Abstract
Blood-brain barrier (BBB) dysfunction might be an important component of many neurodegenerative disorders. In this study, we investigated its role in dementia using large clinical cohorts. The cerebrospinal fluid (CSF)/plasma albumin ratio (Qalb), an indicator of BBB (and blood-CSF barrier) permeability, was measured in a total of 1015 individuals. The ratio was increased in patients with Alzheimer's disease, dementia with Lewy bodies or Parkinson's disease dementia, subcortical vascular dementia, and frontotemporal dementia compared with controls. However, this measure was not changed during preclinical or prodromal Alzheimer's disease and was not associated with amyloid positron emission tomography or APOE genotype. The Qalb was increased in diabetes mellitus and correlated positively with CSF biomarkers of angiogenesis and endothelial dysfunction (vascular endothelial growth factor, intracellular adhesion molecule 1, and vascular cell adhesion molecule 1). In healthy elderly, high body mass index and waist-hip ratio predicted increased Qalb 20 years later. In summary, BBB permeability is increased in major dementia disorders but does not relate to amyloid pathology or APOE genotype. Instead, BBB impairment may be associated with diabetes and brain microvascular damage.
Keywords: Blood-brain barrier, Dementia, Amyloid, APOE ε4, Diabetes, Vascular pathology
1. Introduction
Cerebrovascular pathology is common in the spectrum of dementia disorders (Snyder et al., 2015). One of the manifestations of vascular disease in the brain is blood-brain barrier (BBB) dysfunction (Zlokovic, 2008). The BBB is a selective diffusion barrier at the level of the cerebral microvascular endothelium that maintains homeostasis in the central nervous system (CNS) by regulating ion balance, facilitating nutritional transport, and preventing influx of potentially neurotoxic molecules from the circulation (Chow and Gu, 2015). Thus, BBB failure may have detrimental effects on CNS function, and animal studies have indicated that BBB breakdown could lead to secondary neuronal injury and neurodegeneration (Bell et al., 2010, Winkler et al., 2014).
Accumulating evidence suggests that BBB function is compromised in neurodegenerative disorders. Studies investigating BBB permeability in clinical patient cohorts mainly use 3 experimental approaches: measurement of the cerebrospinal fluid (CSF)/blood albumin ratio, histologic assessment of the blood-derived proteins in the brain tissue, and brain imaging, for example, magnetic resonance imaging (MRI) or positron emission tomography (PET). Such investigations have convincingly shown BBB dysfunction in vascular dementia (VaD) (Skoog et al., 1998, Taheri et al., 2011, Wada, 1998, Wallin et al., 1990). A recent meta-analysis reported elevated CSF/serum albumin ratio is in Alzheimer's disease (AD); however the effect size was small (Olsson et al., 2016). Studies subgrouping AD patients have found that AD cases with evidence of concomitant cerebrovascular pathology have high CSF/serum albumin ratio as a sign of impaired BBB function (Blennow et al., 1990, Blennow et al., 1991). In agreement, imaging techniques have mostly detected slight BBB impairments in AD patients and only in conjunction with vascular pathology (van de Haar et al., 2015). Although evidence of microvascular lesions has been reported in other dementias, for example, dementia with Lewy bodies (DLB), Parkinson's disease with dementia (PDD), and frontotemporal dementia (FTD) (De Reuck et al., 2012, De Reuck et al., 2013), little data are available with respect to BBB function in these conditions (Janelidze et al., 2015, Llorens et al., 2015, Sjogren et al., 2004).
Multiple mechanisms have been suggested to underlie BBB dysfunction in dementia. Accumulation of amyloid β (Aβ) in the vascular wall may lead to endothelial cell damage and disrupt the BBB in AD (Burgmans et al., 2013, Erickson and Banks, 2013). Some studies have indicated that BBB breakdown may be linked to APOE ε4, a major genetic risk factor for nonfamilial AD (Bell et al., 2012, Halliday et al., 2013, Nishitsuji et al., 2011). We recently demonstrated that increased BBB permeability in PD and PDD is related to an abnormal angiogenic CSF profile (Janelidze et al., 2015). In dementia and other disorders linked to high risk of dementia (e.g., diabetes, cardiovascular disease), BBB impairment has also been attributed to adverse effects of oxidative stress and chronic inflammation on endothelial cell function (Di Marco et al., 2015, Raz et al., 2015). However, BBB dysfunction has so far been investigated in experimental models or in small patient cohorts and needs to be validated in larger clinical material.
In this study, we measured BBB permeability using the CSF/plasma albumin ratio (Qalb) in 2 different cohorts of in total 1015 individuals including cognitively healthy controls and patients with subjective cognitive decline (SCD), mild cognitive impairment (MCI), and with 5 major dementias types, AD, PDD, DLB, VaD, and FTD. We assessed if the disruption of BBB was associated with amyloid pathology and the APOE genotype. We also investigated possible risk factors for BBB dysfunction and analyzed CSF biomarkers of angiogenesis, endothelial damage, and neuroinflammation to determine if these factors are related to BBB breakdown in different dementias.
2. Materials and methods
The study was approved by the Regional Ethics Committee in Lund, Sweden, and the patients and controls gave their informed consent for research.
2.1. Study participants
2.1.1. Cohort 1
Seventy-five patients with AD, 34 patients with DLB/PDD, 28 patients with VaD, 41 patients with FTD, and 65 healthy controls were recruited at the Memory Clinic of Skåne University Hospital in Malmö, Sweden. We also included 96 individuals with a baseline diagnosis of MCI. After an average clinical follow-up period of 5.7 years (3.0–9.6), 35 of these patients had converted to AD (MCI-AD), whereas 61 of them remained cognitively stable (sMCI).
All patients with a clinical syndrome of dementia met the Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) (DSM-IIIR) criteria for dementia (American Psychiatric Association and Work Group to Revise DSM-III, 1987) combined with the NINCDS-ADRDA criteria for AD (McKhann et al., 1984), the NINDS-AIREN criteria for VaD (Roman et al., 1993), criteria of probable DLB according to the 2005 consensus criteria (Geser et al., 2005), or the 1998 consensus criteria for FTD (Neary et al., 1998). Patients with MCI at baseline had to fulfill the criteria advocated by Petersen (2004), including (1) memory complaint, preferably corroborated by an informant; (2) objective memory impairment adjusted for age and education, as judged by the physician; (3) preservation of general cognitive functioning, as determined by the clinician's judgment based on a structured interview with the patient and a Mini-Mental State Examination (MMSE) score ≥24; (4) 0 or minimal impairment of daily life activities; and (5) not fulfilling the DSM-III-R criteria of dementia (American Psychiatric Association and Work Group to Revise DSM-III, 1987). The healthy participants were not allowed to have any cognitive complaints or any significant neurological or psychiatric illness, and they needed to have a well-preserved general cognitive functioning. A careful clinical interview, together with an assessment of global function (MMSE), delayed recall (Alzheimer's Disease Assessment Scale Cognitive Subscale, item 3), attention (a quick test of cognitive speed), and visuospatial and executive function (cube-drawing test and clock test), was done to rule out mild cognitive impairment. All subjects were assessed by medical doctors with extensive experience in cognitive disorders. The characteristics of cohort 1 are given in Table 1.
Table 1.
