Summary
Background
Joint trauma is predisposing to the incidence of osteoarthritis (OA) of the knee. There is a limited knowledge on the impact of posttraumatic osteochondral defects on the whole joint. This study was designed to define a critical size osteochondral defect in the knee of rats and to investigate a possible association between osteochondral defects and degeneration of the surrounding joint surface.
Methods
Cylindrical osteochondral defects of different sizes were created in the knee joint of rats. The natural course of these lesions was studied by macroscopic observation, histology, and immunohistochemistry. Gene expression of the articular cartilage surrounding the defects in vivo and of articular chondrocytes cultured in vitro in IL1β and fibroblast growth factor 2 (FGF2) supplemented media was evaluated by quantitative polymerase chain reaction (qPCR).
Results
In defects of 0.9 mm diameter, spontaneous joint surface healing was observed but also upward advancing of the subchondral bone plate at 16 weeks. Larger 1.4 mm diameter defects were critical size, not resulting in successful healing at any time point. Importantly, the articular cartilage surrounding the defects expressed FGF2 and IL1β, but not ACAN and Col2. Chondrocytes cultured in IL1β and FGF2 supplemented media lost the natural fibroblast growth factor receptors – FGFr1/FGFr3 balance and showed decreased viability.
Conclusions
A critical size osteochondral defect was defined as 1.4 mm in diameter in rat. Subchondral bone plate advancement occured rapidly. The articular cartilage surrounding osteochondral defects showed catabolic activity with expression of IL1β, FGF2 and a disturbed FGFr1/FGFr3 balance, potentially initiating a process of early osteoarthritic disease.
Keywords: Tissue regeneration, Knee, Osteoarthritis, Osteochondral
Introduction
Knee arthroscopies performed in over 900,000 patients in the USA revealed osteochondral injuries in up to 60% of patients. From the patient's perspective of quality of life, osteochondral defects were estimated to be as painful and debilitating as late stage osteoarthritis (OA)1, 2, 3. Since articular cartilage has little capacity to repair itself, several treatments have been attempted to restore joint surface defects, such as microfracture, the osteochondral autograft transplant system (OATS), and autologous chondrocyte implantation (ACI)4, 5. The results of OATS showed a high rate of return to sport on the short term, while significantly more failures occurred with OATS than with ACI at 10 years6. ACI gives very reasonable outcomes in prospective randomized studies7, 8, 9, however some challenges remain including fibrocartilage formation with little hyaline cartilage restoration leading to joint deterioration over time and the high cost of this treatment10, 11. Despite new strategies for osteochondral injuries repair have increasingly been attempted, treating these defects is still an unsolved challenge11.
Several animal models have been investigated to be able to address the challenges for osteochondral repair12, 13. An osteochondral defect model in rats seems very attractive in providing proof-of-concept data. First, the rat model displays an economic advantage as rats are relatively cheap and easy to care for. Second, the rat model is perceived as more clinically relevant than the mouse model, since the articular cartilage in rats displays typically also a zonal structure that resembles the one in human joints, less so for the mouse model14. In addition, immune-deficient rats are now also commercially available, providing an opportunity to study the regenerative potential of human cells in this model. Overall, the rat model seems appropriate for initial in vivo testing, however there is a need to better understand the biology of osteochondral defects. The spontaneous repair of osteochondral defects of different sizes has not been studied in a systematic approach15. Since the joint surface has some regeneration ability, a critical size osteochondral defect model for the rat should be defined. Although subchondral bone plate advancement towards the joint surface is a major and growing concern in osteochondral repair, this has not been studied in chronological order in rats16, 17. Currently, no strategy seems to avoid subchondral bone plate advancement and fibrocartilage formation that leads to treatment failure.
As the incidence of OA has been associated with joint trauma, osteochondral defects may not remain restricted to a local wound, and they could also trigger a whole joint disease. In fact, there is some evidence that the presence of asymptomatic osteochondral defects or full thickness chondral defect leads to articular cartilage loss and to the development of early OA18, 19, 20. In this case, the anabolic activity of chondrocytes seems to be reduced in both normal and OA-articular cartilage21, which can be explained, at least partially, by cartilage's limited capacity to recover from damage and exposure to inflammation22. Moreover, fibroblast growth factors (FGF) and fibroblast growth factor receptors (FGFr) also show regulatory effects on both catabolic and anabolic processes of the joint23. Therefore, therapies targeting inflammatory cytokines and FGFs are assumed to be of great relevance in the treatment of joint diseases and in the prevention of trauma related OA. However, our knowledge about the mechanisms triggering degeneration of the cartilage surrounding osteochondral defects is still incomplete and further understanding is required11.
In this study, osteochondral defects of different sizes were used to evaluate the natural course of spontaneous osteochondral healing over time, not only within the defect area but also in the cartilage surrounding the defect. The aim of the present study was: (1) to define the critical size of an osteochondral defect in rats, (2) to define the course of subchondral bone plate advancement, (3) and to examine the association between osteochondral defects and degeneration of the surrounding cartilage.
Materials and methods
Animal experiments
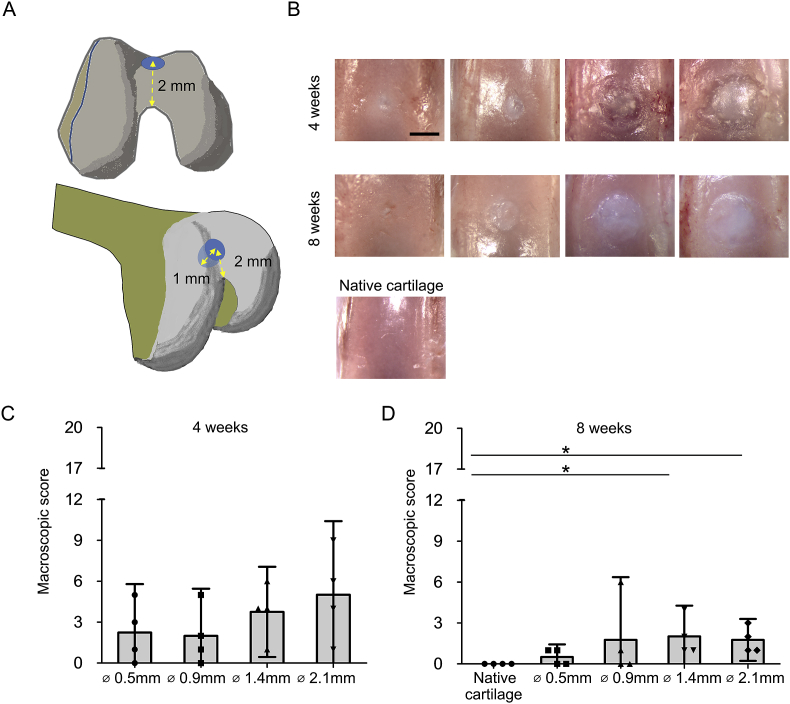
All animal procedures were approved by the local ethical committee for Animal Research (KU Leuven). The animals were housed according to the guidelines of the Animalium Leuven (KU Leuven). Wild-type Lewis male rats (Charles River Laboratories, Den Bosch, Netherlands) at 9–11 weeks olds were used. Each rat received surgery on both knees. A small incision in the articular capsule was made along the patella and patellar tendon. Subsequently, the patella was dislocated to the lateral side according to previous reports24, 25. Cylindrical osteochondral defects of different sizes (0.5 mm, 0.9 mm, 1.4 mm or 2.1 mm diameter × 1.0 mm depth) were created, as indicated in Fig. 1(A). After wound closure in two layers, the rats were allowed to walk freely in the cage before being sacrificed at 4, 8, and 16 weeks (n = 4 for histology and n = 3 for gene expression analyses). Eight age matched rats were sacrificed for non-operated knees.
