Abstract
Background
Acute dyspnea affects a large heterogeneous patient group with high mortality and readmission rates.
Purpose
To investigate if cardiometabolic biomarkers and clinical characteristics predict readmission and death in patients hospitalized for acute dyspnea.
Methods
65 dyspnea patients at a general internal medicine ward were followed for six months. The combined endpoint was readmission or death.
Measurements and results
Cardiometabolic biomarkers at admission were related to the endpoint in Cox proportional hazard models (adjusted for sex, age, oxygen saturation, respiratory rate and C-reactive protein (CRP)). The biomarkers tissue-type plasminogen activator (tPA), prolactin (PRL), tumor necrosis factor receptor superfamily member 6 (FAS) and C-C motif chemokine 3 (CCL3) were independently and significantly related to the endpoint and combined into a biomarker risk score (BRS). Each SD increment of the BRS conferred a hazard ratio (HR) of 2.13 (1.39–3.27) P = 0.001. The top vs bottom tertile of the BRS conferred a HR of 4.75 (1.93–11.68) P = 0.001. Dyspnea severity was also associated with worse outcome, HR = 3.43 (1.28–9.20) P = 0.014. However, when mutually adjusted the BRS remained significant (P = 0.004) whereas dyspnea severity was not. The BRS was related to the endpoint among patients with mild to moderate dyspnea (P = 0.016) but not among those with severe dyspnea.
Conclusion
A score of tPA, PRL, FAS and CCL3 predicts 6-month death and readmission in patients hospitalized for acute dyspnea and may prove useful to optimize length of stay and follow-up. Although the BRS outweighs dyspnea severity in prediction of the endpoint, its prognostic role is strongest in mild-moderate dyspnea.
Keywords: Prognosis, acute dyspnea, biomarker, risk score
1. Introduction
Patients with acute dyspnea are a large and heterogeneous patient group with high mortality and readmission rates [1], [2], [3], [4], [5], [6]. Dyspnea is a common manifestation of diseases in the cardiovascular and respiratory system such as congestive heart failure, myocardial infarction, chronic obstructive pulmonary disease and pulmonary thromboembolism [3], [6]. Due to the diversity, diagnostics is often challenging and delayed. Early risk assessment is crucial for patient outcome but individual risk assessment is difficult and reliable predictive tools are missing [7], [8].
Today, medical history, physical examination and clinical characteristics are often combined with blood chemistry for diagnostic and prognostic evaluation in patients with acute dyspnea. This approach has its limitations and the need for further methods has been addressed to optimize medical care [8]. There are few studies investigating the long-term outcome for patients with acute dyspnea [6], [9]. Improvement of early prognostic judgments in patients admitted for acute dyspnea has the potential of both shortening the length of stay and to stratify patients for intensified treatment or direct for thorough search for the underlying condition if the patient is at high risk of adverse outcome. Importantly, any prognostic role of biomarkers has to yield additional clinical information on top of currently used risk stratification tools. Not at least, a structured classification of the severity of dyspnea needs to be taken into account [8]. In this context, a standardized classification of dyspnea severity is necessary. The NYHA functional classification is a widely used prognostic tool for patients with congestive heart failure [6], [10] but to our knowledge it has not been evaluated for prognosis in unselected patients hospitalized for dyspnea. So far no one has investigated the link between a broad panel of inflammation, immune response, metabolism and cardiovascular stress biomarkers with outcome for patients hospitalized for acute dyspnea. However, with novel multimarker panels recently developed, such broad biomarker characterization has now become feasible.
The purpose of this study is to investigate if circulating cardiometabolic biomarkers can be used to predict outcome of patients hospitalized due to acute dyspnea on top of clinical characteristics used for risk stratification today.
2. Materials and methods
We studied in-patients hospitalized due to acute dyspnea during 2012 and 2013 at a general internal medicine ward at the University Hospital of Skåne in Malmö, Sweden. The inclusion criterion was acute dyspnea on arrival, defined as either “dyspnea” as the preliminary diagnosis on admission to the emergency department or as the presence of dyspnea on arrival to the internal medicine department. A written form of consent was obtained from all study participants. Patients with cognitive dysfunction, defined as a mini mental state examination performance of < 13, were excluded [11]. The study was approved by the regional board of ethics in Lund, Sweden.