Cohort 1: demographic data, clinical characteristics, and CSF levels of biomarkers
| Sample characteristics | Control (n = 65) | sMCI (n = 61) | MCI-AD (n = 35) | AD (n = 75) | DLB/PDD (n = 34) | VaD (n = 28) | FTD (n = 41) |
|---|---|---|---|---|---|---|---|
| Sex F/M | 42/23 | 34/27 | 23/12 | 51/24 | 13/21∗ | 13/15 | 21/20 |
| Age | 75 (6) | 69 (7)∗∗∗ | 75 (8) | 76 (7) | 72 (6) | 75 (8) | 72 (6)∗ |
| MMSE | 28.7 (1.7) | 28.2 (1.2) | 26.4 (1.7)∗∗∗ | 19.5 (3.3) ∗∗∗ | 21.4 (5.1)∗∗∗ | 21.5 (4.5)∗∗∗ | 22.8 (6.1)∗∗∗ |
| APOE 1or 2 ε4 alleles | 34% | 48% | 80%∗∗∗ | 65%∗∗∗ | 55%∗ | 21% | 28%a |
| BMI | 25.9 (3.7) | 25.1 (3.2) | 24.3 (4.2) | 23.7 (3.6) | 25.6 (3.4) | 26.5 (5.8) | 25.4 (4.1) |
| Qalb | 6.3 (2.9) | 6.3 (2.5) | 7.1 (3.3) | 7.6 (3.4)∗∗ | 8.4 (4.6)∗ | 8.7 (3.7)∗∗ | 8.6 (5.5)∗∗ |
| Diabetes, yes/no | 4/61 | 3/58 | 1/34 | 9/66 | 2/32 | 7/21 | 0/32 |
| ICAM-1, ng/mL | 2.0 (0.7) | 1.9 (0.7) | 2.1 (0.7) | 2.2 (0.7) | 2.0 (0.8) | 2.1 (0.7) | 2.0 (1.0) |
| VCAM-1, ng/mL | 5.4 (1.4) | 4.9 (1.5) | 5.4 (1.6) | 5.7 (1.6) | 5.3 (1.4) | 5.5 (1.5) | 4.9 (1.6) |
| VEGF, pg/mL | 58.0 (19.1) | 65.0 (32.0)∗ | 70.9 (21.8)∗∗ | 73.2 (27.7)∗∗ | 67.0 (29.0) | 80.8 (33.5)∗∗ | 74.6 (31.6)∗∗ |
| sVEGFR-1, pg/mL | 149.2 (55.0) | 111.4 (39.7)∗∗∗ | 128.9 (44.7)∗ | 130.3 (41.1)∗∗ | 103.3 (37.4)∗∗∗ | 100.3 (30.3)∗∗∗ | 118.0 (38.7)∗∗ |
| VEGF/sVEGFR-1 | 0.4 (0.2) | 0.6 (0.3)∗∗∗ | 0.6 (0.2)∗∗∗ | 0.6 (0.2)∗∗∗ | 0.7 (0.4)∗∗∗ | 0.9 (0.4)∗∗∗ | 0.7 (0.3)∗∗∗ |
| Aβ42, pg/mL | 675.2 (289.8) | 478.9 (195.0)∗∗∗ | 314.5 (78.9)∗∗∗ | 259.7 (105.0)∗∗∗ | 349.6 (172.7)∗∗∗ | 407.4 (187.0)∗∗∗ | 682.9 (290.5) |
| Aβ40, pg/mL | 5241 (1487) | 3786 (1360)∗∗∗ | 4219 (1327)∗∗∗ | 3899 (1376)∗∗∗ | 3240 (1200)∗∗∗ | 3218 (1345)∗∗∗ | 4530 (1536)∗ |
Data are shown as mean (standard deviation) unless otherwise specified. Demographic factors and clinical characteristics were compared using 1-way analysis of variance and chi-square tests. CSF biomarkers and the Qalb were analyzed with univariate general linear models controlling for age and gender, compared with controls ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Key: Aβ, amyloid β; AD, Alzheimer's disease; BMI, body mass index; CSF, cerebrospinal fluid; DLB/PDD, dementia with Lewy bodies or Parkinson's disease with dementia; F, female; FTD, frontotemporal dementia; ICAM-1, intercellular adhesion molecule 1; M, male; MCI-AD, MCI that progressed to AD; MMSE, Mini-Mental State Examination; Qalb, CSF/plasma albumin ratio; sMCI, stable mild cognitive impairment; sVEGFR-1, soluble VEGF receptor 1; VaD, vascular dementia; VCAM-1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor.
APOE data were only available from 18 FTD patients.
2.1.2. Cohort 2
The study population stemmed from the prospective and longitudinal Swedish BioFINDER study (further information available at www.biofinder.se). Cohort 2 included 292 cognitively normal elderly participants recruited from the population-based Malmö Diet Cancer Study (Berglund et al., 1993), and 384 patients with mild cognitive complaints enrolled consecutively at 3 memory outpatient clinics in Sweden. Cognitively normal elderly were eligible for inclusion if they (1) were aged ≥60 years old; (2) scored 28–30 points on the MMSE (Folstein et al., 1975) at the screening visit; (3) had absence of cognitive symptoms as evaluated by a physician; (4) were fluent in Swedish; and (5) did not fulfill the criteria of MCI or any dementia. The exclusion criteria were (1) presence of significant neurologic or psychiatric disease (e.g., stroke, Parkinson's disease, multiple sclerosis, major depression); (2) significant systemic illness making it difficult to participate; (3) refusing lumbar puncture (LP); and (4) significant alcohol abuse. Data were collected between 2009 and 2014 in accordance with a standardized protocol. The patients with mild cognitive complaints were referred for assessment of their cognitive complaints and were included between 2010 and 2014. They were thoroughly assessed by physicians with special interest in dementia disorders. The inclusion criteria were (1) cognitive symptoms; (2) not fulfilling the criteria for dementia; (3) MMSE score of 24–30 points; (4) age 60–80 years; and (5) fluent in Swedish. The exclusion criteria were (1) cognitive impairment that without doubt could be explained by another condition (other than prodromal dementia); (2) severe somatic disease; and (3) refusing LP or neuropsychological investigation. These criteria resulted in a clinically relevant population where 45% were classified as SCD, 42% as amnestic MCI, and 13% as nonamnestic MCI. The classification was based on a neuropsychological battery assessing the cognitive domains of verbal ability, visuospatial construction, episodic memory, and executive functions and the clinical assessment of a senior neuropsychologist. The characteristics of cohort 2 are given in Table 2.
Table 2.