Fig. 1.
Macroscopic evaluation showed complete filling of the osteochondral defects. (A): Schematic view of operation. Cylindrical osteochondral defects of different sizes (0.5 mm, 0.9 mm, 1.4 mm or 2.1 mm diameter × 1.0 mm depth) were created at 2.0 mm from the top of the intercondylar groove by using a micro drill burr with a stopper for accurate depth control. (B): Representative macroscopic findings of the spontaneous healing of different sizes of the osteochondral defects at 4 and 8 weeks. (Bar = 500 μm). (C): Quantification for healing areas of the osteochondral defects using a scoring system developed by Goebel et al.21, in which a full score (20) indicated the worst possible result and a lower score indicated values closer to the normal cartilage at 4 weeks (n = 4). (D): Quantification for healing areas of the osteochondral defects using a scoring system developed by Goebel et al., at 8 weeks (n = 4, *P < 0.05).
Macroscopic evaluation
The distal part of the femur end was carefully collected at 4, 8 and 12 weeks after operation. The macroscopic appearance of defects was scored using the Goebel's semi-quantitative macroscopic scoring system26.
Histological examination and immunohistochemistry
Safranin-o (SafO) staining and all immunohistochemistry were performed as described previously27, 28. The specimens were visualized using a Leica DMR microscope (Leica, Wetzlar, Germany) using the same settings for all samples. Defects were evaluated using the Sellers's score29, 30. The antibodies used are indicated in Supplementary Table 1.
Automated histomorphometrical measurements
The SafO positive area, subchondral bone area and subchondral bone plate distance were measured automatically from SafO stained slides using ImageJ. A color threshold for positive SafO staining was defined within a reference area of the native articular cartilage at an unaffected site. A region of interest (ROI) of a size comparable to the original defect was drawn. Subsequently, the SafO positive area within the ROI was measured in injured knees using ImageJ software (n = 4). In parallel, an intact control area was measured within the same size of ROI in native intact cartilage. The percentage of SafO positive area was calculated by the SafO positive area in the osteochondral defect divided by the intact control area.
A subchondral bone area was automatically chosen by the color threshold for a SafO negative region (excluding cysts) and measured in the ROI by ImageJ software. The subchondral bone area rate was calculated by the SafO negative area in the defect divided by the native subchondral bone area.
Distance from the surface to the closest point of subchondral bone in the center of the defect was measured. The subchondral bone plate distance was defined by the distance from surface to the closest point of subchondral bone minus 245 nm as the average native cartilage thickness which was measured.
Total ribonucleic acid (RNA) extraction and quantitative reverse transcription–polymerase chain reaction analysis
The regenerated tissue in the defect, the articular cartilage surrounding the defect, and the intact native cartilage (non-operated positive control) were harvested with a surgical knife under an inverted microscope (Supplementary Fig. 1). Isolation of total RNA, synthesizing complementary deoxyribonucleic acid (DNA), and running quantitative polymerase chain reaction (qPCR) were performed as described previously31. Each sample was tested in duplicate and compared with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression that allowed normalization of results. Relative differences in expression were calculated using the 2−ΔΔCT method. The sequences used to construct the primers are listed in Supplementary Table 2.
Isolation and culture of rat chondrocytes
Intact cartilage (non-operated positive control) from rat knee joints was dissected and digested with 0.2% type 2 collagenase (Thermo Fisher Scientific) in DMEM/F12 for 3 h at 37°C. Primary digested chondrocytes obtained from two knees were seeded in 19 cm2 (5 wells in 12 well plates) at an initial cell density of approx. 500 cells/cm2 and cultured in complete culture medium (DMEM/F12; 5% FBS; 1 μl/ml human transferrin; Sodium selenite; penicillin, streptomycin, and amphotericin B; Thermo Fisher Scientific) for 5 days. Subsequently, these cells were cultured in complete culture medium supplemented with or without ILβ1 (0.1 ng/ml or 10 ng/ml) or FGF2 (30 ng/ml) for 5 days at confluency. Cells were lysed by RLT buffer with 2-mercaptoethanol and collected for RNA extraction.
Statistical analysis
The statistical analysis was performed using Kruskal–Wallis test. Statistically significant differences between groups were further investigated using Dunnett multiple comparison test. Results are considered statistically different for P-values lower than 0.05 (*P < 0.05). Data are expressed as confidence intervals (C.I).
Results
Macroscopic evaluation showed complete filling of the osteochondral defects
Macroscopically, all the defects of the small defect group (0.5 mm and 0.9 mm in diameter) were filled with newly formed tissue 4 weeks after surgery. A similar result was observed at 8 weeks after surgery for defects of the large defect group (1.4 mm and 2.1 mm in diameter). At 4 weeks after surgery, 0.5 mm and 0.9 mm defects were completely covered by translucent or white colored repair tissue displaying a smooth surface [Fig. 1(B)]. 1.4 mm and 2.1 mm defects were filled partially by a translucent or white repair tissue and had a rough surface with large fissures and cracks. At 8 weeks after surgery, 0.5 mm and 0.9 mm defects were completely filled with repair tissue that was similar to the neighboring native cartilage and showed a smooth surface [Fig. 1(B)]. However, there was a clear boundary surrounding the defects. At 8 weeks, the 1.4 mm and 2.1 mm defects were mainly filled with white colored tissue suggesting that the repair tissue is of different quality when compared to the neighboring cartilage. When evaluated by the macroscopic score developed by Goebel et al., the small defect group had a trend for better scores than the large defect group at 4 weeks after surgery [Fig. 1(C)]. At 8 weeks after surgery, the large defect group scored lower than the age matched control group [Fig. 1(D)].
