Abstract
Acinetobacter baumannii is an aerobic Gram-negative coccobacillus that causes nosocomial pneumonia in patients on mechanical ventilation or previously treated with broad-spectrum antibiotics. Nevertheless, community-acquired pneumonia (CAP) caused by A. baumannii, especially multi-drug resistant (MDR) strains, is rare. We experienced the first case of CAP caused by MDR A. baumannii in Korea in a 78-year-old man. This case shows that MDR A. baumannii can cause CAP in Korea.
Keywords: Acinetobacter baumannii, Community-acquired pneumonia, Multi-drug-resistance
Introduction
Acinetobacter baumannii is an aerobic Gram-negative coccobacillus [1]. It causes nosocomial pneumonia, especially in patients on mechanical ventilation or after treatment with broad-spectrum antibiotics [1,2]. Interestingly, community-acquired pneumonia (CAP) caused by A. baumannii has a higher mortality rate than that of hospital-acquired pneumonia (HAP), although CAP caused by A. baumannii, especially multi-drug resistant (MDR) A. baumannii, is rare [3]. Recently, MDR A. baumannii has increased as an emerging problem because of restricted therapeutic options [4]. Here, we report the first case of CAP caused by MDR A. baumannii in Korea in a 78-year-old man.
Case report
A 78-year-old man presented to the emergency department with dyspnea and fever for 6 hours. He was not currently employed and had no history of cigarette smoking or alcohol ingestion. He had no underlying disease. Besides, he had no hospital admission, antimicrobial use history and healthcare institution visiting record in 1 year. On admission, he appeared acutely ill. His blood pressure was 104/57 mmHg, respiratory rate 20 breaths per minute, pulse rate 110 beats per minute, and body temperature 38.2°C. A regular heart rhythm was observed. There were coarse breath sounds with crackles in the right lung field.
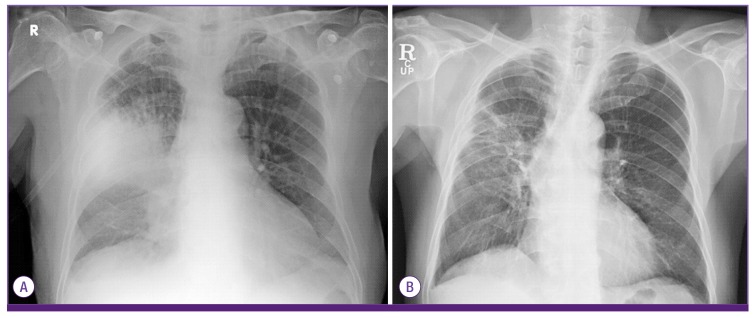
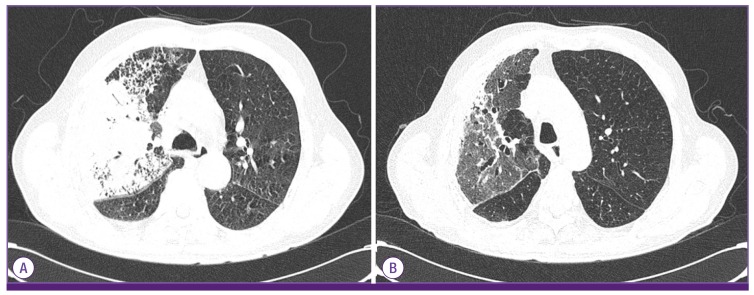
The laboratory results were as follows: white blood cell count 3,900/mm3 (72.5% neutrophils, 20.4% lymphocytes, 4.7% monocytes, 2.82% eosinophils), hemoglobin 12.2 g/dL, platelet count 197,000/mm3, C-reactive protein 1.24 mg/dL, blood urea nitrogen 28.2 mg/dL, creatinine 1.24 mg/dL, aspartate aminotransferase 16 UI/L, alanine aminotransferase 10 IU/L, total bilirubin 0.9 mg/dL, total protein 6.0 g/dL, and albumin 3.5 g/dL. The arterial blood gas analysis breathing room air was pH 7.44, pCO2 24.1 mmHg, pO2 59.0 mmHg, HCO3– 17 mmol/L, and O2 saturation 87%. A plain chest radiograph showed patchy consolidations in the right middle and lower lobes (Fig. 1A). Chest computed tomography (CT) showed lobar pneumonia in the right middle and lower lobes (Fig. 2A). He was diagnosed with CAP. Intravenous piperacillin/tazobactam and ciprofloxacin were administered empirically.
Figure 1.