Cohort 2: demographic data, clinical characteristics and CSF levels of biomarkers
| Sample characteristics | Controls (n = 292) | SCD (n = 171) | MCI (n = 213) |
|---|---|---|---|
| Gender, F/M | 176/116 | 94/77 | 94/119∗∗∗ |
| Age, y | 73 (5) | 70 (6)∗∗∗ | 71 (6)∗∗∗ |
| MMSE | 29.1 (0.9) | 28.4 (1.4)∗∗∗ | 27.0 (1.9)∗∗∗ |
| APOE 1 or 2 ε4 alleles | 29% | 38%∗ | 49%∗∗∗ |
| Qalb | 5.9 (2.2) | 6.0 (2.4) | 6.5 (4.0) |
| Composite SUVRa | 1.3 (0.3) | 1.4 (0.4)∗∗ | 1.7 (0.5)∗∗∗ |
| BMI | 26.5 (4.3) | 25.1 (3.6) | 25.4 (4.2) |
| Homocysteine, μmol/L | 13.5 ± 4.1 | 12.6 ± 3.9 | 13.5 ± 4.1 |
| Diabetes, yes/no | 26/266 | 17/152 | 20/187 |
| Hyperlipidemia, yes/no | 124/168 | 22/147 | 19/188 |
| Aβ42, pg/mL | 561.9 (199.3) | 589.6 (254.6) | 470.5 (233.0)∗∗∗ |
| Aβ40, pg/mL | 4766 (1753) | 4925 (1751) | 4760 (1873) |
Data are shown as mean (standard deviation) unless otherwise specified. Demographic factors and clinical characteristics were compared using 1-way analysis of variance and chi-square tests. Qalb was analyzed with univariate general linear models controlling for age and gender, compared with controls ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Key: Aβ, amyloid β; BMI, body mass index; F, female; M, male; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; Qalb, cerebrospinal fluid/plasma albumin ratio; SCD, subjective cognitive decline; SUVR, standardized uptake value ratio.
Normalized for the cerebellar cortex; amyloid positron emission tomography data were available from 342 subjects (129 cognitively normal elderly, 102 SCD patients, and 111 MCI patients).
In both cohorts, the diagnosis of hypertension, diabetes, hyperlipidemia, and ischemic heart disease made by a medical doctor was available from the medical records.
2.2. CSF sampling and biological assays
For all patients and controls, blood plasma and CSF samples were drawn at some point between 8 AM and 12 AM with the patients nonfasting. CSF was collected in polypropylene tubes and mixed gently to avoid gradient effects. All samples were centrifuged within 30 minutes at +4 °C at 2000 g for 10 minutes to remove cells and debris. Samples were stored in aliquots at −80 °C pending biochemical analysis. The procedure followed The Alzheimer's Association Flow Chart for LP and CSF sample processing (Blennow et al., 2010). CSF Aβ42 and Aβ40 were analyzed by Euroimmun immunoassay (EUROIMMUN AG, Lübeck, Germany).
CSF levels of vascular endothelial growth factor (VEGF), soluble VEGF receptor 1 (sVEGFR-1), intracellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) were measured using Growth Factor I and Vascular Injury II kits according to the manufacturer's protocol with some modifications (Meso Scale Discovery, Gaithersburg, MD, USA). Briefly, 10% bovine serum albumin was added to the blocking buffer for all the assays, and in Growth Factor I assays, samples were incubated overnight at +4 °C. Data were collected and analyzed using SECTOR Imager 6000 reader and Discovery Workbench Software (www.mesoscale.com). All samples were measured in duplicates, and the mean of the duplicated was used in the statistical analysis. Detection limits were 8.1 pg/mL for VEGF, 213.6 pg/mL for sVEGFR-1, 5.6 pg/mL for ICAM-1, and 257.5 pg/mL for VCAM-1. The coefficient of variation was <20% for all assays. The very few samples with coefficient of variation >20% did not affect the results and were therefore included in the statistical analysis.
Albumin levels in plasma and CSF were measured by immunoturbidimetry on a Roche Cobas Analyzer (Roche Diagnostics, Bromma, Sweden). The albumin ratio was calculated as CSF albumin (mg/L)/plasma albumin (g/L) and was used as a measure of BBB function.
2.3. [18F]flutemetamol PET in cohort 2
Cerebral Aβ deposition was visualized with the PET tracer [18F]flutemetamol (approved by the Food and Drug Administration and the European Medical Agency). PET/computed tomography (CT) scanning of the brain was conducted at 2 sites using the same type of scanner (Gemini, Philips Healthcare, Best, The Netherlands). Baseline sum images from 90 to 110 minutes post injection were analyzed using the software NeuroMarQ (provided by GE Healthcare, Cleveland, OH, USA). A volume of interest template was applied for the following 9 bilateral regions: prefrontal, parietal, lateral temporal, medial temporal, sensorimotor, occipital, anterior cingulate, posterior cingulate/precuneus, and a global neocortical composite region (Lundqvist et al., 2013). The standardized uptake value ratio (SUVR) was defined as the uptake in a volume of interest normalized for either the cerebellar cortex or pons uptake. Amyloid PET data were available from 342 subjects (129 cognitively normal elderly, 102 SCD patients, and 111 MCI patients).
2.4. CT and MRI
In cohort 1, 312 cases underwent CT (n = 266) and MRI (n = 46) for assessment of white matter changes (WMCs). Imaging was performed according to the clinical protocols including acquisition of fluid-attenuated inversion recovery images on a 1.5-T scanner (Siemens) for MRI or of noncontrast CT images using multiple CT scanners. WMCs were visually assessed according to the age-related WMC rating scale developed by Wahlund et al. (2001) for rating of both MRI and CT with high agreement between modalities. On CT images, WMC were rated in the left and right frontal and occipital-parietal lobes; temporal lesions were not included because this location is very rare for WMCs (Bronge, 2002). WMCs were graded from 0 to 3 points: 0 = no lesions or lesions <5 mm, 1 = presence of lesion ≥5 mm, 2 = lesions beginning to aggregate, and 3 = confluent lesions involving almost the entire region.
In cohort 2, 618 study participants (269 controls, 159 SCD, and 190 MCI) were examined using a single 3-T MR scanner (Trio, Siemens). Automated segmentation of white matter lesions was performed using the Lesion Segmentation Tool implemented in SPM8 (http://www.applied-statistics.de/lst.html); this generated a total white matter lesion volume (mL) for each individual.
2.5. Statistical analyses
SPSS (IBM, Armonk, NY, USA) was used for statistical analysis. Data for VEGF, sVEGFR-1, ICAM-1, VCAM-1, and albumin were skewed; therefore, all variables were ln transformed before analyses. In addition to CSF concentrations of angiogenesis biomarkers, we also used the VEGF/sVEGFR-1 ratio when investigating associations with clinical data. The rationale for this is that sVEGFR-1 has direct antagonistic effect on VEGF by sequestering the ligands from the membrane receptors (Ambati et al., 2006, Kendall and Thomas, 1993, Qi et al., 2013). Consequently, high VEGF/sVEGFR-1 ratios provide an index of the bioactive levels of VEGF.
In cohort 1, the confounding effects of age, gender, and body mass index (BMI) were tested with Pearson's correlation analysis and Student's t-tests. None of the measured analytes were associated with BMI. However, for most analytes, we found correlations with age and gender differences. Therefore, all subsequent statistical analyses were controlled for age and gender.
For group-wise comparisons, we used univariate general linear models. Linear regressions were used to investigate associations between 2 continuous variables. The study participants were categorized into groups with normal and pathologic PET status using the SUVR cutoff >1.42 when normalized for the cerebellar cortex uptake (Palmqvist et al., 2014). The SUVR cutoff >0.51 with pons as a reference region was derived using mixture modeling (Benaglia et al., 2009). We also categorized the study participants into groups with normal and pathologic CSF signature using the CSF Aβ42/Aβ40 ratio cutoff ≥0.1 (Janelidze et al., 2016). Associations between the Qalb, [18F]flutemetamol SUVR, and vascular risk factors were tested in the total sample with univariate and linear regression models controlling for age, gender, and diagnosis. Alpha level of significance was set at p < 0.05.
