Abstract
Background
Despite declines in mortality and morbidity rates of patients with human immunodeficiency virus (HIV) infection as the result of highly active antiretroviral therapy, liver diseases due to chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are a leading cause of death among HIV-infected patients. However, HIV and HBV or HCV coinfection is still poorly documented, and more information is needed to better understand the characteristics of HIV-infected patients in Korea.
Materials and Methods
A cross-sectional study was performed to investigate clinical characteristics and prevalence of HBV and HCV infection in HIV patients enrolled in the Korea HIV/acquired immune deficiency syndrome (AIDS) cohort study from 17 institutions between December 2006 and July 2013.
Results
Among the 1,218 HIV-infected participants, 541 were included in this study. The prevalence of HBV-HIV and HCV-HIV coinfection was 5.0% (27/541) and 1.7% (9/541), respectively. There was no patient who was positive for both HBs antigen and HCV antibody. In multivariate logistic regression analysis, HBV unvaccinated status was a significant risk factor for HBV-HIV coinfection (odds ratio = 4.95, 95% confidence interval = 1.43–17.13).
Conclusions
HBV and HCV infection was more common in HIV-infected persons enrolled in the Korean HIV/AIDS cohort, than in the general population in Korea.
Keywords: HIV infection, Hepatitis B virus, Hepatitis C virus, Coinfection
Introduction
Therapy for human immunodeficiency virus (HIV)-infected patients has progressed remarkably since the introduction of antiretroviral therapy (ART). Furthermore, since the early start of effective ART, the incidence of opportunistic infections as well as the mortality rate in patients with HIV has decreased [1-3]. Liver disease is currently the major concern in HIV-infected patients coinfected with hepatitis B virus (HBV) or hepatitis C virus (HCV) [4, 5]. HBV-HIV or HCV-HIV patients have more rapid progression of liver disease than those with HBV or HCV mono-infection. End-stage liver disease, such as liver cirrhosis or hepatocellular carcinoma, is commonly observed in patients with HBV-HIV or HCV-HIV coinfection [6-9]. Furthermore, HBV or HCV coinfection may increase the risk of ART-related hepatotoxicity or influence the selection of ART regimen [10].
HIV and HBV or HCV coinfection is still poorly documented in Korea. Although some studies have evaluated viral hepatitis coinfection in HIV-infected patients, these were retrospective single-center studies and reported little data about HBV-HIV coinfection [11, 12]. Thus, more information is needed to better understand the characteristics of HIV-infected patients in Korea.
We therefore conducted a prospective multicenter study to investigate the prevalence and epidemiological features of both HBV and HCV coinfections among HIV-infected Korean patients.
Materials and Methods
1. Study design and population
The Korea HIV/acquired immune deficiency syndrome (AIDS) cohort study is a prospective multicenter study with ongoing enrollment of HIV-infected adult patients older than 18 years from 17 hospitals in South Korea (Gachon University Gil Hospital, Seoul St. Mary's Hospital, Hallym University Kangdong Sacred Heart Hospital, Kyungpook National University Hospital, Korea University Guro Hospital, Korea University Ansan Hospital, Seoul Asan Hospital, Soon Chun Hyang University Hospital Seoul, Ajou University Hospital, Severance Hospital, Wonju Severance Christian Hospital, Ewha Womans University Mokdong Hospital, Inha University Hospital, Chungbuk National University Hospital, Hallym University Anyang Sacred Heart Hospital, Hallym University Kangnam Sacred Heart Hospital, and Yeungnam University Hospital). To evaluate the prevalence and epidemiological features of HBV or HCV coinfection among HIV-infected persons, we investigated the presence of HBV surface antigen (HBs Ag) and anti-HCV antibody (Ab). Patients who tested negative for HBs Ag and anti-HCV Ab were defined as HIV mono-infection patients. Among patients with HIV infection, HBV coinfection was defined based on positive test results for HBs Ag and HCV coinfection was defined based on positive test results for anti-HCV Ab. Trained researchers from all centers prospectively collected information every 6 months using a standardized protocol. Information included medical history, socioeconomic status, physical findings, laboratory findings including immunological and virological status, and opportunistic diseases. All participants provided written informed consent and ethics approval was obtained from the Institutional Review Board of each participating institute. This study analyzed data for 1,218 HIV-infected persons enrolled between December 2006 and July 2013.