Chest radiography. (A) The chest radiograph on admission showed lobar pneumonia in the right upper and middle lobes. (B) On the 48th day after admission, the chest radiograph showed improvement in the consolidations of the right and middle lobes.
Figure 2.
Chest computed tomography (CT). (A) On admission, CT showed lobar pneumonia in the right upper and middle lobes with a parapneumonic effusion. (B) At the 48th day after admission, CT showed improvement in the consolidations of the right upper and middle lobes.
Five hours after admission, his respiratory distress worsened, and he was treated with oxygen at 6 L/min via mask. On the second day after admission, intravenous teicoplanin was administered because of a persistent fever. On the third day, A. baumannii was identified in cultures of both sputum and in two of three pairs of blood samples taken on admission. A. baumannii was identified using Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS, Bruker Daltonics GmbH, Bremen, Germany) on a Microflex spectrometer (Bruker Daltonics GmbH, Germany). Antimicrobial susceptibilities were determined using VITEK-2 system (bioMerieux, Marcy I'Etoile, France). The bacterial isolate was resistant to multiple drugs, except colistin, tigecycline, minocycline, amikacin, and gentamicin (Table 1). Therefore, the antibiotics were changed to intravenous colistin and oral minocycline.
Table 1. The antibiotic susceptibility of the Acinetobacter baumannii isolate.
| Antibiotic | MIC | Susceptibility |
|---|---|---|
| Ampicillin/sulbactam | ≥32 | R |
| Ceftazidime | 4 | R |
| Ciprofloxacin | ≥4 | R |
| Colistin | ≤0.5 | S |
| Cefepime | ≥64 | R |
| Cefotaxime | 32 | I |
| Gentamicin | ≤1 | S |
| Imipenem | ≥16 | R |
| Levofloxacin | ≥8 | R |
| Meropenem | ≥16 | R |
| Minocycline | ≤1 | S |
| Piperacillin | ≥128 | R |
| Piperacillin/tazobactam | ≥128 | R |
| Trimethoprim/sulfamethoxazole | 160 | R |
| Tigecycline | ≤0.5 | S |
MIC, minimum inhibitory concentration; R, resistant; I, intermediate resistance; S, susceptible
On the 6th day after admission, his vital signs stabilized and the fever subsided. Follow-up chest radiography and CT showed improved consolidation. No A. baumannii grew in the follow-up blood cultures. On the 26th day after admission, because of colistin-induced nephropathy, the antibiotics were switched to tigecycline. On the 48th day after admission, the chest radiograph and chest CT showed that the pneumonia had improved (Fig.1B and 2B). The patient was discharged on day 56 and followed as an outpatient with no significant complications.
Discussion
Organisms in the genus Acinetobacter were isolated and first described by the microbiologist Beijerinck in 1911 [2]. A. baumannii is an aerobic Gram-negative coccobacillus that occurs in fresh water and soil [1]. A. baumannii is regarded as a significant pathogen causing nosocomial pneumonia [1,2]. By contrast, community-onset A. baumannii infection is uncommon and usually occurs in patients with solid tumor, prior receipt of antimicrobial agents within 30 days and central venous catheterization [5]. Especially CAP caused by A. baumannii tends to progress rapidly to a fulminate clinical course [1,2,3,6]. Recently, the incidence of carbapenem-resistant A. baumannii has increased [7,8].
Five cases of CAP caused by A. baumannii have been reported in Korea; in all cases, A. baumannii was identified in sputum cultures, blood cultures, or biopsy. Fortunately, the A. baumannii was susceptible to piperacillin/tazobactam, ceftazidime, cefepime, meropenem, and tobramycin [9,10,11,12,13]. In our case, susceptibility tests showed that the A. baumannii was resistant to piperacillin/tazobactam, ceftazidime, cefepime, imipenem, meropenem, and tobramycin but susceptible to colistin, tigecycline, minocycline, amikacin, and gentamicin.
Although this is the first reported case of CAP caused by MDR A. baumannii in Korea, it is expected to increase with the incidence of MDR A. baumannii. MDR A. baumannii should be considered if empirical antibiotics do not work in CAP. Recommended empirical antibiotics for CAP caused by A. baumannii include anti-pseudomonal penicillins, ciprofloxacin, aminoglycoside, and imipenem [14,15,16]. However, A. baumannii resistance to carbapenem has increased recently, limiting the treatment options [4]. MDR A. baumannii has few treatment options and a poor prognosis, although colistin, tigecycline, or minocycline are options [16,17,18]. Our patient responded to colistin (with minocycline) and tigecycline, despite a long treatment period.
