Abstract
Background
Eugenol, a natural compound available in Syzigium aromaticum (cloves), is exploited for various medicinal applications. Eugenol induces apoptosis and functions as an anti‐cancer drug in several types of tumors. We investigated the tumor suppressive role and potential mechanisms of eugenol in human lung cancer cells.
Methods
Human embryonic lung fibroblast MRC‐5 and lung cancer adenocarcinoma A549 cells were incubated with or without various concentrations of eugenol for 24 hours. Cell counting kit 8 and crystal violet staining assays were performed to detect cell viability. The cell migration and invasion abilities were also determined by wound healing and transwell assays. Finally, Western blotting assay was performed to examine the changes in lung cancer cell viability and invasion of downstream targets after treatment with eugenol.
Results
Eugenol could inhibit cell viability in lung cancer cells. Furthermore, eugenol obviously impaired cell migration and invasion. Finally, the expression levels of phosphate‐Akt and MMP‐2 in lung cancer cells were reduced after treatment with eugenol.
Conclusion
Our results demonstrated the tumor suppressive roles of eugenol on lung cancer cell proliferation, migration, and invasion partially through the PI3K/Akt pathway and MMP activity in vitro. These results suggest eugenol as a potential chemotherapeutic agent against human lung cancer.
Keywords: Cell migration and invasion, cell viability, eugenol, MMP, PI3K/Akt
Introduction
Eugenol is a natural compound available in Syzigium aromaticum (cloves), and also exists in other types of aromatic plants, such as basil, cinnamon, and bay leaves.1 In fact, eugenol is widely used in traditional medicine in Asian countries, mainly as an antiseptic, analgesic, and anti‐bacterialagent.2 Because of its anesthetic and analgesic properties, eugenol is also applied in dentistry as a pain reliever and cavity filling cement.2 In addition, studies have reported that eugenol performs several biological roles, including antiviral, antioxidant and anti‐inflammatory actions, which make it useful in pharmaceutical production.3, 4, 5 By inhibiting lipid peroxidation, COX‐2 gene expression, and reactive oxygen species, multiple lines of evidence suggest that eugenol may be effective for cancer prevention and in chemotherapy.6, 7, 8 For instance, it is reported to exert anti‐proliferative effects in an array of cancer cells in a B16 melanoma xenograft model.1, 9, 10 On the other hand, it triggers apoptosis in some cancer cells, such as melanoma and HL‐60 leukemia cells.7, 10 Thus, eugenol might function as a tumor suppressor in most cancer types according to previous in vitro and in vivo studies. However, the effect of eugenol in lung cancer cells remains largely unknown.
Lung cancer is the leading cause of cancer‐related morbidity and mortality worldwide, and remains a serious public health concern.11 Developing countries observe higher rates of incidence because of polluted air.12 Although great advances in surgical, radiotherapy, and chemotherapy approaches have been made during the last two decades, the disease is still a major concern. The five‐year survival rate of non‐small cell lung cancer (NSCLC), the most prevalent type, is about 15%.13 Distant metastasis makes this condition worse. Therefore, further investigation of the molecular mechanisms of lung cancer is critical in order to identify more effective therapeutic methods for this disease.
Methods
Cell lines and reagents
Human embryonic lung fibroblast MRC‐5 and human lung adenocarcinoma A549 cells were purchased from American Type Culture Collection (Rockville, MD, USA). Cells were grown in RPMI‐1640 medium supplemented with 10% fetal bovine serum (FBS), L‐glutamine, penicillin/streptomycin (Life Technologies, Gaithersburg, MD, USA), sodium pyruvate, and non‐essential amino acids. Adherent monolayer cultures were maintained and incubated at 37°C in 5% CO2. Eugenol was obtained from Sigma–Aldrich (St. Louis,MO, USA).