2. Laboratory tests and data collection
HIV infection was screened for using enzyme immunoassays (EIA) and confirmed using western blotting. HBV infection was defined based on HBs Ag positivity without protective antibodies. HCV infection was detected via EIA for anti-HCV Ab presence, and reactive samples were confirmed using recombinant immunoblot assay. To evaluate the prevalence and epidemiological features of HBV or HCV coinfections, we used baseline data at the time of registration in the Korea HIV/AIDS cohort study. These data included age, sex, body mass index, race, socioeconomic status, sexual habit, first year of HIV diagnosis, history of smoking and alcohol use, route of transmission, CD4/CD8 count, HIV viral load, ART history, HBV vaccination status, history of liver disease, and serum chemistry at study enrollment.
3. Statistical analysis
Continuous variables are presented as medians with interquartile ranges (IQR) and categorical variables are expressed as numbers with percentages. To compare variables between two groups, an independent sample t-test or the Mann-Whitney U test was used for continuous variables, and a Brown-Mood median two-sample test or Fisher's exact test was used for categorical variables. Factors associated with HBV or HCV coinfection were analyzed by univariate and multivariate logistic regression models. Variables with a P value less than 0.25 in baseline characteristic analyses were included in univariate analyses, and multivariate analyses included variables with a P value less than 0.16 in univariate analyses. The relationships between clinical factors and HBV or HCV coinfection were summarized by odds ratios (ORs) and 95% confidence intervals (CIs). Statistical analyses were performed using the SPSS, version 16.0 (SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered statistically significant.
Results
1. Baseline characteristics of patients
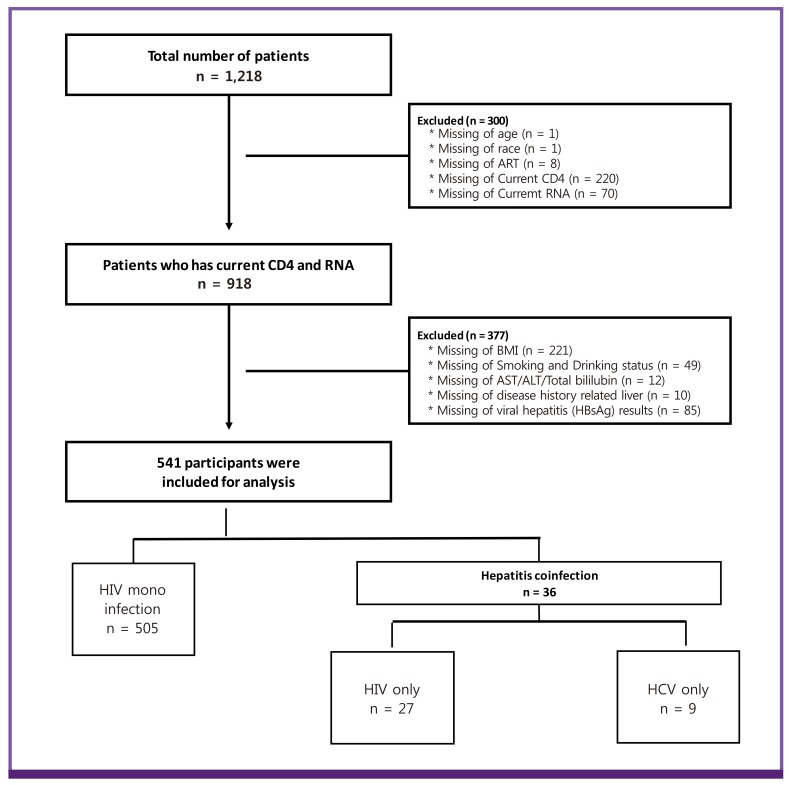
Among 1,218 participants, 677 were excluded because of missing data. 541 participants were included for analysis. The prevalence of HBV and HCV coinfection was 5.0% (27/541) and 1.7% (9/541), respectively (Fig. 1). The baseline characteristics of the 541 HIV-infected patients are shown in Table 1. Most patients were men (92.8%) and of Korean ethnicity (98.7%). The median age was 42 years (IQR, 33–51 years). According to a self-reported questionnaire, HIV was transmitted mainly by sexual contact (97.6%), and the proportion of homosexual patients (33.1%) was similar to that of heterosexual patients (36.4%). The median CD4 cell count was 356 cells/mm3 (IQR, 206–512 cells/mm3) and the median HIV viral load was 157 copies/mL (IQR, 20–19,645 copies/mL). 375 patients (69.3%) were treatment naïve.