Histological examination indicated the critical size of osteochondral defects
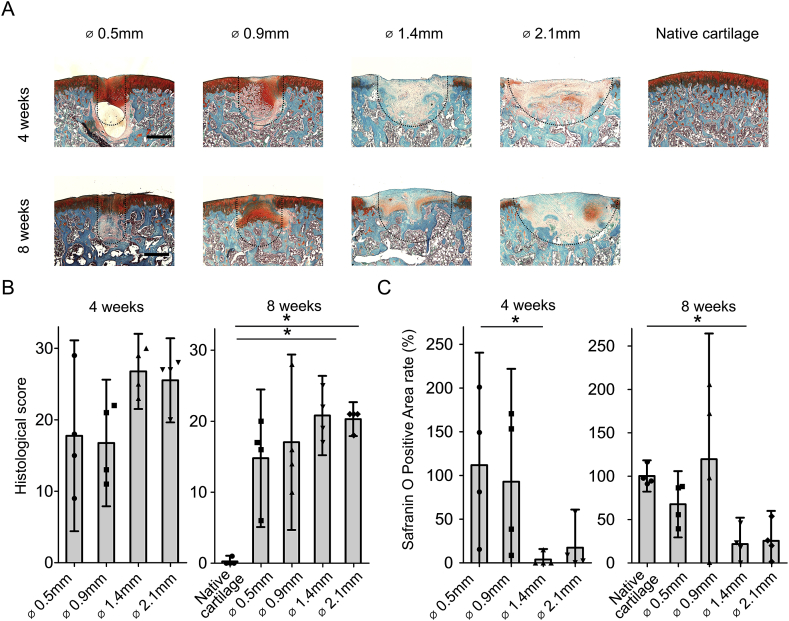
Histological characterization was performed to evaluate the osteochondral repair process. At 4 weeks after surgery, the larger (1.4 and 2.1 mm) osteochondral defects were filled mainly by fibrocartilage tissue displaying negative SafO staining. Small areas of SafO positive tissue were found adjacent to the subchondral bone. Moreover, these samples displayed multiple small fissures. In the case of smaller (0.5 and 0.9 mm) osteochondral defects, the entire articular surface was almost completely covered by SafO positive tissue with moderate or good surface regularity at 4 weeks after surgery [Fig. 2(A)]. However, all the samples from the 0.5 mm group showed cyst formation underneath the repaired articular surface. In the 0.9 mm group, 1 out of 4 had a cyst and 1 out of 4 had a deep cleft. A process of cartilage flow phenomenon, characterized by the flow of cartilage from the edge into the defect, could partially explain the regeneration of small defects at early time points and the appearance of subchondral cysts as a result of articular cartilage closure before subchondral bone healing, as previously reported32.
Fig. 2.
Histological examination indicated the critical size of osteochondral defects in rats (A): Representative SafO staining sections of native cartilage and four different sizes of osteochondral defects at 4 and 8 weeks. Dotted line indicated the original defect borders. (Bar = 500 μm) (B): Sellers's score for histology in which a full score of 31 points indicated no repair response and a lower score indicated values closer to the normal cartilage (n = 4, *P < 0.05)24. (C): Quantification for the rate of SafO positivity in which 100% indicated same size as native cartilage. (n = 4; *P < 0.05).
At 8 weeks after surgery, the larger osteochondral defects were mainly filled by SafO negative tissue, with small areas of SafO positive tissue close to the subchondral bone. Conversely, small osteochondral defects were filled almost completely by SafO positive tissue displaying moderate or good surface regularity. However, the joint surface developed early signs of cartilage degeneration, and the cysts formed underneath the articular surface were still present at 8 weeks after surgery [Fig. 2(A)].
At 4 weeks after surgery, Sellers's histological score confirmed a better outcome in the 0.5 mm and 0.9 mm defect groups when compared to the 1.4 mm and 2.1 mm defect groups without statistical significance [Fig. 2(B)]. Accordingly, at 8 weeks after surgery, the 1.4 mm and 2.1 mm defect groups showed significant lower scores than age matched native cartilage.
At 4 weeks after surgery, the 1.4 mm group had significantly less percentage of SafO positive area than the 0.5 mm group (*P < 0.05) [Fig. 2(C)]. At 8 weeks after surgery, the percentage of SafO positive area within the reparative tissue compared to native cartilage was 67.5% in the 0.5 mm group, 119.3% in the 0.9 mm group, 21.7% in the 1.4 mm group, and 25.4% in the 2.1 mm group, respectively. The 1.4 mm group had significantly less SafO positive area than the age matched control group at 8 weeks. In summary, it was found that the reparative tissues of 1.4 mm and 2.1 mm groups consist mostly of fibrocartilage, therefore confirming that osteochondral defects of 1.4 mm in diameter or larger do not heal spontaneously and can be classified as critical size osteochondral defects.
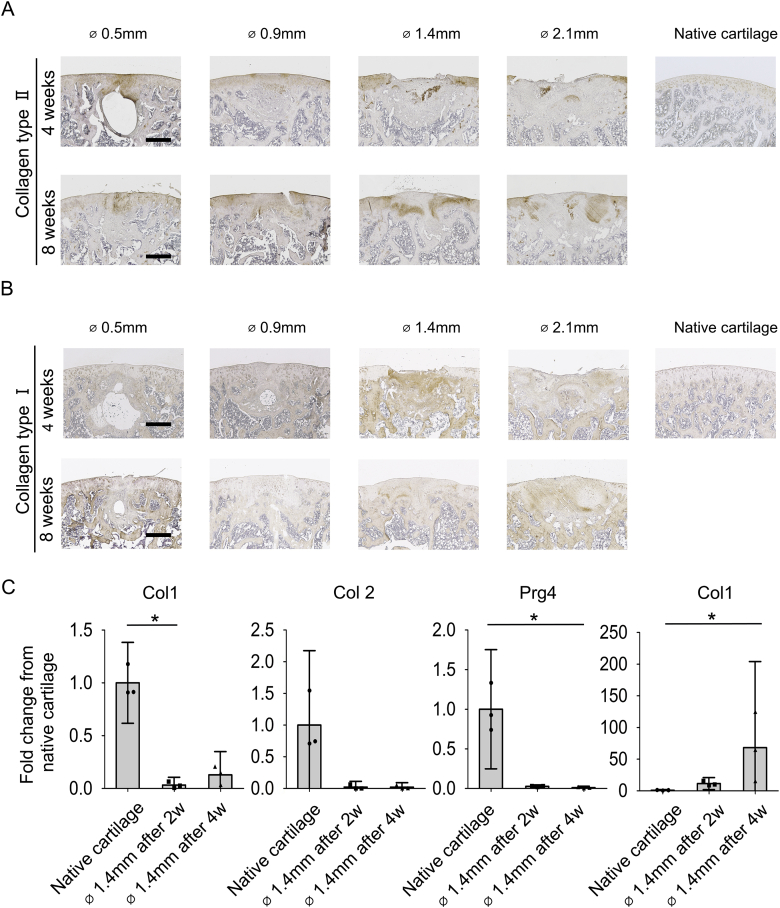
Immunohistochemical and gene expression analysis confirmed fibrocartilage formation at the defect site
Immunohistochemistry and gene expression analysis was performed to further characterize the reparative tissue in osteochondral defects of different sizes. In the small defect group, the newly formed tissue was characterized by a deposition of type 2 collagen similar to the articular cartilage surrounding the defects. Type 1 collagen was found at relative low intensity at 4 and 8 weeks after surgery [Fig. 3(A) and (B)]. The large defect groups displayed either low or no deposition of type 2 collagen and a high content of type 1 collagen at 4 and 8 weeks after surgery. This indicates that osteochondral defects of 1.4 mm in diameter or larger were repaired mostly through accumulation of fibrocartilage. In addition, the reparative tissues were characterized by quantitative gene expression analysis. The relative gene expression of selected cartilage-associated genes in the intact native cartilage was used as a reference value. The results confirmed a decrease of articular cartilage gene markers in the defect area at 2 weeks and 4 weeks after surgery when compared to native cartilage. At 4 weeks after surgery, a decrease was seen in ACAN (0.13 ± 0.05 fold), Col2 (0.02 ± 0.02 fold), and Lubricin/Prg4 (0.01 ± 0.004 fold) expression from native cartilage to the reparative tissue of the 1.4 mm group. Conversely, the fold increase for Col1 compared to native cartilage was 11.5 ± 2.2 at 2 weeks and 68.3 ± 31.6 at 4 weeks after surgery, therefore suggesting that the newly formed tissue in defects of 1.4 mm or larger was fibrocartilage and not hyaline cartilage.