Data collection was performed by review of medical records, blood sample analysis and interviews. Variables recorded were patient baseline characteristics, medical history, social status, functional and physical examinations, vital signs, physical findings, severity of dyspnea, cardiometabolic biomarkers and routine blood chemistry. The variables included are shown in Table 1. We measured circulating cardiometabolic plasma biomarkers using the multiplex immunoassay Proseek Multiplex CVD I biomarker panel (Olink Bioscience, Uppsala, Sweden), (http://www.olink.com/proseek-multiplex/cvd/). More information of the biomarkers in the biomarker panel can be found in the supplementary data. In total, 80 patients were enrolled. Due to missing data on respiratory rate for 14 patients and blood biomarkers for 1 patient, 15 patients were excluded. This yielded 65 study participants.
Table 1.
Patient baseline characteristics, n = 65.
| Age (years), mean (± SD) | 81.9 (± 9.3) |
| Sex (males), n (%) | 36 (55.4) |
| Smoking, n (%) | |
| Current-smoker | 7 (10.8) |
| Former-smoker | 39 (60.0) |
| Never-smoker | 19 (29.2) |
| C-reactive protein (mg/L), median (range) | 17 (209.4) |
| NT-proBNP (ng/L), median (range) | 3754 (34950) |
| Medications, n (%) | |
| ASA or TRC-inhibitors | 34 (52.3) |
| Warfarin or DOACs | 18 (27.7) |
| Beta-antagonists | 44 (67.7) |
| ACE-inhibitors | 25 (38.5) |
| Angiotensin II receptor antagonists | 15 (23.1) |
| Calcium channel antagonists | 23 (35.4) |
| Diuretics | 44 (67.7) |
| Inhalations (SABA, LABA, LAMA, ICS) | 22 (33.8) |
| Nitroglycerin | 18 (27.7) |
| Anxiolytics | 7 (10.8) |
| Anti-diabetics | 10 (15.4) |
| Medical history, n (%) | |
| Congestive heart failure | 46 (70.8) |
| Anemia | 45 (69.2) |
| Ischemic heart disease | 43 (66.2) |
| Hypertension | 42 (64.6) |
| Atrial fibrillation | 35 (53.8) |
| Chronic obstructive pulmonary disease | 23 (35.4) |
| Pneumonia or sepsis | 22 (33.8) |
| Diabetes | 19 (29.2) |
| Chronic kidney disease | 14 (21.5) |
| Pulmonary thromboembolism | 8 (12.3) |
| Asthma | 5 (7.7) |
| Vital parameters, mean (± SD) | |
| Oxygen saturation (%) | 95 (± 3.6) |
| Respiratory rate (min− 1) | 22 (± 4.5) |
| Heart rate (min− 1) | 80 (± 15.9) |
| Systolic blood pressure (mm Hg) | 132 (± 18.6) |
| Diastolic blood pressure (mm Hg) | 75 (± 11.4) |
| Body temperature (°C) | 36.9 (± 0.7) |
| Severity of dyspnea, n (%) | |
| Dyspnea severity score, DSS 1 - no dyspnea | 0 (0) |
| Dyspnea severity score, DSS 2 - mild | 15 (23.1) |
| Dyspnea severity score, DSS 3 - moderate | 30 (46.2) |
| Dyspnea severity score, DSS 4 - severe | 20 (30.8) |
The severity of dyspnea was defined as the impact of dyspnea and measured as a single rating of disability on day 0. The severity was graded into a four-level score with the following values: no dyspnea, mild dyspnea, moderate dyspnea and severe dyspnea. The classification was based on the NYHA functional classification [10] and named dyspnea severity score (DSS). The combined study endpoint was readmission or death within six months. Readmission was considered as a following hospital stay, with the date of readmission as the endpoint date. The date of death was derived from the Swedish national civil registry. Statistical analysis was performed with IBM SPSS statistics 22 (SPSS) (SPSS Inc., Chicago, IL, USA).
