (12%) | |||||
| PTX (6%) | ||||||
| Retrospective cohort study | 28 | Median: 2 (range 1–4) | Nivolumab (86%) | DTX (50%) | ORR: 39% | Schvartsman et al.2 |
| Pemrolizumab (10%) | GEM (21%) | |||||
| Durvalumab (4%) | PEM (11%) | |||||
| MMC (11%) | ||||||
| Other (7%) | ||||||
| Retrospective cohort study | 32 | Median: 2 (range 1–6) | Nivolumab | PTX + RAM (38%) | ORR: 25% | Grigg et al.3 |
| Pembrolizumab | VNR (22%) | |||||
| Atezolizumab | GEM‐based (19%) | |||||
| Durvalumab | CBDCA doublets (13%) | |||||
| Others (8%) | ||||||
| Case study | 2 | 2 | Nivolumab | S − 1 | PR | Present study |
| 3 | Nivolumab | CBDCA/nab PTX | PR |
CI, confidence interval; CBDCA, carboplatin; DTX, docetaxel; GEM, gemcitabine; MMC, mitomycin C; nab‐PTX, albumin‐bound PTX; ORR, overall response rate; PEM, pemetrexed; PR, partial response; PTX, paclitaxel; RAM, ramucirumab; VNR, vinorelbine.
In one of two cases, the pretreatment lung cancer cells highly expressed PD‐L1 and were accompanied by predominantly infiltrating CD8+ lymphocytes; regulatory T cells and TIM‐3+ cells were also included. Nivolumab recruits tumor‐infiltrating lymphocytes and upregulates interferon‐γ‐related chemokines, independent of nivolumab treatment status.10 Nevertheless, nivolumab failure may partially be a result of the co‐existence of regulatory T‐cells and TIM‐3+ cells.11, 12 Several chemotherapeutic agents are known to decrease the number of regulatory T‐cells and restore the anti‐tumor activity of CD8+ cells.13, 14 Of note, in PD‐1 knockout mice with favorable survival, paclitaxel enhanced the expression of major histocompatibility complex class I on cancer cells and recruited more cancer‐specific CD8+ cells.15 Therefore, chemotherapy following nivolumab therapy may improve the balance of immunostimulator and immunosuppressive cells.
The influence of the timing and sequence of chemotherapy on this balance are important to evaluate. In cancer patients, carboplatin/paclitaxel therapy decreases the proportion of circulating regulatory T cells after around 12–14 days.16 However, the immunologic effects are eventually restored to initial pre‐chemotherapy values despite repeated chemotherapy.17 These findings suggest the possible need for immunologic interventions that will break the existing homeostatic mechanisms of immune escape. Immunologic examinations of tissue biopsy specimens before and after nivolumab failure may shed light on this issue.
Although the definition and exact mechanisms of pseudoprogression following nivolumab therapy remain undetermined, we considered this possibility in our cases. In Case 1, the tumor enlarged and respiratory symptom worsened after the 18‐week observation period during nivolumab therapy. On review of previous clinical trials for NSCLC, symptomatic pseudoprogression was uncommon at nine weeks after the initiation of nivolumab.18, 19 In Case 2, the serum CYFRA 21‐1 level increased, along with enlargement of the tumor. A case report on pseudoprogression in two lung cancer patients receiving nivolumab showed that tumor size initially increased independent of a decrease in serum tumor maker levels and that tumor‐infiltrating lymphocytes persisted even after cancer cells have disappeared.20 These findings suggest an unusual response to chemotherapy in our cases, rather than pseudoprogression.
In conclusion, physicians should be cautious when treating patients after progression on nivolumab because nivolumab‐refractory lung cancer could show a remarkable response to salvage chemotherapy. Further investigation with a large cohort is required to elucidate the extent of the benefits of salvage chemotherapy and how these responders can be selected.
Disclosure
No authors report any conflicts of interest.