Figure 1.
Profile of enrolled patients.
ART, antiretroviral therapy; RNA, ribonucleic acid; BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase; HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV, hepatitis C virus.
Table 1. Patients' baseline characteristics.
| Variables | n (%) | P value | ||||
|---|---|---|---|---|---|---|
| Total | HIV mono infection | Hepatitis coinfection | ||||
| n | 541 (100.0) | 505(93.4) | 36(6.7) | <0.0001 | ||
| Age (yrs)a | 42 (33 - 51) | 41 (33 - 50) | 45 (37 - 51.5) | 0.039b | ||
| <30 | 91(16.8) | 86(17.0) | 5(13.9) | 0.077 | ||
| <40 | 151(27.9) | 145(28.7) | 6(16.7) | |||
| <60 | 252(46.6) | 228(45.2) | 24(66.7) | |||
| ≥60 | 47(8.7) | 46(9.1) | 1(2.8) | |||
| Sex | ||||||
| Male | 502(92.8) | 472(93.5) | 30(83.3) | 0.037c | ||
| Female | 39(7.2) | 33(6.5) | 6(16.7) | |||
| Race | ||||||
| Korean | 534(98.7) | 500(99.0) | 34(94.4) | 0.073c | ||
| Foreigner | 7(1.3) | 5(1.00) | 2(5.7) | |||
| Transmission route of HIV | ||||||
| Reception of blood/product | 1(0.2) | 0(0.0) | 1(0.2) | 0.042c | ||
| Others (include unknowing)d | 12(2.2) | 11(2.2) | 1(2.8) | |||
| Sexual contact | 528(97.6) | 494(97.8) | 34(94.4) | |||
| Homosexual | 179(33.1) | 173(33.7) | 6(22.2) | 0.179 | ||
| Heterosexual | 197(36.4) | 182(35.4) | 15(55.6) | |||
| Bisexual | 152(28.1) | 146(28.4) | 6(22.2) | |||
| Baseline CD4 cell count (cells/mm3)a | 356 (206 - 512) | 360 (208 - 512) | 310 (175 - 501.5) | 0.306b | ||
| Baseline HIV RNA (copies/mL)a | 157 (20 - 19,645) | 196 (20 - 17,415) | 74.5 (20 - 26,574) | 0.307b | ||
| Treatment naïve | 375(69.3) | 348(68.9) | 27(75.0) | 0.444 | ||
| Body mass index (BMI, kg/m2) | ||||||
| Mean ± SD | 22.2 ± 2.91 | 22.2 ± 2.91 | 21.9 ± 2.94 | 0.305b | ||
| Median (IQR) | 21.8 (20.31 - 23.88) | 21.85 (20.32 - 23.88) | 21.56 (19.72 - 23.94) | |||
| Smoking history | ||||||
| Current | 267(49.4) | 251(49.7) | 16(44.4) | 0.086 | ||
| Previous | 99(18.3) | 96(19.0) | 3(8.3) | |||
| Non smoker | 175(32.4) | 158(31.3) | 17(47.2) | |||
| Alcohol history | ||||||
| Current | 285(52.7) | 270(53.5) | 15(41.7) | 0.380 | ||
| Previous | 91(16.8) | 84(16.6) | 7(19.4) | |||
| Non drinker | 165(30.5) | 151(29.9) | 14(38.9) | |||
| Laboratory resultsa | ||||||
| AST (IU/L) | 22 (18 - 30) | 22 (18 - 30) | 23 (20 - 28.5) | 0.660b | ||