Venous blood samples were obtained on admission day 0. Routine blood chemistry was analyzed using a Radiometer ABL800 Flex machine or Afinion AS100 Analyzer System [12] (http://www.alere.com/en/home/product-details/afinion-as100-analyzer-us.html). Three 7 mL EDTA blood samples and three 5 mL serum blood samples were drawn from each study participant for cardiometabolic biomarker analysis with Proseek. The samples were transferred into immunoassay plates with 24 plasma aliquots and 12 serum aliquots. The immunoassays were centrifuged 3000 turns per minute in 10 min and stored in − 80 °C for later analysis made by Proseek in Uppsala, Sweden. Detailed instructions of the methods in this analysis is found at the homepage of the manufacturer (http://www.olink.com/proseek-multiplex/cvd/)
For biomarkers with skewed data distribution natural logarithms were used to transform data to standardized scales and express the results per one standard deviation increment. All parameters were related to the endpoint by using Cox proportional hazard ratios adjusted for sex, age, peripheral oxygen saturation (SpO2), respiratory rate and C-reactive protein (CRP). A P value of < 0.05 (95% CI) was considered significant. Biomarkers independently related to outcome were analyzed using Cox regression with stepwise backward elimination. Significant biomarkers were combined into a biomarker risk score (BRS). The standardized values of significant biomarkers were weighted by their respective beta-coefficients and summed up to comprise the BRS. The BRS was also ranked and patients were categorized into tertiles according to the BRS, with the bottom tertile (lowest risk) used as the reference group.
3. Results
The mean age of in-patients with acute dyspnea was 81.9 (± 9.3) years. The proportion of men was 36 (55.4%). A medical history of earlier chronic diseases was common (Table 1). During the six months of follow up, 27 (41.5%) of the patients experienced a first readmission and 17 (26.2%) deceased. Main diagnosis at discharge is shown in Table 2.
Table 2.
Main diagnoses at discharge, n (%).
| Heart failure | 29 (44.6) |
| COPD/asthma | 13 (20.0) |
| Pneumonia/sepsis | 8 (12.3) |
| Acute coronary syndrome | 2 (3.1) |
| Pulmonary thromboembolism | 2 (3.1) |
| Malignancy | 1 (1.5) |
| Others | 10 (15.4) |
Oxygen saturation level was marginally lowered (95%) and respiratory rate elevated (22 ± 4.5), (Table 1). Most of the patients had moderate dyspnea 30 (46.2%) (DSS 3) but a substantial number suffered from severe dyspnea 20 (30.8%) (DSS 4). No patient had DSS 1.
The biomarkers tissue-type plasminogen activator (tPA), prolactin (PRL), tumor necrosis factor receptor superfamily member 6 (FAS) and C-C motif chemokine 3 (CCL3) were independently significant by Cox regression hazard analysis (Table 3) and combined into a biomarker risk score (BRS). Among others, the biomarkers Adrenomedullin (ADM), Natriuretic peptides B (BNP) and Interleukin-6 (IL-6) were not related to outcome (Supplementary Table 1). The prognostic impact of the biomarker risk score's tertiles in relation to outcome is seen in Fig. 1. For patients in tertile 3 of the BRS, the 6-month mortality and readmission rate was 87%. Each standard deviation increment of the score by multivariate analysis conferred a hazard ratio (HR) of 2.13 (1.39–3.27) P = 0.001 (Table 4). The top versus bottom tertile conferred a HR of 4.75 (1.93–11.68) P = 0.001. High severity of dyspnea was also associated with worse outcome, HR 3.43 (1.28–9.20) P = 0.014 (Table 4) but when the BRS and DSS were entered into the same model, the BRS remained highly significant (HR 1.94 per SD increment (1.24–3.02) P = 0.004) whereas DSS did not remain a significant independent determinant of the endpoint (NS). In addition, male gender was an independent risk factor for poorer outcome with a HR of 2.21 (1.08–4.54) P = 0.031.