3. Results
Demographic and clinical characteristics of cohorts 1 and 2 and raw untransformed concentrations of CSF analytes are shown in Tables 1 and 2.
3.1. The Qalb in dementias
3.1.1. Cohort 1 (dementia)
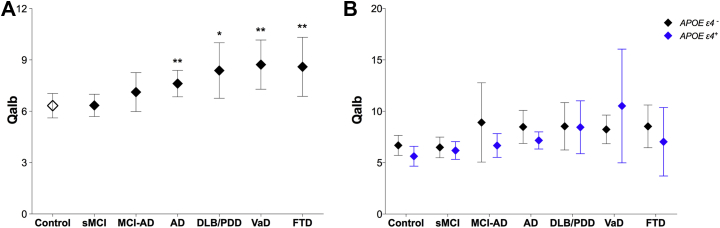
We found that the Qalb differed significantly between the diagnostic groups. Specifically, the ratio was higher in AD (p = 0.007), DLB/PDD (p = 0.037), VaD (p = 0.004), and FTD (p = 0.004) patients compared with controls (Fig. 1A and Table 1). However, there were no differences between patients with stable MCI (p = 0.690) or in patients with MCI who subsequently progressed to AD (p = 0.186) compared with controls. These results were similar in regression models additionally adjusting for the confounding effects of WML.
Fig. 1.
The cerebrospinal fluid/plasma albumin ratio (Qalb) in different diagnostic groups and APOE genotypes in cohort 1. (A) The Qalb in cognitively healthy controls and patients with stable mild cognitive impairment (sMCI), MCI that progressed to Alzheimer's disease (MCI-AD), AD, dementia with Lewy bodies or Parkinson's disease with dementia (DLB/PDD), vascular dementia (VaD), and frontotemporal dementia (FTD). (B) The Qalb in different diagnostic groups stratified according to APOE genotype (APOE ε4 carriers vs. noncarriers). Data are presented as mean ±95% confidence interval; p-values are from univariate general linear models controlling for age and gender: ∗p < 0.05 and ∗∗p < 0.01, compared with controls.
3.2. The Qalb and Aβ pathology
3.2.1. Cohort 2 (biofinder)
Next we sought to determine if the Qalb was altered in prodromal and preclinical stages of AD in a large cohort of cognitively healthy individuals and patients with SCD or MCI (cohort 2). To this end, we compared diagnostic subgroups with pathologic CSF signature (control-P, SCD-P, and MCI-P; CSF Aβ42/Aβ40 ratio <0.1) with control subjects showing normal CSF status (control-N; CSF Aβ42/Aβ40 ratio ≥0.1). In addition, we investigated if changes in the Qalb were associated with cortical amyloid deposition measured using [18F]flutemetamol PET. There were no differences in the Qalb between any of the diagnostic subgroups (control-N, control-P, SCD-P, and MCI-P; all p > 0.172, Table 3) and no significant correlations between the Qalb and composite [18F]flutemetamol SUVR (β = −0.111, p = 0.052 and β = −0.097, p = 0.081 with the cerebellar cortex or pons as reference regions, respectively). Moreover, there were no differences in the ratio between study subjects with normal versus pathologic amyloid PET (p = 0.315 and p = 0.385 with the cerebellar cortex or pons as reference regions, respectively).
Table 3.
The Qalb in individuals with normal (CSF Aβ42/Aβ40 ratio ≥0.1) and pathologic (CSF Aβ42/Aβ40 ratio <0.1) CSF signature
| Sample characteristics | Controls (N), n = 214 | Control (P), n = 77 | SCD (P), n = 57 | MCI (P), n = 121 |
|---|---|---|---|---|
| Gender F/M | 124/90 | 52/25 | 26/31 | 60/61 |
| Age, y | 72 (5) | 74 (5)∗∗ | 71 (5) | 72 (5) |
| MMSE | 29.2 (0.9) | 29.0 (0.9) | 28.1 (1.5)∗∗∗ | 26.8 (1.8)∗∗∗ |
| APOE 1 or 2 ε4 alleles | 20% | 53%∗∗∗ | 67%∗∗∗ | 68%∗∗∗ |
| Qalb | 6.0 (2.1) | 5.8 (2.4) | 5.7 (2.2) | 6.6 (4.8) |
Data are shown as mean (standard deviation) unless otherwise specified. Demographic factors and clinical characteristics were compared using 1-way analysis of variance and chi-square tests. Qalb was analyzed with univariate general linear models controlling for age and gender, compared with controls ∗∗p < 0.01, and ∗∗∗p < 0.001.
Key: Aβ, amyloid β; CSF, cerebrospinal fluid; F, female; M, male; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; N, normal CSF signature; P, pathologic CSF signature; Qalb, CSF/plasma albumin ratio; SCD, subjective cognitive decline.
3.3. The Qalb and APOE genotype
Previous studies have indicated that APOE might play a role in maintaining the integrity of the BBB (Zhao et al., 2015). To explore that association, we compared the Qalb between different APOE genotypes in cohorts 1 and 2.
3.3.1. Cohort 1 (dementia)
The Qalb did not differ between the APOE ε4 carriers and noncarriers in any of the diagnostic groups in cohort 1 (all p > 0.081, Fig. 1B). We did not find significant differences in the Qalb between APOE ε4 carriers and noncarriers (p = 0.062) or between carriers of 1 ε4 allele, 2 ε4 alleles, and noncarriers (all p > 0.087) in the total sample using univariate regression models adjusting for age, gender, and diagnosis.
3.3.2. Cohort 2 (biofinder)
Confirming our findings in cohort 1, there were no differences in the Qalb between APOE ε4 carrier and noncarriers in control (p = 0.720), SCD (p = 0.634), or MCI (p = 0.874) groups in cohort 2. Furthermore, there were no differences comparing carriers of 1 ε4 allele, 2 ε4 alleles, and noncarriers (all p > 0.228). The Qalb did not differ comparing younger (≤65 years) and older (≥66 years) APOE ε4 carrier and noncarriers in SCD and MCI groups (all p > 0.380).
3.4. The Qalb and CSF biomarkers of angiogenesis or endothelial damage
Altered Qalb may be related to abnormal angiogenesis and endothelial cell function (Janelidze et al., 2015, Zlokovic, 2008). Therefore, we investigated associations between the ratio and CSF biomarkers of angiogenesis and endothelial dysfunction.
3.4.1. Cohort 1 (dementia)
High Qalb was associated with increased CSF levels of ICAM-1 and VCAM-1 (markers of endothelial dysfunction) in all diagnostic groups (Table 4). Moreover, the Qalb positively correlated with CSF levels of VEGF in control, sMCI, MCI-AD, AD, DLB/PDD, and FTD groups and with the CSF VEGF/sVEGFR-1 ratio in sMCI, AD, DLB/PDD, and FTD groups. The associations remained significant after additionally adjusting for WMLs.
Table 4.