Fig. 3.
Immunohistochemical characterization and gene expression of the reparative tissues (A): Representative immunohistochemically staining of native cartilage and four different sizes of osteochondral defects for type 2 collagen at 4 and 8 weeks (n = 4). (B): Representative immunohistochemically staining of native cartilage and four different size of osteochondral defects for type 1 collagen at 4 and 8 weeks (n = 4). (C): Quantitative gene expression analysis the newly formed tissue in 1.4 mm osteochondral defects and non-operative native cartilage (n = 3). (Bar = 500 μm).
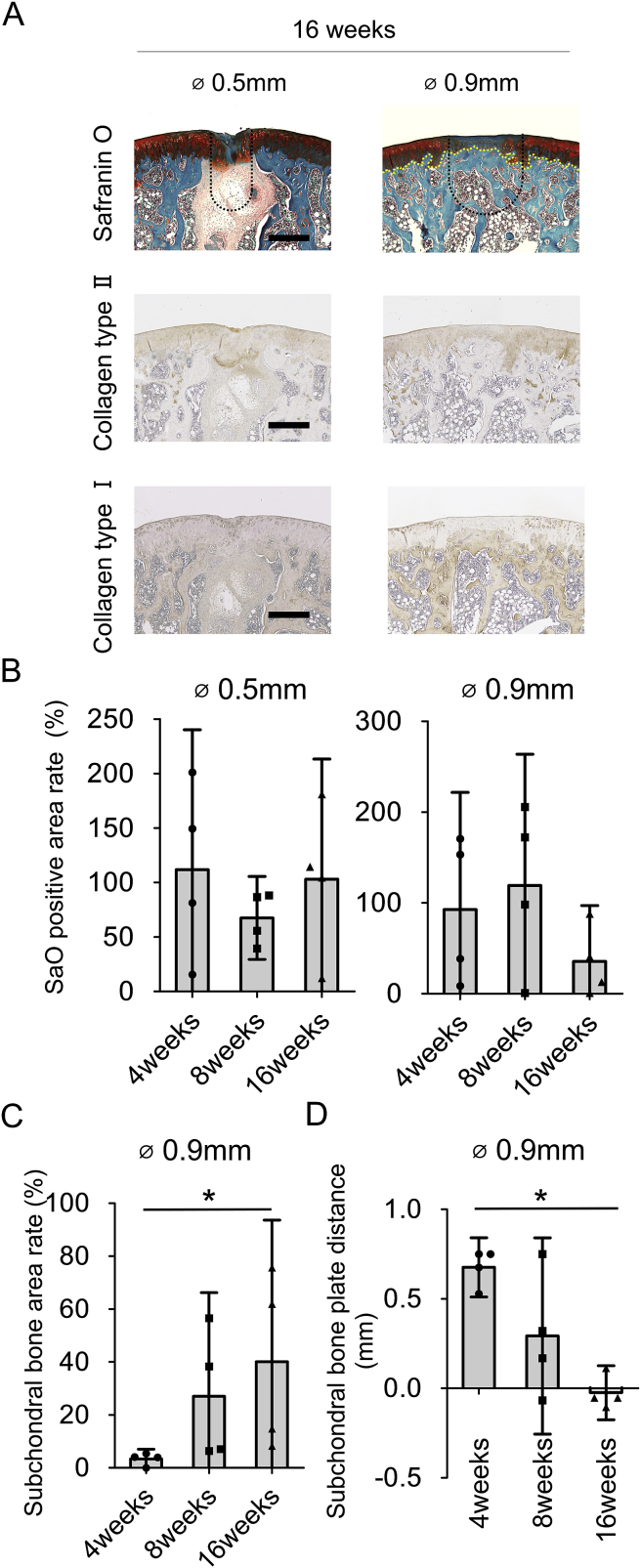
Instability of the newly formed cartilage tissue and subchondral bone plate migration in small size osteochondral defects
Since the small defect groups showed de novo hyaline-like cartilage at 4 and 8 weeks after surgery, we were interested to investigate the durability of the repair process.
At 16 weeks after surgery, 2 out of 4 samples in the 0.5 mm group were almost completely covered by SafO positive tissue and showed good surface regularity [Fig. 4(A)]. However, 2 out of 4 samples lost partially their SafO positive area. Also, all samples in the 0.5 mm group maintained the cysts formed at early time-points underneath the repair cartilage tissue. All samples in the 0.9 mm group lost their SafO positive staining in most of the articular surface and displayed signs of cartilage degeneration. Immunohistochemistry showed overlapping type 2 collagen deposition and SafO positive tissue, while type 1 collagen immunostaining confirmed the subchondral bone plate migration towards the articular surface in the 0.9 mm defect group. The percentage of SafO positive area was clearly reduced to 35.6% in the 0.9 mm group at 16 weeks after surgery [Fig. 4(B)].
Fig. 4.