Table 3.
Individual cardiometabolic biomarkers related to readmission or deatha.
| N°Events/N° | HR (95% CI) | PP | |
|---|---|---|---|
| FAS | 44/65 | 1.553 (1.094–2.205) | 0.014 |
| CCL3 | 44/65 | 1.604 (1.084–2.374) | 0.018 |
| tPA | 44/65 | 1.483 (1.018–2.160) | 0.040 |
| PRL | 44/65 | 0.736 (0.544–0.995) | 0.046 |
Adjusted for sex, age, respiratory rate, peripheral oxygen saturation and C-reactive protein.
Fig. 1.
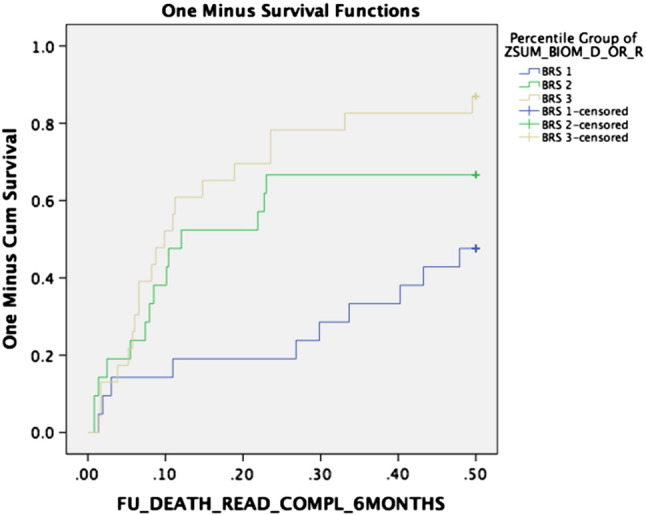
Kaplan-Meier cumulative curves for the three tertiles of cardiometabolic biomarker score – risk of death or readmission during the six-month follow up period.
Table 4.
Cardiometabolic biomarker score and severity of dyspnea by tertile categorizationa.
| HR (95% CI) | P-trend | HR (95% CI) |
P | |||
|---|---|---|---|---|---|---|
| Per 1 SD increment | Tertile 1 10/21 | Tertile 2 14/21 | Tertile 3 20/23 | |||
| Biomarker score (BRS) | 2.13 (1.39–3.27) | 0.001 | REF (1.0) | 2.53 (1.04–6.16) | 4.75 (1.93–11.69) | 0.003 |
| DSS 2 7/15 | DSS 3 21/30 | DSS 4 16/20 | ||||
|---|---|---|---|---|---|---|
| Dyspnea severity score (DSS) | NA | NA | REF (1.0) | 2.26 (0.93–5.51) | 3.43 (1.28–9.20) | 0.050 |
Adjusted for sex, age, respiratory rate, peripheral oxygen saturation and C-reactive protein.
When the BRS was stratified by dyspnea severity, the strength of the BRS's effect estimate remained strong and was independently significant for patients with low-moderate severity of dyspnea, HR = 2.14 (1.15–3.98) P = 0.016, but not for patients with severe dyspnea (Table 5). The BRS also remained significant with virtually no change in the effect size (HR per 1 SD increment 2.05 (1.32–3.18) P = 0.001) when NT-proBNP was entered on top of the multivariate analysis.
Table 5.
Cardiometabolic biomarker score stratified by dyspnea severitya.
| N°Events/N° | HR (95% CI) | P | |
|---|---|---|---|
| Biomarker score in DSS 2 & 3 | 28/45 | 2.14 (1.15–3.98) | 0.016 |
| Biomarker score in DSS 4 | 16/20 | 1.97 (0.67–5.74) | NS |
Adjusted for sex, age, respiratory rate, peripheral oxygen saturation and C-reactive protein.