Cohort 1: associations between the Qalb and CSF biomarkers of angiogenesis or endothelial damage
| CSF biomarkers | Control | sMCI | MCI-AD | AD | DLB/PDD | VaD | FTD |
|---|---|---|---|---|---|---|---|
| ICAM-1 | β = 0.637∗∗∗ | β = 0.733∗∗∗ | β = 0.776∗∗∗ | β = 0.650∗∗∗ | β = 0.756∗∗∗ | β = 0.729∗∗∗ | β = 0.833∗∗∗ |
| VCAM-1 | β = 0.411∗∗ | β = 0.470∗∗∗ | β = 0.620∗∗∗ | β = 0.512∗∗∗ | β = 0.431∗ | β = 0.627∗∗ | β = 0.679∗∗∗ |
| VEGF | β = 0.402∗∗ | β = 0.482∗∗∗ | β = 0.685∗∗∗ | β = 0.458*∗∗ | β = 0.719∗∗∗ | β = 0.240 | β = 0.346∗ |
| sVEGFR-1 | β = 0.240∗ | β = −0.021 | β = 0.365∗ | β = 0.092 | β = −0.054 | β = 0.220 | β = −0.075 |
| VEGF/sVEGFR-1 | β = 0.060 | β = 0.358∗∗ | β = 0.235 | β = 0.370∗∗ | β = 0.518∗∗ | β = 0.013 | β = 0.305∗ |
p-Values are derived from linear regression controlling for age and gender; significant results are shown in bold; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Key: AD, Alzheimer's disease; CSF, cerebrospinal fluid; DLB/PDD, dementia with Lewy bodies or Parkinson's disease with dementia; FTD, frontotemporal dementia; ICAM-1, intercellular adhesion molecule 1; MCI-AD, MCI that progressed to AD; Qalb, CSF/plasma albumin ratio; sMCI, stable mild cognitive impairment; sVEGFR-1, soluble VEGF receptor 1; VaD, vascular dementia; VCAM-1, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; β, standardized coefficient.
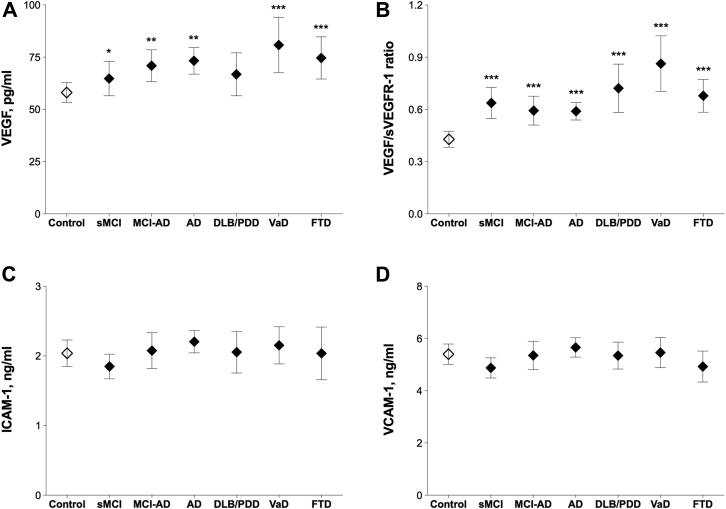
Comparing with control subjects, we found that VEGF levels were increased in sMCI (p = 0.021), MCI-AD (p = 0.008), AD (p = 0.002), VaD (p = 0.001), and FTD (p = 0.001) patients, whereas the VEGF/sVEGFR-1 ratio was increased across all the groups (all p < 0.001, Fig. 2A and B and Table 1). There were no changes in CSF levels of ICAM-1 or VCAM-1 (Fig. 2C and D and Table 1).
Fig. 2.
Cerebrospinal fluid (CSF) biomarkers of angiogenesis or endothelial damage in cohort 1. CSF vascular endothelial growth factor (VEGF) (A), the VEGF/soluble VEGF receptor 1 (sVEGFR-1) ratio (B), intercellular adhesion molecule 1 (ICAM-1) (C), and vascular cell adhesion molecule 1 (VCAM-1) (D) were measured in cognitively healthy controls and patients with stable mild cognitive impairment (sMCI), MCI that progressed to Alzheimer's disease (MCI-AD), AD, dementia with Lewy bodies or Parkinson's disease with dementia (DLB/PDD), vascular dementia (VaD), and frontotemporal dementia (FTD). Data are presented as mean ±95% confidence interval; p values are from univariate general linear models controlling for age and gender: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 compared with controls.
3.5. Risk factors for abnormal Qalb
Finally, we examined associations between the Qalb and potential vascular risk factors in dementia cohorts 1 and 2.
3.5.1. Cohort 1 (dementia)
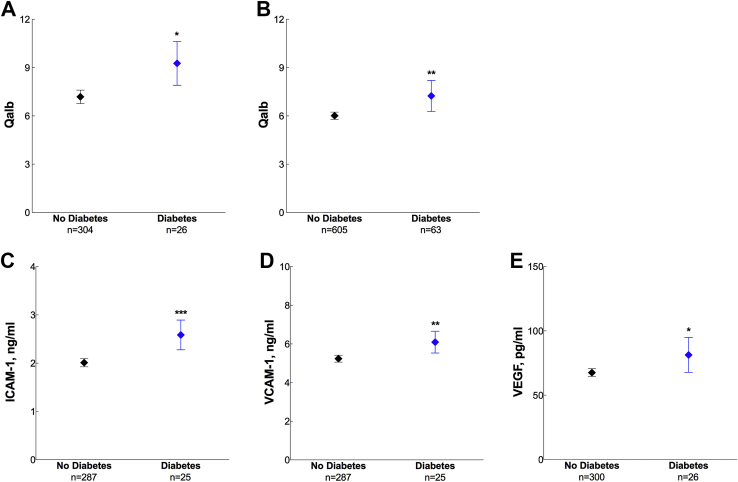
In cohort 1, the Qalb was increased in individuals with diabetes (diagnosed with diabetes or taking antidiabetic medications) compared with those without diabetes (p = 0.015) (Fig. 3A). Furthermore, diabetes was associated with high CSF levels of ICAM-1 (p < 0.001), VCAM-1 (p = 0.007), and VEGF (p = 0.024) (Fig. 3C–E). These results remained significant after additionally adjusting for WMLs. We did not find any associations with hypertension (p = 0.633) or ischemic heart disease (p = 0.801).
Fig. 3.
The CSF/plasma albumin ratio (Qalb) and cerebrospinal fluid (CSF) biomarkers of angiogenesis or endothelial damage in diabetes. The Qalb in patients with and without diabetes in cohort 1 (A) and cohort 2 (B). CSF levels of intercellular adhesion molecule 1 (ICAM-1) (C), vascular cell adhesion molecule 1 (VCAM-1) (D), and vascular endothelial growth factor (VEGF) (E) in patients with and without diabetes in cohort 1. Data are presented as mean ±95% confidence interval; p values are from univariate general linear models controlling for age, gender, and diagnosis: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3.5.2. Cohort 2 (biofinder)
Similar to findings in cohort 1, the Qalb was increased in individuals with diabetes in cohort 2 (p = 0.041) (Fig. 3B). Whereas there was no association with ischemic heart disease (p = 0.281), plasma homocysteine (p = 0.608), and hyperlipidemia (p = 0.414), the ratio was higher in patients with hypertension (p = 0.012). In this cohort, linear regression models revealed no effects of WMLs on the Qalb (β = 0.018, p = 0.692); therefore, we did not include WML variable in the statistical analysis. The cognitively healthy elderly in cohort 2 were recruited from the Malmö Diet Cancer Study where the first assessments of the study participants were conducted 20.1 ± 1.5 (mean ± standard deviation [SD]) years previous the present study. In this group, linear regression analysis revealed that high BMI (24.8 ± 3.5, mean ± SD) and the waist-hip ratio (0.8 ± 0.09, mean ± SD) in the middle age (53.9 ± 4.7, mean ± SD) predicted increase in the Qalb 20 years later (BMI, β = 0.144, p = 0.013; waist-hip ratio, β = 0.304, p = 0.003).