| ALT (IU/L) | 21 (15 - 32) | 21 (15 - 33) | 20.5 (15.6 - 26.0) | 0.403b | ||
| Total bilirubin (mg/dL) | 0.60 (0.43 - 0.90) | 0.60 (0.43 - 0.90) | 0.57 (0.455 - 0.805) | 0.122b | ||
| Liver disease | ||||||
| Fatty liver | 6(1.1) | 5(1.0) | 1(2.8) | 0.267c | ||
| Liver cirrhosis | 4(0.7) | 1(0.2) | 3(8.3) | 0.001c | ||
Values are presented as medians (interquartile ranges (IQR)) or numbers (percentages).
HIV, human immunodeficiency virus; RNA, ribonucleic acid; SD, standard deviation; IQR, interquartile range; AST, aspartate transaminase; ALT, alanine transaminase.
aMedian (Interquartile range, IQR)
bBrown-Mood's median two-sample test
cFisher's exact test
dOthers include vertical transmission and unknowing.
Among the 541 HIV patients who had results of HBs Ag and anti-HCV Ab testing, 36 patients (6.7%) had HBV or HCV coinfection. There were significant differences in age, sex, transmission route of HIV, history of liver cirrhosis, and HBV vaccination status between HIV mono-infection patients and HBV or HCV coinfection patients. Patients with HBV or HCV coinfection were older than HIV mono-infection patients (median, 45 years; IQR, 37–51 years vs. median, 41 years; IQR, 33–50 years, P <0.039). Patients with liver cirrhosis were more common in the HBV or HCV coinfection group than the HIV mono-infection group (8.3% vs. 0.2%, P = 0.001). In contrast, male sex was more common in the HIV mono-infection patients than the HBV or HCV coinfection patients (93.5% vs. 83.3%, P = 0.037), and the rate of HBV vaccination was significantly different between the HIV mono-infection group and HBV or HCV coinfection group (P = 0.0002).
2. Factors associated with HBV and HCV coinfection
Univariate logistic regression analysis showed that age, sex, sexual contact, history of liver cirrhosis, and HBV vaccination status were associated with HBV coinfection in HIV patients (Table 2). Sexuality and history of liver cirrhosis were not included in multivariate analysis because of unmatched number of participants. In multivariate analysis, HBV unvaccinated status was an independent risk factor for HBV coinfection (OR = 4.95, 95% CI = 1.43–17.13). Factors associated with HCV coinfection were not analyzed because of small number of HCV coinfection patients.
Table 2. Factors associated with hepatitis B virus coinfection.