We also subdivided the material into 31 “cardiac dyspnea patients” (29 heart failure and 2 acute coronary syndrome patients) and 23 “pulmonary dyspnea patients” (13 COPD/Asthma, 8 pneumonia/sepsis and 2 pulmonary thromboembolism patients). The BRS was significantly associated with 6-month mortality and readmission in both the cardiac dyspnea patients (HR per 1 SD increment of the BRS = 3.05; 95% CI 1.50–6.20; P = 0.002) and in the pulmonary dyspnea patients (HR per 1 SD increment of the BRS = 2.99; 95% CI 1.24–7.19; P = 0.015). Thus, the association seems to be similar in both groups.
4. Discussion
This study shows that a score of tPA, PRL, FAS and CCL3 (BRS) and dyspnea severity (DSS) predict 6-month readmission and death in patients hospitalized due to acute dyspnea. Both the BRS and the DSS significantly predicted the risk of readmission and death, independently of potential confounders. However, only the BRS remained significant when combined with the DSS in multivariate analysis, suggesting that the BRS adds more prognostic information and that it partially confers the prognostic information of the DSS. The highest predictive value of the BRS was seen for patients with mild-moderate dyspnea and risk assessment in these patients are often the most difficult.
Readmission and mortality rates were high, but vital parameters including peripheral oxygen saturation were little affected and showed no prognostic value. There is a lack of reliable tools for risk stratification in unselected patients with acute dyspnea. Progress in diagnostics and prognostics for patients with selected and specific diagnoses in acute dyspnea, such as heart failure, has been made with the use of natriuretic peptides as biomarkers that increase in response to myocardial stress [13], [14], [15]. Neurohormones and circulating peptides associated with the diseases causing dyspnea are potential prognostic biomarkers that to our knowledge have not been studied in a broad biomarker panel. The cardiometabolic biomarker panel in Proseek contains pro- and anti-inflammatory markers, markers of myocyte injury and stress, markers of remodeling, neurohormones and novel/exploratory biomarkers (http://www.olink.com/proseek-multiplex/cvd/). Patients with poorer outcome in the study tended to have a pro-inflammatory phenotype. Further studies are warranted to test if the biomarkers in the BRS may be causally involved in the development of the diseases causing dyspnea.
A few studies have investigated the association of the biomarkers FAS, CCL3, tPA and PRL with patient outcome, often focusing on cardiovascular disease or congestive heart failure. Recently, we published data on a cohort of patient with acute dyspnea in the emergency department, where inflammatory biomarkers were related to 90-day mortality [16]. Of the 25 included biomarkers, 20 biomarkers showed independently significant prognostic information for 90-day mortality. The biomarkers FAS, CCL3, tPA and PRL were not included, but the results indicate that circulating inflammatory biomarkers may be used to predict end stage disease or severe illness.
The pathways and mechanisms to inflammation are complex and theories are often based on in vitro studies. There are however some studies that have investigated the levels of the biomarkers in the BRS in vivo. The known biology behind the included biomarkers needs to be explained further. Earlier results have shown that activation of the tumor necrosis factor receptor superfamily member 6 (FAS) induces apoptosis [17]. Cytokines acting on endothelial and myocardial cells by inflammation and remodeling seems to be involved in the development of heart failure. High levels of FAS have been found in patients with severe heart failure and FAS plays a central role in the cytokine-hypothesis of heart failure [18], [19], [20]. FAS has in earlier studies been proposed to identify patients at future risk of heart failure and to be a valuable biomarker for patient risk stratification [21]. C-C motif chemokine 3 (CCL3) is involved in the acute phase of inflammation. The chemokine seems to be released upon specific stimulation and to be involved in neutrophil extravasation and T-cell homing towards areas of injury [22]. It increases during cardiac ischemic events, rapidly decreases during follow-up and may be a marker for future cardiovascular events with strong predictive power for a next episode of acute coronary syndrome [23]. The CCL3 level has also been found to increase in congestive heart failure irrespective of cause in comparison to healthy subjects, with the highest concentrations observed in patients with NYHA class IV [24]. Tissue-type plasminogen activator (tPA) is a biomarker involved in the breakdown of blood coagulates. High tPA activity can result in bleeding and low tPA activity can result in thrombosis or embolism. tPA is released by the appropriate cellular stimulation in response to venous stasis and possibly during arterial and venous thromboembolic events like myocardial infarction and pulmonary embolism. Some studies have associated increased tPA levels with recurrent thrombosis while others have found no relationship [25]. tPA may also act as pro-inflammatory cytokine and among other functions, regulates lipoprotein metabolism [26]. Prolactin (PRL) is besides its function in reproduction involved in the metabolism and the immune response. There is a link of PRL and high activity in Metalloproteinases to the risk of post-partum cardiomyopathy, partly explained by PRL acting as a cardiomyocyte-toxic agent [27]. However in this study, low levels of PRL related to poorer outcome. If a hypothetical relation to heart failure and extrapolation of earlier results can be made for PRL in unselected patients with acute dyspnea is unknown.