Instability of the newly formed cartilage tissue and subchondral bone plate migration in small size defects (A): Representative SafO staining and immunohistochemical staining for type 2 collagen and type 1 collagen of small osteochondral defects at 16 weeks. (Bar = 500 μm. Yellow dot line in 0.9 mm group indicates subchondral bone plate. Black dotted line indicated the original defect borders) (B): Quantification of proportion of SafO positivity in which 100% indicated same size as native cartilage in the small defect size group until 16 weeks. (C): Quantification of the relative proportion of the subchondral bone area in which 100% indicated same size as the drilling size in the 0.9 mm group until 16 weeks. (D): Quantification for areas of subchondral bone plate distance in which 0 mm indicated same depth as native subchondral bone plate depth in the 0.9 mm group until 16 weeks. (n = 4, *P < 0.05).
The relative proportion of the subchondral bone area in the 0.9 mm group significantly increased in chronological order from 4.6% at 4 weeks to 55% at 16 weeks [Fig. 4(C)]. In the same group, subchondral bone plate distance significantly decreased from 0.68 mm at 4 weeks to −0.03 mm at 16 weeks after surgery [Fig. 4(D)], suggesting that the subchondral bone plate started to expand at 16 weeks after surgery. Altogether, these results suggest that reparative hyaline cartilage formed in the small defect group degenerates in a time dependent manner and is associated with subchondral bone plate migration towards the surface at later time points.
Surrounding native cartilage undergoes catabolic degeneration
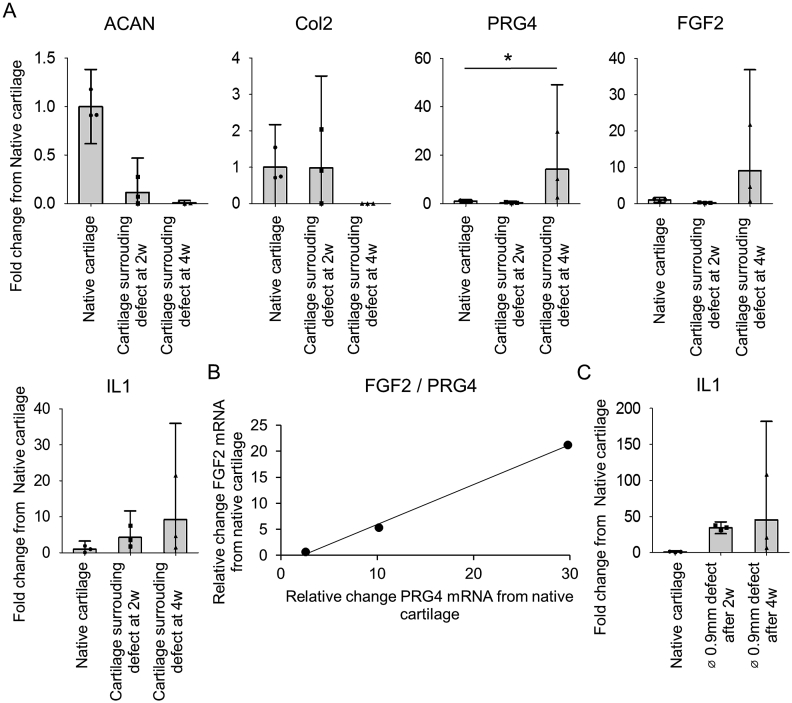
To investigate the potential harmful effect of osteochondral defects to the surrounding native cartilage, we studied both the catabolic and anabolic activity of cartilage surrounding the defect area and native cartilage of non-operated knees (positive controls). These results showed a lower expression of articular extracellular matrix (ECM) proteins in the cartilage surrounding the defects when compared to native cartilage [Fig. 5(A)]. Conversely, Prg4, FGF2, and IL1β increased with a 14.2 ± 8.1 fold, 9.1 ± 6.4 fold, and 9.2 ± 6.2 fold, respectively. Moreover, FGF2 and Prg4 expression levels displayed a very strong correlation when individual replicates were analyzed [Fig. 5(B)]. Since inflammation has been implicated in cartilage degeneration and progressive joint deterioration, we investigated whether IL1β could be correlated with the surrounding cartilage degeneration process. IL1β gene expression in the defect was upregulated from 2 to 4 weeks after surgery [Fig. 5(C)].
Fig. 5.
Gene expression in surrounding native cartilage (A): Quantitative gene expression analysis of the cartilage surrounding 0.9 mm defects at 2 weeks and 4 weeks and non-surgical native cartilage. (B): Correlation of FGF2 and Prg4 expression level of the cartilage surrounding 0.9 mm defects at 4 weeks (R2 = 0.9971). (C): Quantitative gene expression analysis of the newly formed tissue in 0.9 mm defect and non-surgical native cartilage (n = 3; *P < 0.05).
The mechanism of anabolic to catabolic shift in articular chondrocytes involves IL1β and FGF2
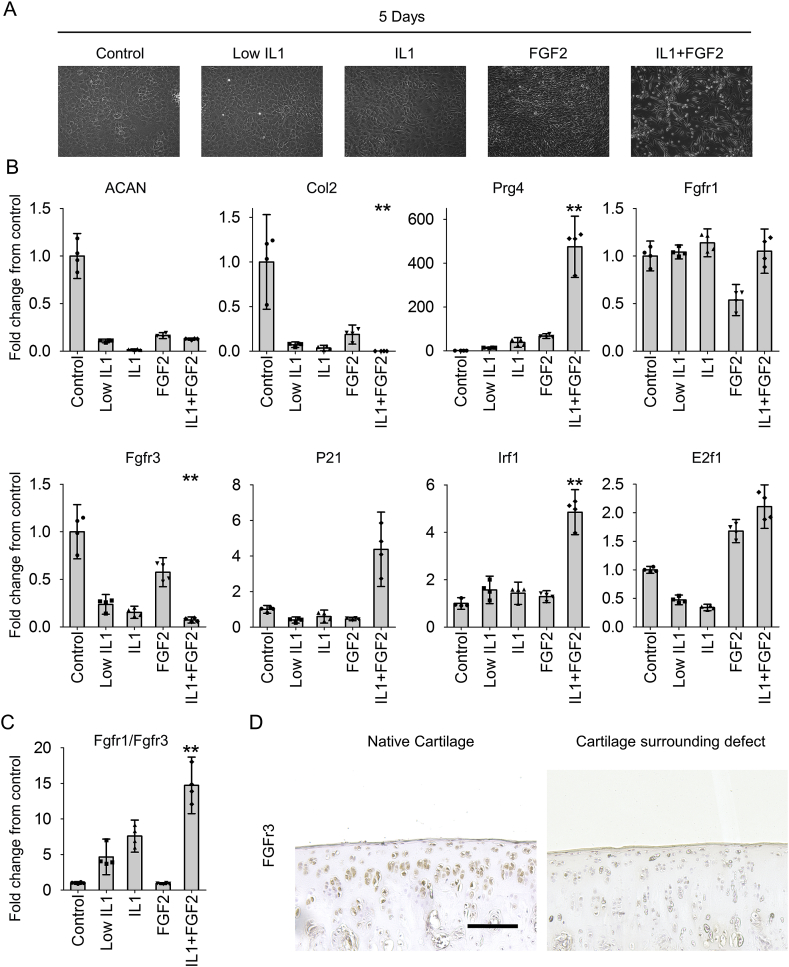
To investigate the potential synergistic catabolic effect of IL1β and FGF2 in articular cartilage surrounding an osteochondral defect, rat articular chondrocytes were isolated from normal knees and cultured in vitro in expansion medium supplemented with IL1β, FGF2 or IL1β + FGF2. Figure 6 shows the cell morphology of chondrocytes cultured in adherent monolayers under different conditions [Fig. 6(A)]. Chondrocytes cultured in expansion medium showed a polygonal shape throughout the experiment. The same morphology was found in chondrocytes cultured in either 0.1 ng/mL IL1β or 10 ng/mL IL1β. FGF2 supplementation changed the chondrocyte morphology to spindle shape. Interestingly, when cultured in expansion medium supplemented with IL1β + FGF2, adherent chondrocytes showed spindle shape morphology, but a large number of cells detached from the culture vessel. Cell density was also diminished in IL1β + FGF2 condition when compared to FGF2 condition.