| Variables | HBV coinfection (n = 104) | ||||
|---|---|---|---|---|---|
| Univariate OR (95 % CI) | P value | Multivariate OR (95% CI) | P value | ||
| Age (years) (vs. <42a) | |||||
| ≥42 | 2.06 (0.91–4.68) | 0.083 | 1.52 (0.63–3.65) | 0.353 | |
| Male sex (vs. female sex) | 0.42 (0.114–1.28) | 0.128 | 0.59 (0.17–2.05) | 0.405 | |
| Race (vs. foreigner) | |||||
| Korean | 0.31 (0.04–2.65) | 0.283 | |||
| Sexuality (vs. homosexual) | |||||
| Heterosexual | 2.38 (0.90–6.27) | 0.587 | |||
| Bisexual | 1.19 (0.37–3.75) | 0.05 | |||
| Smoking history (vs. nonsmoker) | |||||
| Current | 0.76 (0.33–1.74) | 0.799 | |||
| Previous | 0.47 (0.13–1.71) | 0.313 | |||
| Liver disease (vs. no) | |||||
| Liver cirrhosis | 64.10 (6.43–639.12) | 0.0004 | |||
| HBV vaccination (vs. yes) | |||||
| No | 6.28 (1.85–21.32) | 0.0005 | 4.95 (1.43–17.13) | 0.001 | |
| Unknown | 1.09 (0.18–6.63) | 0.286 | 0.69 (0.10–4.79) | 0.17 | |
Values are presented as medians (interquartile ranges) or numbers (percentages).
HBV, hepatitis B virus; OR, odds ratio; CI, confidence interval.
aMedian criteria
Discussion
In this study, we investigated the prevalence and epidemiological features of HBV and HCV coinfection in HIV-infected Korean patients. The rate of HBV or HCV coinfection in HIV patients was 6.7%. The prevalence of HBV-HIV coinfection and HCV-HIV coinfection was 5.0% and 1.7%, respectively. Multivariate logistic regression analysis showed that the risk of HBV coinfection was associated with HBV unvaccinated status. Factors associated with HCV infection were not identified in this study because of small number of HCV coinfection patients.
Previous studies have reported hepatitis virus coinfection among HIV-infected Korean patients [11, 12]. However, they did not report the demographic characteristics of patients with HBV-HIV coinfection. To the best of our knowledge, our study is the first to evaluate the prevalence and demographic characteristics of both HBV-HIV and HCV-HIV coinfections in HIV-infected Korean patients.
HBV or HCV infection in HIV patients is more common than in the general population [13]. This may be caused by similarities in routes of transmission and risk factors between HBV or HCV and HIV, for example, injection drug use, sexual contact, and reception of blood products [14-16]. Our study also showed a higher prevalence of HBV or HCV infection in HIV patients than in the general population. According to the Korea National Health and Nutrition Examination Survey, the HBV infection rate in the general population is 2.9% [17]. Kim et al. reported that the nationwide prevalence of HCV in Korea was 0.78% [18].
The rates of HBV or HCV coinfection among HIV patients have been studied in several countries [19-23]. The prevalence of HBV-HIV coinfection reported in our study is similar to that of other countries [19]. However, we found a lower prevalence of HCV-HIV coinfection than that reported in previous studies [24, 25]. A possible cause is that the regions from which patients were drawn for this study did not include regions with a high prevalence of anti-HCV. A Korean study, implemented in a region with a high prevalence of anti-HCV, reported an HCV-HIV coinfection prevalence of 5.2% [12].
Our study had several limitations. First, we did not show the factors associated with HCV-HIV coinfection because of small number of patients with HCV-HIV coinfection. Second, our HCV-HIV coinfection data is insufficient to represent the characteristics of HCV-HIV coinfection throughout Korea because we did not include the institutions in regions with a high prevalence of HCV-HIV coinfection. Third, a large number of participants were excluded due to missing data. Therefore, there is a possibility of selection bias. Also, there is the potential for recall bias because the history of HBV vaccination was confirmed by questionnaire to the participants. Further studies are required to be conducted with nationwide and accurate data for HBV and HCV coinfection.
In conclusion, this study showed that the prevalence of HBV and HCV infection in HIV patients was higher than that in the Korean general population. The prevalence of HBV-HIV coinfection was higher than that of HCV-HIV coinfection. HBV unvaccinated status was an independent risk factor for HBV-HIV coinfection. A nationwide study is needed to analyze the prevalence and epidemiological features of HCV-HIV coinfection in the Korean population as a whole.