4. Discussion
In the present study, we demonstrate that the Qalb is increased in patients with AD, DLB/PDD VaD, and FTD but not during preclinical or prodromal AD stages. In 2 cohorts comprising a total of 1015 individuals, we did not find any associations between the Qalb and APOE genotype. However, in both cohorts, the ratio was associated with coexisting diabetes mellitus. Further, the ratio positively correlated with CSF biomarkers of angiogenesis and endothelial dysfunction, including VEGF, the VEGF/sVEGFR-1 ratio, ICAM-1, and VCAM-1. VEGF or the VEGF/sVEGFR-1 ratio was increased in all dementias and in MCI, whereas patients with diabetes showed increased CSF levels of VEGF, ICAM-1, and VCAM-1. Last, in the longitudinal cohort, a high Qalb was related to increased BMI and a higher waist-hip ratio at the middle age.
Although an elevated Qalb is a consistent finding in VaD (Skoog et al., 1998, Taheri et al., 2011, Wada, 1998, Wallin et al., 1990), the evidence is not clear-cut when it comes to AD (Erickson and Banks, 2013). Nonstringent clinical categorization of AD and VaD (especially in earlier investigations), bias related to sample size and effects of age on the ratio have been suggested to account for the conflicting results (Blennow et al., 1990, Blennow et al., 1991, Erickson and Banks, 2013). Nevertheless, a recent meta-analysis came out positive with a small but statistically significant 1.1-fold (95% confidence interval 1.01–1.20) increase of Qalb in AD patients compared with control individuals (Olsson et al., 2016). In the present study, we compared the Qalb in a relatively large and well-characterized cohort of cognitively healthy controls and patients with major dementia disorders using statistical methods adjusting for confounding effect of age and gender. We found increased Qalb not only in VaD but also in AD, DLB/PDD, and FTD suggesting that dysfunction of the BBB is common across different dementia disorders (Janelidze et al., 2015, Sjogren et al., 2004). Compromised BBB has recently been demonstrated in the hippocampus of patients with MCI (Montagne et al., 2015). In contrast, we did not detect any differences in the Qalb between control and MCI groups. Furthermore, the ratio was not altered in cognitively healthy individuals who showed a pathologic CSF AD biomarker profile and did not correlate with cortical amyloid deposition. Altogether these results speak against a significant causative link between amyloid pathology and BBB dysfunction at least during the early disease stages. In fact, the BBB appears intact in the murine models of AD displaying significant amyloid pathology (Bien-Ly et al., 2015).
Some evidence from preclinical models and from human disease implicated ApoE4 in BBB dysfunction in AD. In transgenic mice, production of human ApoE4 by astrocytes has been shown to induce BBB leakage and neurodegeneration (Bell et al., 2012). The Qalb was found to be higher in cognitively healthy individuals carrying APOE ε4 than in noncarriers and to increase with age in APOE ε4 carriers only (Halliday et al., 2013). However, analysis of the Qalb in the considerably larger cohort in the present study showed no differences between APOE ε4 carriers and noncarriers or between younger and older APOE ε4 carriers. In agreement with our findings, several studies reported no associations between APOE ε4 and BBB breakdown (Bien-Ly et al., 2015, Bowman et al., 2007, Karch et al., 2013), thus suggesting that APOE genotype is unlikely to play a role in BBB dysfunction.
Our previous work indicated that in PD high Qalb was associated with increased CSF levels of angiogenic factors including VEGF (Janelidze et al., 2015). In addition to its central role in physiological and pathologic angiogenesis, VEGF is known to regulate vascular permeability. In rats, administration of VEGF induces leakage of the BBB (Zhang et al., 2000). Increased production of VEGF has also been shown to cause BBB breakdown in experimental models of MS and cerebral ischemia (Argaw et al., 2012, Suzuki et al., 2015). In the present study, we found elevated VEGF or the VEGF/sVEGFR-1 ratio (an index of the bioactive levels of VEGF) in the CSF of patients with different forms of dementia and positive correlation between VEGF and the Qalb. Interestingly, CSF levels of VEGF were increased in MCI and MCI-AD patients who showed no difference in the Qalb compared with control individuals. These findings suggest that abnormal VEGF production may precede BBB breakdown in dementia and provide further support for the link between aberrant VEGF signaling and BBB dysfunction. We also found an association between elevated CSF levels of VEGF and coexisting diabetes. Notably, VEGF pathways have been recently shown to contribute to impaired BBB and functional recovery in experimental model of comorbid diabetes and stroke (Reeson et al., 2015).
In accordance with the earlier studies (Hawkins et al., 2007, Starr et al., 2003), we observed associations between disrupted BBB and diabetes in 2 different cohorts. Obesity is a risk factor for type-2 diabetes and constitutes an important component of the metabolic syndrome, and we also show that increased markers for obesity (BMI and the waist-hip ratio) at the middle age predicts high Qalb 20 years later. Endothelial and vascular pathology induced by chronic inflammation and oxidative stress are major complications of diabetes (Tousoulis et al., 2013). Changes in endothelial cells include upregulation of adhesion molecules ICAM-1 and VCAM-1, which are considered biomarkers of peripheral vascular dysfunction in diabetes (Meigs et al., 2004, Tousoulis et al., 2013). Here, we demonstrate for the first time increased CSF levels of ICAM-1 and VCAM-1 in diabetic patients and positive correlations between the Qalb and the CSF levels of adhesion molecules indicating that diabetes may lead to endothelial damage in the cerebral vasculature. However, the number of individuals with diabetes was small in the present study; thus, the results require further validation in larger population.
Another limitation of the present study is that we used Qalb as a measure for the permeability of the BBB, which is common practice (Nagga et al., 2014, Reiber, 1994, Tibbling et al., 1977). However, factors other than disruption of the barrier might affect levels of albumin in the CSF. In particular, the turnover rate of CSF is slowed with advancing age and in patients with AD that has been hypothesized to influence the CSF/serum albumin ratio, with resulting higher CSF levels of albumin (Erickson and Banks, 2013, Reiber and Peter, 2001). Although we did adjust for the potentially confounding effects of age when analyzing our material, we cannot entirely rule out that changes in CSF turnover have contributed to the observed high Qalb. Some researchers also caution against describing Qalb as a BBB marker and state that it actually reflects the blood-CSF barrier at the choroid plexus (Reiber and Peter, 2001). We cannot exclude the possibility that, for example, vascular changes in the choroid plexus microvessels may impair function and thereby lower CSF production rate with a reduced CSF flow rate that would affect the CSF/serum albumin ratio. On the other hand, in for example stroke, leaving the choroid plexus intact but injuring cerebrovascular endothelial cells, the CSF/serum albumin ratio is increased (Brouns et al., 2011), suggesting that CSF/serum albumin ratio probably is a marker of both barriers. Altogether, direct assessments of BBB function, such as using dynamic contrast enhanced MRI and labeled tracers, are warranted to confirm the results of the present study.