Fig. 6.
The mechanism towards catabolism in chondrocytes (A): Chondrocyte morphology in complete medium with or without low IL1β, IL1β, FGF2, or IL1β + FGF2. (B): Quantitative gene expression analysis of chondrocytes in complete medium with or without low IL1β, IL1β, FGF2, or IL1β + FGF2. (n = 4; **P < 0.05 to control group). (C): Quantitative FGFr1/FGFr3 gene expression ratio in all groups (n = 4; **P < 0.05 to control group). (D): Representative immunohistochemically staining of native cartilage and cartilage surrounding 0.9 mm defects for FGFr3 at 4 weeks (n = 4). (Bar = 500 μm).
At the gene expression level, IL1β or FGF2 supplementation in vitro reduced ACAN and Col2 expression [Fig. 6(B)]. Conversely, IL1β + FGF2 stimulated Prg4, corroborating the in vivo results.
In chondrocytes, FGF2 signals through two different FGF receptors, FGFr1 leading to a catabolic activity and FGFr3 associated with an anabolic activity23. In our experiments, FGF2 suppressed the expression of FGFr1 while IL1β or IL1β + FGF2 did not change the expression of FGFr1. Instead, IL1β or IL1β + FGF2 reduced the expression of FGFr3. This result showed that the FGFr1/FGFr3 balance was dynamically altered in IL1β + FGF2 supplementation (fold change from control = 14.7 ± 1.2) [Fig. 6(C)]. Immunohistochemistry confirmed a decreased FGFR3 expression in articular cartilage surrounding the defect [Fig. 6(D)].
Downstream targets of FGF2 were analyzed to confirm FGF signaling in articular chondrocytes. P21 and Irf1 expression, which are known to block proliferation in chondrocytes, increased after IL1β + FGF2 supplementation. Expression of E2f1, which leads to apoptosis, increased after IL1β + FGF2 supplementation. These results suggested a critical role of IL1β and FGF2 in triggering articular cartilage catabolism.
Discussion
In this study, osteochondral defects of different sizes were investigated in rats to define critical size defects and study their spontaneous healing capacity. Uniquely, the natural course of osteochondral repair was evaluated overtime not only in the defect area, but also in the cartilage surrounding the defect. We found that osteochondral defects of 1.4 mm in diameter (or larger) and 1.0 mm in depth did not spontaneously restore the osteochondral unit, therefore defining the critical size of osteochondral damage in a rat knee. Interestingly, small osteochondral defects were filled with hyaline cartilage at early time points, indicating spontaneous healing of the joint surface. However subchondral bone plate advancement above its normal level was found at 16 weeks after surgery. Importantly, analysis of gene expression of cartilage surrounding the defect indicated a shift from anabolic to catabolic activity after osteochondral injury. IL1β and FGF2 were implicated with an altered FGFr1/FGFr3 balance in chondrocytes after injury, resulting in loss of articular cartilage homeostasis.
Despite its limited regenerative capacity, articular cartilage retains some regeneration ability depending on the animal species, age, and the size of the defect33. The critical size of osteochondral defects in rabbit, dog, and sheep is 3.0 mm, 4.0 mm, and 7.0 mm diameter, respectively15, 25. In rats, the critical size of an osteochondral defect had not been defined, however osteochondral defects smaller than 0.7 mm in diameter were reported to spontaneously regenerate with hyaline-like cartilage34, 35. On the other hand, several experiments have been published showing that osteochondral defects larger than 1.5 mm in diameter do not heal spontaneously in rats36, 37, 38. Therefore, our results extend previous observations and define the new threshold of critical-size defect of 1.4 mm in diameter.
The osteochondral model used in this study has the advantage of allowing the investigation of subchondral bone plate advancement and potential treatment failure in a shorter period when compared to other animals16, 39. In this model, the subchondral bone plate advanced above its normal level at 16 weeks after surgery. In rabbits, it can take up to 1 year for the subchondral bone plate to advance above its normal level after the natural healing of osteochondral defects17. Therefore, rats are a suitable small size animal model to investigate subchondral bone migration after osteochondral damage. This is believed to be of potential clinical relevance, as repair failure with subchondral bone overgrowth is observed in 62% of patients after microfracture40.
Herein, we showed that osteochondral defects affected the anabolic and catabolic activity of native cartilage surrounding the defect. This finding supports the concept that osteochondral defects may not only be restricted to a focal defect but also affect the whole joint. Also, these results may help to explain why one-third of the joints treated with a micro fracture procedure for cartilage defects show early radiographic signs of OA after 5 years18. In this study, Col2 and ACAN expression levels decreased in the articular cartilage surrounding the defect at 4 weeks after injury. This result corroborates other reports showing that anabolic gene expression in non OA lesioned cartilage is chronologically reduced in early OA knees21, 41. On the other hand, Prg4 and FGF2 expression levels increased in native cartilage surrounding the defect at 4 weeks after injury. Increased lubricin biosynthesis was reported to be an early transient response of the superficial cartilage to compression and excessive mechanical loading42, 43. Furthermore, increased IL1β expression was found in both the fibrocartilage filling the defect and cartilage surrounding the defect, confirming previous observations of injured or inflamed knees44. Overall, the gene expression shift observed in chondrocytes surrounding osteochondral defects is similar to early OA or injured knees.