Acknowledgments
We thank the members of the Korea HIV/AIDS cohort study, Moon Won Kang, Min Ja Kim, Jun Hee Woo, Sang Il Kim, Youn Jeong Kim, Dae Won Park, Won Suk Choi, Jang Wook Sohn, Seong Han Kim, Seong-Heon Wie, Ji-An Hur, Yeon Joon Park, Shin-Woo Kim, Hyun-Ha Chang, Yoo Joo Kim, Joon Young Song, Joong Shik Eom, Jin Seo Lee, Jacob Lee, Hye Won Jeong, Jin Soo Lee, Hee Jung Choi, Seung Soon Lee, June Myung Kim, Jun Yong Choi, Sang Hoon Han, Nam Su Ku, Jin Young Ahn, Hyo-Youl Kim, Young Keun Kim, Yong Kyun Cho, Yoon Soo Park, Seung Kwan Lim, Young Hwa Choi, Choi Bo Youl, Hee Suk Park, Mee-Kyung Kee, Joo Shil Lee, and Sung Soon Kim.
Footnotes
Funding: This work was supported by a fund of the Chronic Infectious Disease Cohort Study [grant number 4800-4859-304, 2016-E51003-00] by Research of Korea Centers for Disease Control and Prevention.
Conflict of Interest: No conflicts of interest
Supplementary Material
Supplementary data including one table can be found with this article online http://www.icjournal.org/src/sm/ic-49-268-s001.pdf.
Hepatitis B virus vaccination status and serologies of hepatitis B virus and hepatitis C virus
References
- 1.Buchacz K, Baker RK, Palella FJ, Jr, Chmiel JS, Lichtenstein KA, Novak RM, Wood KC, Brooks JT HOPS Investigators. AIDS-defining opportunistic illnesses in US patients, 1994-2007: a cohort study. AIDS. 2010;24:1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 2.Schwarcz L, Chen MJ, Vittinghoff E, Hsu L, Schwarcz S. Declining incidence of AIDS-defining opportunistic illnesses: results from 16 years of population-based AIDS surveillance. AIDS. 2013;27:597–605. doi: 10.1097/QAD.0b013e32835b0fa2. [DOI] [PubMed] [Google Scholar]
- 3.Kim MJ, Chang HH, Kim SI, Kim YJ, Park DW, Kang C, Kee MK, Choi JY, Kim SM, Choi BY, Kim WJ, Kim JM, Choi JY, Choi YH, Lee JS, Kim SW, Korea H Korea HIV/AIDS Cohort Study. Trend of CD4+ Cell Counts at Diagnosis and Initiation of Highly Active Antiretroviral Therapy (HAART): Korea HIV/AIDS Cohort Study, 1992-2015. Infect Chemother. 2017;49:101–108. doi: 10.3947/ic.2017.49.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(Suppl):S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402–409. doi: 10.1016/S1473-3099(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 7.Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, Moschidis Z, Sypsa V, Zavitsanos X, Kalapothaki V, Hatzakis A. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis. 2009;48:1763–1771. doi: 10.1086/599110. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Quijano A, Andreu J, Gavilán F, Luque F, Abad MA, Soto B, Muñoz J, Aznar JM, Leal M, Lissen E. Influence of human immunodeficiency virus type 1 infection on the natural course of chronic parenterally acquired hepatitis C. Eur J Clin Microbiol Infect Dis. 1995;14:949–953. doi: 10.1007/BF01691375. [DOI] [PubMed] [Google Scholar]
- 9.Soto B, Sánchez-Quijano A, Rodrigo L, del Olmo JA, García-Bengoechea M, Hernández-Quero J, Rey C, Abad MA, Rodríguez M, Sales Gilabert M, González F, Mirón P, Caruz A, Relimpio F, Torronteras R, Leal M, Lissen E. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 10.Rockstroh JK. Influence of viral hepatitis on HIV infection. J Hepatol. 2006;44(Suppl):S25–S27. doi: 10.1016/j.jhep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Kim KH, Lee SG, Chen DH, Jung DS, Moon CS, Park JY, Chung JS, Kwak IS, Cho GJ. Trends of mortality and cause of death among HIV-infected patients in Korea, 1990-2011. J Korean Med Sci. 2013;28:67–73. doi: 10.3346/jkms.2013.28.