References
- 1. Leger PD, Rothschild S, Castellanos E, Narayana Pillai R, York SJ, Horn L. Response to salvage chemotherapy following exposure to immune checkpoint inhibitors in patients with non‐small cell lung cancer. 2017 ASCO Annual Meeting Proceedings. J Clin Oncol 2017; 35: Abstract 9084. [Google Scholar]
- 2. Schvartsman G, Peng A, Bis G et al Response rates to single‐agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non‐small cell lung cancer. Lung Cancer 2017. https://doi.org/10.1016/j.lungcan.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 3. Grigg C, Reuland BD, Sacher AG, Yeh R, Rizvi NA, Shu CA. Clinical outcomes of patients with non‐small cell lung cancer (NSCLC) receiving chemotherapy after immune checkpoint blockade. 2017 ASCO Annual Meeting Proceedings. J Clin Oncol 2017; 35: Abstract 9082. [Google Scholar]
- 4. Nishio M, Mok TSK, Nakagawa K et al EAST‐LC: Randomized controlled phase III trial of S‐1 versus docetaxel in patients with non‐small‐cell lung cancer who had received a platinum‐based treatment. Ann Oncol 2016; 27 (Suppl 6): 1218PD. [Google Scholar]
- 5. Higuchi M, Takagi H, Owada Y et al Efficacy and tolerability of nanoparticle albumin‐bound paclitaxel in combination with carboplatin as a late‐phase chemotherapy for recurrent and advanced non‐small‐cell lung cancer: A multi‐center study of the Fukushima Lung Cancer Association Group of Surgeons. Oncol Lett 2017; 13: 4315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Girard N, Jacoulet P, Gainet M et al Third‐line chemotherapy in advanced non‐small cell lung cancer: Identifying the candidates for routine practice. J Thorac Oncol 2009; 4: 1544–9. [DOI] [PubMed] [Google Scholar]
- 7. Geng ZY, Jiao SC, Liu SC et al Third‐line therapy in advanced non‐small cell lung cancer. J BUON 2013; 18: 899–907. [PubMed] [Google Scholar]
- 8. Kanda S, Goto K, Shiraishi H et al Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non‐small‐cell lung cancer: A four arms phase Ib study. Ann Oncol 2016; 27: 2242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langer CJ, Gadgeel SM, Borghaei H et al Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: A randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol 2016; 17: 1497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choueiri TK, Fishman MN, Escudier B et al Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res 2016; 22: 5461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang C, Thudium KB, Han M et al In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res 2014; 2: 846–56. [DOI] [PubMed] [Google Scholar]
- 12. Anderson AC, Joller N, Kuchroo VK. Lag‐3, Tim‐3, and TIGIT: Co‐inhibitory receptors with specialized functions in immune regulation. Immunity 2016; 44: 989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G et al The secret ally: Immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11: 215–33. [DOI] [PubMed] [Google Scholar]
- 14. Zheng Y, Dou Y, Duan L et al Using chemo‐drugs or irradiation to break immune tolerance and facilitate immunotherapy in solid cancer. Cell Immunol 2015; 294: 54–9. [DOI] [PubMed] [Google Scholar]
- 15. Peng J, Hamanishi J, Matsumura N et al Chemotherapy induces programmed cell death‐ligand 1 overexpression via the nuclear factor‐κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res 2015; 75: 5034–45. [DOI] [PubMed] [Google Scholar]
- 16. Wu X, Feng QM, Wang Y, Shi J, Ge HL, Di W. The immunologic aspects in advanced ovarian cancer patients treated with paclitaxel and carboplatin chemotherapy. Cancer Immunol Immunother 2010; 59: 279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park A, Govindaraj C, Xiang SD et al Substantially modified ratios of effector to regulatory T cells during chemotherapy in ovarian cancer patients return to pre‐treatment levels at completion: Implications for immunotherapy. Cancers (Basel) 2012; 4: 581–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanizaki J, Hayashi H, Kimura M et al Report of two cases of pseudoprogression in patients with non‐small cell lung cancer treated with nivolumab‐including histological analysis of one case after tumor regression. Lung Cancer 2016; 102: 44–8. [DOI] [PubMed] [Google Scholar]