In conclusion, we show that a compromised BBB appears to be a feature of several different dementias but is not directly associated with Aβ pathology or the APOE ε4 genotype. Our data link BBB dysfunction with abnormal angiogenic pathways, endothelial damage, and possibly with diabetes mellitus. These findings prompt for future studies investigating molecular mechanisms of BBB breakdown in dementia disorders and impact of therapeutic interventions that target these mechanisms.
Disclosure statement
Drs Janelidze, Hertze, Nägga, Nilsson K., Nilsson C., Wennström, Van Westen, and Hansson report no disclosures. Drs Blennow and Zetterberg are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Venture-based platform company at the University of Gothenburg. Dr. Blennow has served at advisory boards or as a consultant for Eli-Lilly, Fujirebio Europe, Novartis, and Roche Diagnostics.
Acknowledgements
The authors would like to thank the collaborators of this study and the entire BioFINDER Study Group (www.biofinder.se), including Susanna Vestberg for classifying the MCI-AD patients into MCI subgroups and Per Wollmer and Douglas Hägerström for help with [18F]flutemetamol PET imaging. Work in the authors' laboratory was supported by the European Research Council (311292), the Swedish Research Council (K2014-62X-22525-01-4), the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson's disease) at Lund University, the Crafoord Foundation, the Swedish Brain Foundation, the Swedish Alzheimer Foundation, the Torsten Söderberg Foundation, Skåne Research Hospital research funds, Greta and Johan Kock Foundation, Koch’s Foundation, and the Swedish federal government under the ALF (Medical Training and Research Agreement) agreement.
References
- Ambati B.K., Nozaki M., Singh N., Takeda A., Jani P.D., Suthar T., Albuquerque R.J., Richter E., Sakurai E., Newcomb M.T., Kleinman M.E., Caldwell R.B., Lin Q., Ogura Y., Orecchia A., Samuelson D.A., Agnew D.W., St Leger J., Green W.R., Mahasreshti P.J., Curiel D.T., Kwan D., Marsh H., Ikeda S., Leiper L.J., Collinson J.M., Bogdanovich S., Khurana T.S., Shibuya M., Baldwin M.E., Ferrara N., Gerber H.P., De Falco S., Witta J., Baffi J.Z., Raisler B.J., Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Work Group to Revise DSM-III . third ed. American Psychiatric Association; Washington, DC: 1987. Diagnostic and Statistical Manual of Mental Disorders: DSM-iii-r. [Google Scholar]
- Argaw A.T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J.N., Mahase S., Dutta D.J., Seto J., Kramer E.G., Ferrara N., Sofroniew M.V., John G.R. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.D., Winkler E.A., Sagare A.P., Singh I., LaRue B., Deane R., Zlokovic B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R.D., Winkler E.A., Singh I., Sagare A.P., Deane R., Wu Z., Holtzman D.M., Betsholtz C., Armulik A., Sallstrom J., Berk B.C., Zlokovic B.V. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaglia T., Chauveau D., Hunter D.R., Young D.S. Mixtools: an R Package for analyzing finite mixture models. J. Stat. Softw. 2009;32:1–29. [Google Scholar]
- Berglund G., Elmstahl S., Janzon L., Larsson S.A. The Malmo diet and cancer study. Design and feasibility. J. Intern. Med. 1993;233:45–51. doi: 10.1111/j.1365-2796.1993.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Bien-Ly N., Boswell C.A., Jeet S., Beach T.G., Hoyte K., Luk W., Shihadeh V., Ulufatu S., Foreman O., Lu Y., DeVoss J., van der Brug M., Watts R.J. Lack of widespread BBB disruption in Alzheimer's disease models: focus on therapeutic antibodies. Neuron. 2015;88:289–297. doi: 10.1016/j.neuron.2015.09.036. [DOI] [PubMed] [Google Scholar]
- Blennow K., Hampel H., Weiner M., Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- Blennow K., Wallin A., Fredman P., Karlsson I., Gottfries C.G., Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer's disease is related to vascular factors. Acta Neurol. Scand. 1990;81:323–326. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Blennow K., Wallin A., Uhlemann C., Gottfries C.G. White-matter lesions on CT in Alzheimer patients: relation to clinical symptomatology and vascular factors. Acta Neurol. Scand. 1991;83:187–193. doi: 10.1111/j.1600-0404.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- Bowman G.L., Kaye J.A., Moore M., Waichunas D., Carlson N.E., Quinn J.F. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronge L. Magnetic resonance imaging in dementia. A study of brain white matter changes. Acta Radiol. Suppl. 2002;428:1–32. doi: 10.1034/j.1600-0455.43.s.428.1.x. [DOI] [PubMed] [Google Scholar]
- Brouns R., Wauters A., De Surgeloose D., Marien P., De Deyn P.P. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur. Neurol. 2011;65:23–31. doi: 10.1159/000321965. [DOI] [PubMed] [Google Scholar]
- Burgmans S., van de Haar H.J., Verhey F.R., Backes W.H. Amyloid-beta interacts with blood-brain barrier function in dementia: a systematic review. J. Alzheimers Dis. 2013;35:859–873. doi: 10.3233/JAD-122155. [DOI] [PubMed] [Google Scholar]
- Chow B.W., Gu C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 2015;38:598–608. doi: 10.1016/j.tins.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuck J., Deramecourt V., Cordonnier C., Auger F., Durieux N., Bordet R., Maurage C.A., Leys D., Pasquier F. Detection of microbleeds in post-mortem brains of patients with frontotemporal lobar degeneration: a 7.0-Tesla magnetic resonance imaging study with neuropathological correlates. Eur. J. Neurol. 2012;19:1355–1360. doi: 10.1111/j.1468-1331.2012.03776.x. [DOI] [PubMed] [Google Scholar]
- De Reuck J., Deramecourt V., Cordonnier C., Leys D., Pasquier F., Maurage C.A. Prevalence of cerebrovascular lesions in patients with Lewy body dementia: a neuropathological study. Clin. Neurol. Neurosurg. 2013;115:1094–1097. doi: 10.1016/j.clineuro.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Di Marco L.Y., Venneri A., Farkas E., Evans P.C., Marzo A., Frangi A.F. Vascular dysfunction in the pathogenesis of Alzheimer's disease—a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015;82:593–606. doi: 10.1016/j.nbd.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Erickson M.A., Banks W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J. Cereb. Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geser F., Wenning G.K., Poewe W., McKeith I. How to diagnose dementia with Lewy bodies: state of the art. Mov. Disord. 2005;20(Suppl 12):S11–S20. doi: 10.1002/mds.20535. [DOI] [PubMed] [Google Scholar]