Our current investigation suggests that cartilage homeostasis is partially regulated by inflammatory cytokines and FGF2. The limited capacity of articular cartilage to recover from inflammation has been described as a risk factor that often leads to cartilage degeneration22. Moreover, it has been shown that a delicate balance between FGF ligands and FGF receptors in articular cartilage is essential to control chondrocyte homeostasis, especially to maintain a proper balance between catabolic and anabolic activity of chondrocytes23. Our in vitro experiments showed a decreased expression of ACAN and Col2 genes in chondrocytes exposed to FGF2 and IL1β, which represented a similar trend of that observed in chondrocytes surrounding the osteochondral defect in vivo. These results suggest that in vitro culture of chondrocytes in the presence FGF2 and IL1β in vitro mimics some of the influence of the defect on the surrounding cartilage in vivo. FGF2 has been shown to exhibit both catabolic effects and suppression of ECM synthesis through FGFr1 and anabolic effects through FGFr3 in articular chondrocytes45. In our study, the FGFr1/FGFr3 ratio increased in IL1β supplemented groups in vitro. Therefore, the otherwise anabolic effect of FGF2 changed to catabolic in the presence of IL1β, as indicated by the decrease in ACAN and Col2. Moreover, FGF2 is also involved in the control of proliferation via p21 and IRF1 and apoptosis via Akt and E2f146. In our system, regulation of some of the FGF2 downstream targets, such as p21, IRF1, and Akt indicated that FGF2 induced a blockade in cell proliferation while increasing apoptosis. These results were in line with the low proliferation and presence of many detached cells in the in vitro culture conditions supplemented with IL1β + FGF2. Overall, these results suggest that IL1β and FGF2 change the FGFr1/FGFr3 balance in articular chondrocytes, leading to loss of homeostasis and a phenotypic shift that mimics early signs of OA.
Author contributions
HK conceived the study and performed all experiments and participated in its design.
MLF participated in its design and performed analysis.
Dr. Luyten FP had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors read and approved the final manuscript.
Conflict of interest
There is no conflict of interest.
Acknowledgements
This work is part of Prometheus, the Katholieke Universiteit Leuven R&D division for skeletal tissue engineering (www.kuleuven.be/prometheus). The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007–2013)/ERC grant agreement 294191.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.joca.2017.05.006.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
List of antibodies.
List of primers.
Supplementary Fig. 1.
(A): Representative macroscopic view of a knee used to harvest tissues for RNA extraction. Repair tissue was harvested from the original defect area (within solid black line). The articular cartilage surrounding the defect was harvested from the area adjacent to the defect on the patellofemoral groove (within black dotted line). (B): Representative histological findings after harvesting tissues for RNA extraction. Repair tissue in the defect was harvested from the original defect area (within solid black line). Articular cartilage surrounding the defect was harvested from the joint surface adjacent to the defect and above subchondral bone (within black dotted line). (Bar = 1 mm).
References
- 1.Heir S., Nerhus T.K., Rotterud J.H., Loken S., Ekeland A., Engebretsen L. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231–237. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 2.Kim S., Bosque J., Meehan J.P., Jamali A., Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Jt Surg Am. 2011;93:994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 3.Widuchowski W., Widuchowski J., Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 5.Devitt B.M., Bell S.W., Webster K.E., Feller J.A., Whitebread T.S. Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee. 2016 doi: 10.1016/j.knee.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Bentley G., Biant L.C., Vijayan S., Macmull S., Skinner J.A., Carrington R.W. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Jt Surg Br. 2012;94:504–509. doi: 10.1302/0301-620X.94B4.27495. [DOI] [PubMed] [Google Scholar]
- 7.Saris D.B., Vanlauwe J., Victor J., Almqvist K.F., Verdonk R., Bellemans J. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10s–19s. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 8.Van Assche D., Staes F., Van Caspel D., Vanlauwe J., Bellemans J., Saris D.B. Autologous chondrocyte implantation versus microfracture for knee cartilage injury: a prospective randomized trial, with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:486–495. doi: 10.1007/s00167-009-0955-1. [DOI] [PubMed] [Google Scholar]
- 9.Vanlauwe J., Saris D.B., Victor J., Almqvist K.F., Bellemans J., Luyten F.P. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39:2566–2574. doi: 10.1177/0363546511422220. [DOI] [PubMed] [Google Scholar]
- 10.Falah M., Nierenberg G., Soudry M., Hayden M., Volpin G. Treatment of articular cartilage lesions of the knee. Int Orthop. 2010;34:621–630. doi: 10.1007/s00264-010-0959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunziker E.B., Lippuner K., Keel M.J., Shintani N. An educational review of cartilage repair: precepts & practice–myths & misconceptions–progress & prospects. Osteoarthritis and Cartilage. 2015;23:334–350. doi: 10.1016/j.joca.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Gregory M.H., Capito N., Kuroki K., Stoker A.M., Cook J.L., Sherman S.L. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012 doi: 10.1155/2012/764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu C.R., Szczodry M., Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16:105–115. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahern B.J., Parvizi J., Boston R., Schaer T.P. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis and Cartilage. 2009;17:705–713. doi: 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Cook J.L., Hung C.T., Kuroki K., Stoker A.M., Cook C.R., Pfeiffer F.M. Animal models of cartilage repair. Bone Jt Res. 2014;3:89–94. doi: 10.1302/2046-3758.34.2000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orth P., Madry H. Advancement of the subchondral bone plate in translational models of osteochondral repair: implications for tissue engineering approaches. Tissue Eng Part B Rev. 2015;21:504–520. doi: 10.1089/ten.TEB.2015.0122. [DOI] [PubMed] [Google Scholar]
- 17.Orth P., Cucchiarini M., Kaul G., Ong M.F., Graber S., Kohn D.M. Temporal and spatial migration pattern of the subchondral bone plate in a rabbit osteochondral defect model. Osteoarthritis and Cartilage. 2012;20:1161–1169. doi: 10.1016/j.joca.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Knutsen G., Drogset J.O., Engebretsen L., Grontvedt T., Isaksen V., Ludvigsen T.C. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Jt Surg Am. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 19.Schinhan M., Gruber M., Vavken P., Dorotka R., Samouh L., Chiari C. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J Orthop Res. 2012;30:214–220. doi: 10.1002/jor.21521. [DOI] [PubMed] [Google Scholar]