Cell viability assay
MRC‐5 or A549 cells were plated in 96‐well plates at a density of 2 × 103 cells in 200 μL of medium per well and incubated at 37°C. After 24 hours, cells were treated with different dosages of eugenol (0, 50, 100, 200, 400, 800 and 1000 μM) for 24 hours or at different time points (0, 12, 24 and 48 hours) at a dosage of 400 μM. Control cells were treated with dimethyl sulfoxide. Cell viability assay was performed using a cell counting kit 8 (CCK‐8) (Dojindo, Kumamoto, Japan) as per the manufacturer's protocol. Absorbance was detected at 590 nm using a microplate reader. The experiments were performed in triplicate.
Crystal violet staining
The viability of MRC‐5 and A549 cells was also detected by crystal violet staining. The eugenol‐treated cells grown in six‐well plates were fixed in 4% paraformaldehyde for 20 minutes. After washing twice with phosphate buffered saline (PBS), the cells were stained with 0.5% crystal violet for 20 minutes. The plates were then aspirated, washed twice, and allowed to air dry, and were then photographed.
Wound healing assay
A549 cells were seeded in a six‐well plate and allowed to grow until 100% confluence. A wound was generated by scratching a straight line using a 1 mL pipette tip. The cells were washed twice with PBS and cultured in cell culture medium with 5% FBS for a further 24 hours. The migration of A549 cells into denuded areas was monitored and visualized using a 40 × magnification phase contrast microscope. The experiments were performed in triplicate.
Cell invasion assay
Transwell chambers were coated with BD Matrigel matrix (BD Bioscience, San Jose, CA, USA) according to the manufacturer's protocol. A549 cells were seeded on top of the Matrigel in the upper chamber. The lower chamber was filled with cell culture medium containing 10% FBS. Cells that invaded through the Matrigel‐coated membrane after 24 hours were fixed with 4% paraformaldehyde, followed by staining with crystal violet solution, and were then photographed under a microscope. The experiments were performed in triplicate.
Western blotting
MRC‐5 and A549 cells were treated with different dosages of eugenol or at different time points. Cells were harvested and lysed in RIPA buffer with 1 mM phenylmethylsulfonyl fluoride (Beyotime Biotechnology, Shanghai, China). Cell extracts were separated on a 9% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to nitro cellulose membrane. The membrane was then blocked with 5% fat‐free milk in PBS with Tween‐20. The blocked membrane was probed with the appropriate antibodies using an electrochemiluminescence method. Anti‐phosphate‐Akt, anti‐MMP‐2, and anti‐β‐actin antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). β‐actin was used as internal controls.
Statistical analysis
Data were presented as mean ± standard deviation. Significance was determined using a grouped Student's t‐test. The difference between the experimental groups was considered significant at P < 0.05.
Results
Inhibition of cell proliferation in lung cancer cells
MRC‐5 and A549 cells were used to reveal drug sensitivity and specificity in normal lung fibroblast and lung cancer cells after eugenol treatment. Different dosages of eugenol (0, 50, 100, 200, 400, 800 and 1000 μM) were added into the medium and allowed to incubate for 24 hours. CCK‐8 assay showed the cytotoxicity of eugenol in tested cells, with inhibitory concentrations (IC)50 of 800 μM in MRC‐5 cells and 400 μM in A549 cells (Fig 1a). The effect of eugenol on A549 cell viability was also time dependent. As shown in Fig 1b, the cell viability of A549 cells treated with 400 μM of eugenol was further decreased with the extension of incubation time. Crystal violet staining for MRC‐5 and A549 cells treated with 400 μM of eugenol for 24 hours further indicated the anti‐survival effect of eugenol.
Figure 1.