BNP lacked prognostic value. This was a surprising finding and may reflect a fragile population. A potential explanation is that the majority of patients had increased NT-proBNP values. Only four patients had a NT-proBNP value below the upper reference of normal according to our hospital laboratory (< 300 ng/L). As most patients had abnormal values and there were only a small proportion of patients with normal values to compare with, the discriminatory value of NT-proBNP for the outcome may have been limited. In addition, many of the patients with cardiac disease commonly suffered from other diseases contributing to dyspnea. Perhaps NT-proBNP has little additional prognostic value in an aged population with multiple chronic diseases.
The study has several limitations. The study size is small and limited to one hospital department, making the reliability and applicability of results weaker. Not all patients with acute dyspnea participated in the study. However, patients were consecutively and randomly enrolled. There is a substantial amount of missing data in respiratory rate and to a minor extent in biomarker analysis (1 patient). Missing data was interpreted as random errors as it is routine practice to register vital signs for all in-patients. Despite the limitations, the patient characteristics resemble those of other studies of patients suffering from acute dyspnea [9], [13], [28].
Patients hospitalized due to acute dyspnea tend to be old, suffer from respiratory and cardiovascular diseases and often need medical care. The clinical picture is often complex and the presence of comorbidity is high. Cardiac and thromboembolic events may to a larger extent than expected be involved in acute dyspnea among patients with risk factors, as suggested by our results. High-risk patients could thus benefit from more aggressive diagnostic and treatment strategies. Despite efforts, patients with acute dyspnea are a patient group with high readmission and mortality rates. Better prognostic tools are necessary to improve patient outcome. If the results can be replicated and the biomarkers become easily available for the clinician at a reasonable cost, the biomarker risk score may guide in decision making for patients with acute dyspnea that is often difficult to accurately assess.
To conclude, a biomarker score of tPA, PRL, FAS and CCL3 predicts 6-month death and readmission in patients hospitalized for acute dyspnea. The strongest prognostic value of the score is seen in patients with mild and moderate dyspnea. The biomarkers may be used to improve risk stratification and guide in decision making for patients with acute dyspnea.
Author contributions
Conception and design: NL, KG, OM, CW.
Analysis and interpretation: NL, KG, OM.
Drafting the manuscript: NL, KG, OM.
Acknowledgements & sources of support
Swedish Research Council, ERC StG 282285, Swedish Heart and Lung Foundation, Novo Nordisk Foundation, Göran Gustafsson Foundation, Knut and Alice Wallenberg Foundation, and Region Skåne.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ajem.2016.12.048.