- 20.Cicuttini F., Ding C., Wluka A., Davis S., Ebeling P.R., Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52:2033–2039. doi: 10.1002/art.21148. [DOI] [PubMed] [Google Scholar]
- 21.Chambers M.G., Bayliss M.T., Mason R.M. Chondrocyte cytokine and growth factor expression in murine osteoarthritis. Osteoarthritis and Cartilage. 1997;5:301–308. doi: 10.1016/s1063-4584(97)80034-9. [DOI] [PubMed] [Google Scholar]
- 22.Utomo L., Bastiaansen-Jenniskens Y.M., Verhaar J.A., van Osch G.J. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthritis Cartilage. 2016;24:2162–2170. doi: 10.1016/j.joca.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Ellman M.B., Yan D., Ahmadinia K., Chen D., An H.S., Im H.J. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem. 2013;114:735–742. doi: 10.1002/jcb.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasson S.S., Blanchet T.J., Morris E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Lietman S.A., Miyamoto S., Brown P.R., Inoue N., Reddi A.H. The temporal sequence of spontaneous repair of osteochondral defects in the knees of rabbits is dependent on the geometry of the defect. J Bone Jt Surg Br. 2002;84:600–606. doi: 10.1302/0301-620x.84b4.11631. [DOI] [PubMed] [Google Scholar]
- 26.Goebel L., Orth P., Muller A., Zurakowski D., Bucker A., Cucchiarini M. Experimental scoring systems for macroscopic articular cartilage repair correlate with the MOCART score assessed by a high-field MRI at 9.4 T–comparative evaluation of five macroscopic scoring systems in a large animal cartilage defect model. Osteoarthritis and Cartilage. 2012;20:1046–1055. doi: 10.1016/j.joca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Katagiri H., Muneta T., Tsuji K., Horie M., Koga H., Ozeki N. Transplantation of aggregates of synovial mesenchymal stem cells regenerates meniscus more effectively in a rat massive meniscal defect. Biochem Biophys Res Commun. 2013;435:603–609. doi: 10.1016/j.bbrc.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Bolander J., Chai Y.C., Geris L., Schrooten J., Lambrechts D., Roberts S.J. Early BMP, Wnt and Ca(2+)/PKC pathway activation predicts the bone forming capacity of periosteal cells in combination with calcium phosphates. Biomaterials. 2016;86:106–118. doi: 10.1016/j.biomaterials.2016.01.059. [DOI] [PubMed] [Google Scholar]
- 29.Sellers R.S., Peluso D., Morris E.A. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Jt Surg Am. 1997;79:1452–1463. doi: 10.2106/00004623-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Orth P., Zurakowski D., Wincheringer D., Madry H. Reliability, reproducibility, and validation of five major histological scoring systems for experimental articular cartilage repair in the rabbit model. Tissue Eng Part C Methods. 2012;18:329–339. doi: 10.1089/ten.TEC.2011.0462. [DOI] [PubMed] [Google Scholar]
- 31.Mendes L.F., Tam W.L., Chai Y.C., Geris L., Luyten F.P., Roberts S.J. Combinatorial analysis of growth factors reveals the contribution of bone morphogenetic proteins to chondrogenic differentiation of human periosteal cells. Tissue Eng Part C Methods. 2016;22:473–486. doi: 10.1089/ten.TEC.2015.0436. [DOI] [PubMed] [Google Scholar]
- 32.Nosewicz T.L., Reilingh M.L., van Dijk C.N., Duda G.N., Schell H. Weightbearing ovine osteochondral defects heal with inadequate subchondral bone plate restoration: implications regarding osteochondral autograft harvesting. Knee Surg Sports Traumatol Arthrosc. 2012;20:1923–1930. doi: 10.1007/s00167-011-1831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng H., Martin J.A., Duwayri Y., Falcon G., Buckwalter J.A. Impact of aging on rat bone marrow-derived stem cell chondrogenesis. J Gerontol A Biol Sci Med Sci. 2007;62:136–148. doi: 10.1093/gerona/62.2.136. [DOI] [PubMed] [Google Scholar]
- 34.Anraku Y., Mizuta H., Sei A., Kudo S., Nakamura E., Senba K. The chondrogenic repair response of undifferentiated mesenchymal cells in rat full-thickness articular cartilage defects. Osteoarthritis and Cartilage. 2008;16:961–964. doi: 10.1016/j.joca.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Yanagisawa H., Hoshi K., Asawa Y., Ejiri S., Sato K., Ozawa H. Matrix remodeling and cytological changes during spontaneous cartilage repair. J Electron Microsc (Tokyo) 2012;61:237–248. doi: 10.1093/jmicro/dfs044. [DOI] [PubMed] [Google Scholar]
- 36.Xue D., Zheng Q., Zong C., Li Q., Li H., Qian S. Osteochondral repair using porous poly(lactide-co-glycolide)/nano-hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model. J Biomed Mater Res A. 2010;94:259–270. doi: 10.1002/jbm.a.32691. [DOI] [PubMed] [Google Scholar]
- 37.Nishimori M., Deie M., Kanaya A., Exham H., Adachi N., Ochi M. Repair of chronic osteochondral defects in the rat. A bone marrow-stimulating procedure enhanced by cultured allogenic bone marrow mesenchymal stromal cells. J Bone Jt Surg Br. 2006;88:1236–1244. doi: 10.1302/0301-620X.88B9.17810. [DOI] [PubMed] [Google Scholar]
- 38.Ko J.Y., Kim K.I., Park S., Im G.I. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials. 2014;35:3571–3581. doi: 10.1016/j.biomaterials.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Jackson D.W., Lalor P.A., Aberman H.M., Simon T.M. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Jt Surg Am. 2001;83-a:53–64. doi: 10.2106/00004623-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Silverwood V., Blagojevic-Bucknall M., Jinks C., Jordan J.L., Protheroe J., Jordan K.P. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis and Cartilage. 2015;23:507–515. doi: 10.1016/j.joca.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Aigner T., Vornehm S.I., Zeiler G., Dudhia J., von der Mark K., Bayliss M.T. Suppression of cartilage matrix gene expression in upper zone chondrocytes of osteoarthritic cartilage. Arthritis Rheum. 1997;40:562–569. doi: 10.1002/art.1780400323. [DOI] [PubMed] [Google Scholar]
- 42.Jones A.R., Chen S., Chai D.H., Stevens A.L., Gleghorn J.P., Bonassar L.J. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum. 2009;60:133–142. doi: 10.1002/art.24143. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa H., Kozhemyakina E., Hung H.H., Grodzinsky A.J., Lassar A.B. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev. 2014;28:127–139. doi: 10.1101/gad.231969.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jotanovic Z., Mihelic R., Sestan B., Dembic Z. Role of interleukin-1 inhibitors in osteoarthritis: an evidence-based review. Drugs Aging. 2012;29:343–358. doi: 10.2165/11599350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Vincent T.L. Fibroblast growth factor 2: good or bad guy in the joint? Arthritis Res Ther. 2011;13:127. doi: 10.1186/ar3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su N., Jin M., Chen L. Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res. 2014;2:14003. doi: 10.1038/boneres.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of antibodies.
List of primers.