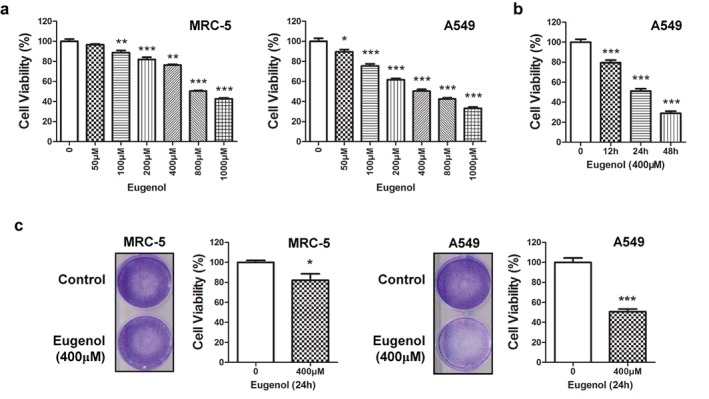
Eugenol inhibited cell viability in normal lung fibroblast and lung cancer cells. Inhibition of cell viability in (a) MRC‐5 or A549 cells after treatment with eugenol at different dosages (0, 50, 100, 200, 400, 800 and 1000 μM) for 24 hours, and (b) A549 cells after treatment with 400 μM eugenol at different time points (0, 12, 24 and 48 hours). (c) After treatment with eugenol at 400 μM for 24 hours, MRC‐5 or A549 cells were stained with crystal violet.*P < 0.05, **P < 0.01, ***P < 0.001.
Inhibition of migration and invasion in lung cancer cells
In order to investigate the possible role of eugenol on lung cancer cell migration and invasion, we treated A549 cells with low (100 μM) or high dosages (500 μM) of eugenol for 24 hours. The cells were then seeded for wound healing and transwell assays. Figure 2a,b shows that eugenol obviously impaired cell migration and invasion, with the high dosage exhibiting more significant repression on migration/invasion. These findings suggested the possibility that low doses of eugenol may represent a novel anti‐metastasis agent in lung cancer cells.
Figure 2.
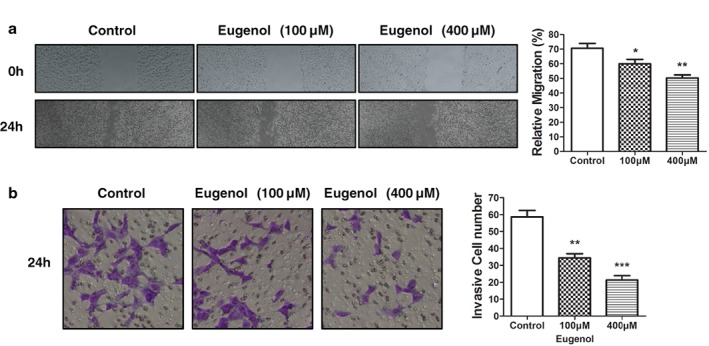
Eugenol inhibited cell migration and invasion in lung cancer cells. (a) Wound healing and (b) cell invasion assays demonstrated that eugenol inhibited lung cancer cell migration in a dose‐dependent manner (low dosage 100 μM, high dosage 400 μM). The right panel shows the quantitative results. *P < 0.05, **P < 0.01, ***P < 0.001.
Alteration of cell viability and invasion marker expression
From these results, we posited that eugenol exerted anti‐survival and anti‐metastasis roles in lung cancer cells, thus we analyzed the underlying mechanisms. Phosphate‐Akt and MMP‐2 expression, the hallmarks of cancer cell viability and metastasis, were examined by Western blotting assay. We found that expression of both phosphate‐Akt and MMP‐2 were decreased in eugenol‐treated A549 cells (Fig 3a). Furthermore, the level of phosphate‐Akt and MMP‐2 in the eugenol‐treated cells decreased with the extension of treatment (Fig 3b).These results suggested that eugenol inhibited lung cancer cell viability, migration, and invasion at least partially by inhibiting the PI3K/Akt pathway and MMP activity in vitro.
Figure 3.