Appendix A. Supplementary data
Supplementary material
References
- 1.Currow D.C. A community population survey of prevalence and severity of dyspnea in adults. J Pain Symptom Manage. 2009;38(4):533–545. doi: 10.1016/j.jpainsymman.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Frostad A. Respiratory symptoms as predictors of all-cause mortality in an urban community: a 30-year follow-up. J Intern Med. 2006;259(5):520–529. doi: 10.1111/j.1365-2796.2006.01631.x. [DOI] [PubMed] [Google Scholar]
- 3.Frostad A. Respiratory symptoms and long-term cardiovascular mortality. Respir Med. 2007;101(11):2289–2296. doi: 10.1016/j.rmed.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Ho S.F. Dyspnoea and quality of life in older people at home. Age Ageing. 2001;30(2):155–159. doi: 10.1093/ageing/30.2.155. [DOI] [PubMed] [Google Scholar]
- 5.Johnson M.J. Breathlessness in elderly adults during the last year of life sufficient to restrict activity: prevalence, pattern, and associated factors. J Am Geriatr Soc. 2016;64(1):73–80. doi: 10.1111/jgs.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ammar K.A. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115(12):1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 7.Pang P.S. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. Eur Heart J. 2008;29(6):816–824. doi: 10.1093/eurheartj/ehn048. [DOI] [PubMed] [Google Scholar]
- 8.Parshall M.B. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berraho M. Dyspnea: a strong independent factor for long-term mortality in the elderly. J Nutr Health Aging. 2013;17(10):908–912. doi: 10.1007/s12603-013-0347-6. [DOI] [PubMed] [Google Scholar]
- 10.Little and B. Co . The criteria committee of New York Heart Association. 9th ed. Boston Mass; 1994. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels; pp. 253–256. [Google Scholar]
- 11.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.ABL800 FLEX reference manual. 201206. Code number: 989–963, Version 6.10. Edition J.
- 13.Maisel A. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (biomarkers in acute heart failure) trial. J Am Coll Cardiol. 2010;55(19):2062–2076. doi: 10.1016/j.jacc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 14.Omland T. Prognostic value of B-type natriuretic peptides in patients with stable coronary artery disease: the PEACE trial. J Am Coll Cardiol. 2007;50(3):205–214. doi: 10.1016/j.jacc.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Braunwald E. Heart failure. JACC Heart Fail. 2013;1(1):1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Wiklund K. Inflammatory biomarkers predicting prognosis in patients with acute dyspnea. Am J Emerg Med. 2016;34(3):370–374. doi: 10.1016/j.ajem.2015.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brint E., O'Callaghan G., Houston A. Life in the Fas lane: differential outcomes of Fas signaling. Cell Mol Life Sci. 2013;70(21):4085–4099. doi: 10.1007/s00018-013-1327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuyama M. Serum levels of soluble form of Fas molecule in patients with congestive heart failure. Am J Cardiol. 1997;79(12):1698–1701. doi: 10.1016/s0002-9149(97)00228-2. [DOI] [PubMed] [Google Scholar]
- 19.Seta Y. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2(3):243–249. doi: 10.1016/s1071-9164(96)80047-9. [DOI] [PubMed] [Google Scholar]
- 20.Levine B. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 21.Vasan R.S. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 22.Reichel C.A. C-C motif chemokine CCL3 and canonical neutrophil attractants promote neutrophil extravasation through common and distinct mechanisms. Blood. 2012;120(4):880–890. doi: 10.1182/blood-2012-01-402164. [DOI] [PubMed] [Google Scholar]
- 23.de Jager S.C. CCL3 (MIP-1 alpha) levels are elevated during acute coronary syndromes and show strong prognostic power for future ischemic events. J Mol Cell Cardiol. 2008;45(3):446–452. doi: 10.1016/j.yjmcc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Aukrust P. Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation. 1998;97(12):1136–1143. doi: 10.1161/01.cir.97.12.1136. [DOI] [PubMed] [Google Scholar]
- 25.Brandt J.T. Plasminogen and tissue-type plasminogen activator deficiency as risk factors for thromboembolic disease. Arch Pathol Lab Med. 2002;126(11):1376–1381. doi: 10.5858/2002-126-1376-PATTPA. [DOI] [PubMed] [Google Scholar]
- 26.Lin L., Hu K. Tissue plasminogen activator and inflammation: from phenotype to signaling mechanisms. Am J Clin Exp Immunol. 2014;3(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- 27.Sliwa K. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 28.Mueller C. The use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647–654. doi: 10.1056/NEJMoa031681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