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Lee SH, Lee SJ, Kim KH, Lee JE, Cho H, Lee SG, Chung JS, Kwak IS. Incidence and risk factors of hepatitis C virus infection among human immunodeficiency virus (HIV) patients in a large HIV clinic in South Korea. Korean J Intern Med. 2016;31:772–778. doi: 10.3904/kjim.2015.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adekunle AE, Oladimeji AA, Temi AP, Adeseye AI, Akinyeye OA, Taiwo RH. Baseline CD4+ T lymphocyte cell counts, hepatitis B and C viruses seropositivity in adults with Human Immunodeficiency Virus infection at a tertiary hospital in Nigeria. Pan Afr Med J. 2011;9:6. doi: 10.4314/pamj.v9i1.71178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellerman SE, Hanson DL, McNaghten AD, Fleming PL. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571–577. doi: 10.1086/377135. [DOI] [PubMed] [Google Scholar]
- 16.Sungkanuparph S, Vibhagool A, Manosuthi W, Kiertiburanakul S, Atamasirikul K, Aumkhyan A, Thakkinstian A. Prevalence of hepatitis B virus and hepatitis C virus co-infection with human immunodeficiency virus in Thai patients: a tertiary-care-based study. J Med Assoc Thai. 2004;87:1349–1354. [PubMed] [Google Scholar]
- 17.Korea Centers for Disease Control and Prevention (KCDC) Korea health statistics 2013: Korea National Health and Nutrition Examination Survey (KNHANES VI-1) [Accessed 30 January, 2017]. Available at: http://knhanes.cdc.go.kr/knhanes/index.do.
- 18.Kim DY, Kim IH, Jeong SH, Cho YK, Lee JH, Jin YJ, Lee D, Suh DJ, Han KH, Park NH, Kang HY, Jung YK, Kim YS, Kim KA, Lee YJ, Lee BS, Yim HJ, Lee HJ, Baik SK, Tak WY, Lee SJ, Chung WJ, Choi SK, Cho EY, Heo J, Kim DJ, Song BC, Kim MW, Lee J, Chae HB, Choi DH, Choi HY, Ki M. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int. 2013;33:586–594. doi: 10.1111/liv.12108. [DOI] [PubMed] [Google Scholar]
- 19.Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection--a global challenge. N Engl J Med. 2012;366:1749–1752. doi: 10.1056/NEJMp1201796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin J, Kaye M, Skidmore S, Pillay D, Cooper DA, Dore GJ. HIV and hepatitis C coinfection within the CAESAR study. HIV Med. 2004;5:174–179. doi: 10.1111/j.1468-1293.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 21.Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub-Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta-analysis. Int J Infect Dis. 2010;14:e1024–e1031. doi: 10.1016/j.ijid.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Moreira M, Ramos A, Netto EM, Brites C. Characteristics of co-infections by HCV and HBV among Brazilian patients infected by HIV-1 and/or HTLV-1. Braz J Infect Dis. 2013;17:661–666. doi: 10.1016/j.bjid.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, He JM, Ding LS, Zhang GQ, Zou XB, Zheng J. Prevalence of hepatitis B virus and hepatitis C virus in patients with human immunodeficiency virus infection in Central China. Arch Virol. 2013;158:1889–1894. doi: 10.1007/s00705-013-1681-z. [DOI] [PubMed] [Google Scholar]
- 24.Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, Yanny I, Razavi H, Vickerman P. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 25.Rao VB, Johari N, du Cros P, Messina J, Ford N, Cooke GS. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:819–824. doi: 10.1016/S1473-3099(15)00006-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hepatitis B virus vaccination status and serologies of hepatitis B virus and hepatitis C virus