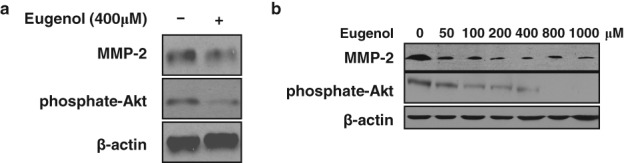
Eugenol altered cell viability and invasion marker expression in lung cancer cells. Western blotting showed the changes of viability and invasion markers in A549 cells treated with (a) 400 μM eugenol for 24 hours, and (b) different dosages of eugenol (0, 50, 100, 200, 400, 800, and 1000 μM) for 24 hours.
Discussion
Our results showed that eugenol exhibits strong anti‐lung cancer features. Indeed, we present clear evidence that eugenol may be considered as a potential chemotherapeutic agent against human lung cancer, for the following reasons.
First, a low dosage of eugenol significantly inhibited lung cancer cell viability. However, non‐tumoral MRC‐5 cells exhibited some resistance to 100 μM of eugenol, as assessed by both CCK‐8 and crystal violet staining assays. We found that higher dosages of eugenol (such as 1000 μM) killed both normal and lung cancer cells (anti‐survival and pro‐apoptosis). This data suggested the cytotoxicity of this agent when used at high dosages.
Second, eugenol exhibited the capability to prevent metastasis in lung cancer cells. As is well known, the leading cause of lung cancer‐related death is distant metastasis.14 Tumor metastasis involves several steps, including separation, migration, invasion, and the formation of a new tumor nodule.14 Metastatic subclones can emerge both early and late in the life of the primary tumor, which remains largely incurable. Our results showed that low concentrations of eugenol could effectively inhibit lung cancer cell migration and invasion in vitro. Only one previous study has examined a correlation between eugenol and cancer metastasis, in which gelatin zymography and Western‐blot confirmed that eugenol can inhibit MMP‐9 expression in human fibrosarcoma cells.15However, the previous study did not provide direct evidence. Herein, we provided valid data that eugenol exerted anti‐metastasis abilities in in lung cancer cells. Thus, combined with the abovementioned anti‐proliferative role, eugenol may represent an excellent agent for the prevention of lung cancer growth and metastasis.
Third, phosphate‐Akt and MMP‐2 expression levels in lung cancer cells were both reduced after treatment with eugenol. Further understanding of the mechanisms behind eugenol‐induced anti‐proliferative and anti‐metastasis roles in lung cancer cells may promote eugenol as a valid anti‐cancer agent. The PI3K/Akt pathway is altered in multiple cellular processes, such as cell differentiation, proliferation, survival, invasion and intracellular trafficking, in a variety of cancers, including lung cancer.16, 17 Thus, it is an excellent target for lung cancer therapy.
MMPs are a pivotal family of zinc enzymes responsible for degradation of extracellular matrix components, which are closely related to tumor metastasis. Excessive MMP expression may contribute to the pathogenesis of tissue‐destructive processes in a wide variety of diseases, including lung cancer.18 Our data indicated that eugenol may inhibit lung cancer cell viability, migration and invasion by negatively regulating the PI3K/Akt pathway and MMP‐2 expression. Although we have limited evidence and our in vivo data was lacking, our results still suggest the potential of eugenol as an anti‐metastasis agent in lung cancer.
Our results illustrated the tumor suppressive roles of eugenol in lung cancer cell viability, migration, and invasion, which might largely function through repression of the PI3K/Akt pathway and reduction of MMP‐2 activity. Our findings support the use of eugenol as a promising external chemotherapeutic agent against human lung cancer.
Disclosure
No authors report any conflict of interest.
Both authors contributed equally to this work.