- Halliday M.R., Pomara N., Sagare A.P., Mack W.J., Frangione B., Zlokovic B.V. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013;70:1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins B.T., Lundeen T.F., Norwood K.M., Brooks H.L., Egleton R.D. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- Janelidze S., Lindqvist D., Francardo V., Hall S., Zetterberg H., Blennow K., Adler C.H., Beach T.G., Serrano G.E., van Westen D., Londos E., Cenci M.A., Hansson O. Increased CSF biomarkers of angiogenesis in Parkinson disease. Neurology. 2015;85:1834–1842. doi: 10.1212/WNL.0000000000002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S., Stomrud E., Palmqvist S., Zetterberg H., van Westen D., Jeromin A., Song L., Hanlon D., Tan Hehir C.A., Baker D., Blennow K., Hansson O. Plasma beta-amyloid in Alzheimer's disease and vascular disease. Sci. Rep. 2016;6:26801. doi: 10.1038/srep26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch A., Manthey H., Ponto C., Hermann P., Heinemann U., Schmidt C., Zerr I. Investigating the association of ApoE genotypes with blood-brain barrier dysfunction measured by cerebrospinal fluid-serum albumin ratio in a cohort of patients with different types of dementia. PLoS One. 2013;8:e84405. doi: 10.1371/journal.pone.0084405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall R.L., Thomas K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. U. S. A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens F., Schmitz M., Gloeckner S.F., Kaerst L., Hermann P., Schmidt C., Varges D., Zerr I. Increased albumin CSF/serum ratio in dementia with Lewy bodies. J. Neurol. Sci. 2015;358:398–403. doi: 10.1016/j.jns.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Lundqvist R., Lilja J., Thomas B.A., Lotjonen J., Villemagne V.L., Rowe C.C., Thurfjell L. Implementation and validation of an adaptive template registration method for 18F-flutemetamol imaging data. J. Nucl. Med. 2013;54:1472–1478. doi: 10.2967/jnumed.112.115006. [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meigs J.B., Hu F.B., Rifai N., Manson J.E. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., Toga A.W., Jacobs R.E., Liu C.Y., Amezcua L., Harrington M.G., Chui H.C., Law M., Zlokovic B.V. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagga K., Hansson O., van Westen D., Minthon L., Wennstrom M. Increased levels of hyaluronic acid in cerebrospinal fluid in patients with vascular dementia. J. Alzheimers Dis. 2014;42:1435–1441. doi: 10.3233/JAD-141200. [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S., Freedman M., Kertesz A., Robert P.H., Albert M., Boone K., Miller B.L., Cummings J., Benson D.F. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nishitsuji K., Hosono T., Nakamura T., Bu G., Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J. Biol. Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Lautner R., Andreasson U., Ohrfelt A., Portelius E., Bjerke M., Holtta M., Rosen C., Olsson C., Strobel G., Wu E., Dakin K., Petzold M., Blennow K., Zetterberg H. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- Palmqvist S., Zetterberg H., Blennow K., Vestberg S., Andreasson U., Brooks D.J., Owenius R., Hagerstrom D., Wollmer P., Minthon L., Hansson O. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Qi J.W., Qin T.T., Xu L.X., Zhang K., Yang G.L., Li J., Xiao H.Y., Zhang Z.S., Li L.Y. TNFSF15 inhibits vasculogenesis by regulating relative levels of membrane-bound and soluble isoforms of VEGF receptor 1. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13863–13868. doi: 10.1073/pnas.1304529110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz L., Knoefel J., Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2015;36:172–186. doi: 10.1038/jcbfm.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeson P., Tennant K.A., Gerrow K., Wang J., Weiser Novak S., Thompson K., Lockhart K.L., Holmes A., Nahirney P.C., Brown C.E. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J. Neurosci. 2015;35:5128–5143. doi: 10.1523/JNEUROSCI.2810-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber H. Flow rate of cerebrospinal fluid (CSF)—a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 1994;122:189–203. doi: 10.1016/0022-510x(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Reiber H., Peter J.B. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J. Neurol. Sci. 2001;184:101–122. doi: 10.1016/s0022-510x(00)00501-3. [DOI] [PubMed] [Google Scholar]
- Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H., Amaducci L., Orgogozo J.M., Brun A., Hofman A., Moody D.M., O'Brien M.D., Yamaguchi T., Grafman J., Drayer B.P., Bennett D.A., Fisher M., Ogata J., Kokmen E., Bermejo F., Wolf P.A., Gorelick P.B., Bick K.L., Pajeau A.K. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Sjogren M., Folkesson S., Blennow K., Tarkowski E. Increased intrathecal inflammatory activity in frontotemporal dementia: pathophysiological implications. J. Neurol. Neurosurg. Psychiatry. 2004;75:1107–1111. doi: 10.1136/jnnp.2003.019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog I., Wallin A., Fredman P., Hesse C., Aevarsson O., Karlsson I., Gottfries C.G., Blennow K. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- Snyder H.M., Corriveau R.A., Craft S., Faber J.E., Greenberg S.M., Knopman D., Lamb B.T., Montine T.J., Nedergaard M., Schaffer C.B., Schneider J.A., Wellington C., Wilcock D.M., Zipfel G.J., Zlokovic B., Bain L.J., Bosetti F., Galis Z.S., Koroshetz W., Carrillo M.C. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr J.M., Wardlaw J., Ferguson K., MacLullich A., Deary I.J., Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Nagai N., Yamakawa K., Muranaka Y., Hokamura K., Umemura K. Recombinant tissue-type plasminogen activator transiently enhances blood-brain barrier permeability during cerebral ischemia through vascular endothelial growth factor-mediated endothelial endocytosis in mice. J. Cereb. Blood Flow Metab. 2015;35:2021–2031. doi: 10.1038/jcbfm.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S., Gasparovic C., Huisa B.N., Adair J.C., Edmonds E., Prestopnik J., Grossetete M., Shah N.J., Wills J., Qualls C., Rosenberg G.A. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbling G., Link H., Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand. J. Clin. Lab. Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- Tousoulis D., Papageorgiou N., Androulakis E., Siasos G., Latsios G., Tentolouris K., Stefanadis C. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J. Am. Coll. Cardiol. 2013;62:667–676. doi: 10.1016/j.jacc.2013.03.089. [DOI] [PubMed] [Google Scholar]
- van de Haar H.J., Burgmans S., Hofman P.A., Verhey F.R., Jansen J.F., Backes W.H. Blood-brain barrier impairment in dementia: current and future in vivo assessments. Neurosci. Biobehav Rev. 2015;49:71–81. doi: 10.1016/j.neubiorev.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Wada H. Blood-brain barrier permeability of the demented elderly as studied by cerebrospinal fluid-serum albumin ratio. Intern. Med. 1998;37:509–513. doi: 10.2169/internalmedicine.37.509. [DOI] [PubMed] [Google Scholar]
- Wahlund L.O., Barkhof F., Fazekas F., Bronge L., Augustin M., Sjogren M., Wallin A., Ader H., Leys D., Pantoni L., Pasquier F., Erkinjuntti T., Scheltens P., European Task Force on Age-Related White Matter Changes A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- Wallin A., Blennow K., Fredman P., Gottfries C.G., Karlsson I., Svennerholm L. Blood brain barrier function in vascular dementia. Acta Neurol. Scand. 1990;81:318–322. doi: 10.1111/j.1600-0404.1990.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Winkler E.A., Sengillo J.D., Sagare A.P., Zhao Z., Ma Q., Zuniga E., Wang Y., Zhong Z., Sullivan J.S., Griffin J.H., Cleveland D.W., Zlokovic B.V. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E1035–E1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.G., Zhang L., Jiang Q., Zhang R., Davies K., Powers C., Bruggen N., Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Nelson A.R., Betsholtz C., Zlokovic B.V. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]