This case is an example of the successful treatment of CAP caused by MDR A. baumannii, in a patient without medical comorbidities, and is the first reported case of CAP caused by MDR A. baumannii in Korea. MDR A. baumannii should be considered as a pathogen causing community-acquired pneumonia, and further epidemiological studies are needed to control infection.
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 2.Towner KJ. Acinetobacter: an old friend, but a new enemy. J Hosp Infect. 2009;73:355–363. doi: 10.1016/j.jhin.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 3.Leung WS, Chu CM, Tsang KY, Lo FH, Lo KF, Ho PL. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest. 2006;129:102–109. doi: 10.1378/chest.129.1.102. [DOI] [PubMed] [Google Scholar]
- 4.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang SJ, Kang CI, Park SY, Ha YE, Joo EJ, Chung DR, Peck KR, Lee NY, Song JH. Epidemiology and clinical features of community-onset Acinetobacter baumannii infections. Infect control Hosp Epidemilo. 2012;33:1053–1055. doi: 10.1086/667739. [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Karveli EA, Kelesidis I, Kelesidis T. Community-acquired Acinetobacter infections. Eur J Clin Microbiol Infect Dis. 2007;26:857–868. doi: 10.1007/s10096-007-0365-6. [DOI] [PubMed] [Google Scholar]
- 7.Richet HM, Mohammed J, McDonald LC, Jarvis WR. Building communication networks: international network for the study and prevention of emerging antimicrobial resistance. Emerg Infect Dis. 2001;7:319–322. doi: 10.3201/eid0702.010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, Park YJ, Yong D, Jeong SH, Chong Y KONSAR Group. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med J. 2011;52:793–802. doi: 10.3349/ymj.2011.52.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han SH, Na DJ, Yoo YW, Kim DG, Moon YR, Moon KM, Lee YD, Cho YS, Han MS, Yoon HJ. A case of probable community acquired Acinetobacter baumannii pneumonia. Tuberc Respir Dis (Seoul) 2007;63:273–277. [Google Scholar]
- 10.Kang CI, Song JH, Oh WS, Ko KS, Chung DR, Peck KR Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group. Clinical outcomes and risk factors of community-acquired pneumonia caused by gram-negative bacilli. Eur J Clin Microbiol Infect Dis. 2008;27:657–661. doi: 10.1007/s10096-008-0485-7. [DOI] [PubMed] [Google Scholar]
- 11.Na JY, Min BW, Chung SH, Kim MY, Lee YJ, Park JT, Kim HS. A probable community-acquired pneumonia due to Acinetobacter baumannii infection presenting the positive pneumothorax test at autopsy. Korean J Leg Med. 2010;34:125–128. [Google Scholar]
- 12.Lee Y, Yoon S, Lee HS, Kang BH, An J, Kim YJ, Hong SB, Choi SH. A case of severe community-acquired Acinetobacter baumannii pneumonia with bacteremia. Infect Chemother. 2012;44:71–74. [Google Scholar]
- 13.Oh YJ, Song SH, Baik SH, Lee HH, Han IM, Oh DH. A case of fulminant community-acquired Acinetobacter baumannii pneumonia in Korea. Korean J Intern Med. 2013;28:486–490. doi: 10.3904/kjim.2013.28.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong CW, Lye DC, Khoo KL, Chua GS, Yeoh SF, Leo YS, Tambyah PA, Chua AC. Severe community-acquired Acinetobacter baumannii pneumonia: an emerging highly lethal infectious disease in the Asia-Pacific. Respirology. 2009;14:1200–1205. doi: 10.1111/j.1440-1843.2009.01630.x. [DOI] [PubMed] [Google Scholar]
- 15.Anstey NM, Currie BJ, Withnall KM. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin Infect Dis. 1992;14:83–91. doi: 10.1093/clinids/14.1.83. [DOI] [PubMed] [Google Scholar]
- 16.Kempf M, Rolain JM. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents. 2012;39:105–114. doi: 10.1016/j.ijantimicag.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Lee CS, Doi Y. Therapy of infections due to crbapenem-rsistant Gram-ngative pthogens. Infect Chemother. 2014;46:149–164. doi: 10.3947/ic.2014.46.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zavascki AP, Bulitta JB, Landersdorfer CB. Combination therapy for carbapenem-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther. 2013;11:1333–1353. doi: 10.1586/14787210.2013.845523. [DOI] [PubMed] [Google Scholar]