References
- 1. Pisano M, Pagnan G, Loi M et al Antiproliferative and pro‐apoptotic activity of eugenol‐related biphenyls on malignant melanoma cells. Mol Cancer 2007; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pramod K, Ansari SH, Ali J. Eugenol: A natural compound with versatile pharmacological actions. Nat Prod Commun 2010; 5: 1999–2006. [PubMed] [Google Scholar]
- 3. Waldman SA, Hyslop T, Schulz S et al Association of GUCY2C expression in lymph nodes with time to recurrence and disease‐free survival in pN0 colorectal cancer. JAMA 2009; 301: 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogata M, Hoshi M, Urano S, Endo T. Antioxidant activity of eugenol and related monomeric and dimeric compounds. Chem Pharm Bull (Tokyo) 2000; 48: 1467–9. [DOI] [PubMed] [Google Scholar]
- 5. Benencia F, Courrèges MC. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother Res 2000; 14: 495–500. [DOI] [PubMed] [Google Scholar]
- 6. Toda S, Ohnishi M, Kimura M, Toda T. Inhibitory effects of eugenol and related compounds on lipid peroxidation induced by reactive oxygen. Planta Med 1994; 60: 282. [DOI] [PubMed] [Google Scholar]
- 7. Okada N, Hirata A, Murakami Y, Shoji M, Sakagami H, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase‐2 gene expression by eugenol‐related compounds. Anticancer Res 2005; 25: 3263–9. [PubMed] [Google Scholar]
- 8. Ou HC, Chou FP, Lin TM, Yang CH, Sheu WH. Protective effects of eugenol against oxidized LDL‐induced cytotoxicity and adhesion molecule expression in endothelial cells. Food Chem Toxicol 2006; 44: 1485–95. [DOI] [PubMed] [Google Scholar]
- 9. Slamenová D, Horváthová E, Wsólová L, Sramková M, Navarová J. Investigation of anti‐oxidative, cytotoxic, DNA‐damaging and DNA‐protective effects of plant volatiles eugenol and borneol in human‐derived HepG2, Caco‐2 and VH10 cell lines. Mutat Res 2009; 677: 46–52. [DOI] [PubMed] [Google Scholar]
- 10. Ghosh R, Nadiminty N, Fitzpatrick JE, Alworth WL, Slaga TJ, Kumar AP. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J Biol Chem 2005; 280: 5812–9. [DOI] [PubMed] [Google Scholar]
- 11. How SH, Ng TH, Kuan YC, Jamalludin AR, Fauzi AR. Survival of lung cancer patients in a resource‐limited country. Asia Pac J Clin Oncol 2015; 11: 221–7. [DOI] [PubMed] [Google Scholar]
- 12. Yue S, Wang Y, Wang J, Chen J. Relationships between lung cancer incidences and air pollutants. Technol Health Care 2017; 25 (Suppl 1): 411–22. [DOI] [PubMed] [Google Scholar]
- 13. Spira A, Ettinger DS. Multidisciplinary management of lung cancer. (Published erratum appears in N Engl J Med 2004; 360: 1917) N Engl J Med 2004; 350: 379–92. [DOI] [PubMed] [Google Scholar]
- 14. Turajlic S, Swanton C. Metastasis as an evolutionary process. Science 2016; 352: 169–75. [DOI] [PubMed] [Google Scholar]
- 15. Nam H, Kim MM. Eugenol with antioxidant activity inhibits MMP‐9 related to metastasis in human fibrosarcoma cells. Food Chem Toxicol 2013; 55: 106–12. [DOI] [PubMed] [Google Scholar]
- 16. Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol 2014; 90: 197–207. [DOI] [PubMed] [Google Scholar]
- 17. Pérez‐Ramírez C, Cañadas‐Garre M, Molina MÁ, Faus‐Dáder MJ, Calleja‐Hernández MÁ. PTEN and PI3K/AKT in non‐small‐cell lung cancer. Pharmacogenomics 2015; 16: 1843–62. [DOI] [PubMed] [Google Scholar]
- 18. Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci 2002; 3: 409–21. [DOI] [PubMed] [Google Scholar]


