Abstract
Recently, carboxamide-type synthetic cannabinoids have been distributed globally as new psychoactive substances (NPS). Some of these compounds possess asymmetric carbon, which is derived from an amide moiety composed of amino acid derivatives (i.e., amides or esters of amino acids). In this study, we synthesized both enantiomers of synthetic cannabinoids, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(2-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA 2-fluorobenzyl isomer), N-(1-amino-1-oxo-3-phenylpropan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide (APP-CHMINACA), ethyl [1-(5-fluoropentyl)-1H-indazole-3-carbonyl]valinate (5F-EMB-PINACA), ethyl [1-(4-fluorobenzyl)-1H-indazole-3-carbonyl]valinate (EMB-FUBINACA), and methyl 2-[1-(4-fluorobenzyl)-1H-indole-3-carboxamido]-3,3-dimethylbutanoate (MDMB-FUBICA), which were reported as NPS found in Europe from 2014 to 2015, to evaluate their activities as CB1/CB2 receptor agonists. With the exception of (R) MDMB-FUBICA, all of the tested enantiomers were assumed to be agonists of both CB1 and CB2 receptors, and the EC50 values of the (S)-enantiomers for the CB1 receptors were about five times lower than those of (R)-enantiomers. (R) MDMB-FUBICA was shown to function as an agonist of the CB2 receptor, but lacks CB1 receptor activity. To the best of our knowledge, this is the first report to show that the (R)-enantiomers of the carboxamide-type synthetic cannabinoids have the potency to activate CB1 and CB2 receptors. The findings presented here shed light on the pharmacological properties of these carboxamide-type synthetic cannabinoids in forensic cases.
Keywords: Carboxamide-type synthetic cannabinoid, Enantiomeric difference in activity, CB1/CB2 receptor agonist, AB-FUBINACA 2-fluorobenzyl isomer, APP-CHMINACA, 5F-EMB-PINACA
Introduction
In recent years, several new psychoactive substances (NPS) have been newly identified from illicit drug products, one after another, and have been abused as legal alternatives to scheduled drugs [1]. One of the most popular classes of NPS is synthetic cannabinoids, which are recreationally used as substitutes for Cannabis sativa. Forensic cases of synthetic cannabinoid use have increased recently; the adverse effects of indazole-carboxamide-type synthetic cannabinoids, especially, present severe social problems [2].
In a previous study, we developed a method to separate enantiomers of synthetic cannabinoids by liquid chromatography–mass spectrometry (LC–MS) [3]. In the case of NPS classified into cathinone and phenethylamine groups, several reports have concluded that (S)-enantiomers are more potent than (R)-enantiomers [4–7]. Among the carboxamide-type synthetic cannabinoids, some compounds possess asymmetric carbon, which is derived from an amide moiety composed of amino acid derivatives (i.e., amides or esters of amino acids). These classes of compounds were first developed by Pfizer as potential therapeutic drugs; however, their patent only includes (S)-enantiomers, even though the structures of these compounds have chiral centers [8]. Some previous studies reported the cannabimimetic activities of the chiral synthetic cannabinoids, but they all investigated only the (S)-enantiomers, and thus the pharmaceutical activities of the (R)-enantiomers remain unknown [9].
In this study, we synthesized both enantiomers of N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(2-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA 2-fluorobenzyl isomer), N-(1-amino-1-oxo-3-phenylpropan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide (APP-CHMINACA), ethyl [1-(5-fluoropentyl)-1H-indazole-3-carbonyl]valinate (5F-EMB-PINACA), ethyl [1-(4-fluorobenzyl)-1H-indazole-3-carbonyl]valinate (EMB-FUBINACA), and methyl 2-[1-(4-fluorobenzyl)-1H-indole-3-carboxamido]-3,3-dimethylbutanoate (MDMB-FUBICA) to evaluate their potency to activate CB1 and CB2 cannabinoid receptors.
Materials and methods
Reagents
Methyl 1H-indazole-3-carboxylate and (bromomethyl)cyclohexane were purchased from Sigma-Aldrich (St. Louis, MO, USA); 1-bromo-5-fluoropentane from Fluorochem Ltd. (Hadfield, UK); d-valine ethyl ester hydrochloride, l-valine ethyl ester hydrochloride, and d-valine amide hydrochloride from Combi-Blocks Inc. (San Diego, CA, USA); d-phenylalanine amide hydrochloride from Watanabe Chemical Industries Ltd. (Hiroshima, Japan). All other reagents used in this study were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance (NMR) spectra were recorded using an ECZS-400 spectrometer (JEOL Resonance, Tokyo, Japan) with dimethyl sulfoxide (DMSO)-d 6 as the solvent. The chemical shifts δ were recorded in ppm relative to tetramethylsilane (1H: δ = 0 ppm, 13C: δ = 0 ppm) or the solvent (13C: δ = 39.5 ppm) as an internal standard. The compounds were assigned by 1H NMR, 13C NMR, distortionless enhancement by polarization transfer, 1H-13C heteronuclear multiple quantum coherence, 1H-13C heteronuclear multiple-bond correlation, and 1H-1H correlation spectroscopy.
Chiral chromatography
Chirality of the compounds was confirmed by LC–MS as described previously [3]. A CHIRALPAK AZ-3R column (3.0 μm particle size, 150 × 2.1 mm i.d.) (Daicel Corporation, Osaka, Japan) was used, and the mobile phase was composed of H2O/acetonitrile (55:45, v/v) under isocratic conditions. The flow rate of the mobile phase was 0.3 mL/min, and the injection volume was 1 μL. The column temperature was 40 °C. The stock standard solutions were prepared as described previously [3]. When necessary, the sample solutions and the stock standard solutions were diluted by the mobile phase for the liquid chromatography–high-resolution-mass spectrometry (LC–HR-MS) analysis [3].
Chemical synthesis
The synthetic pathway of these enantiomers is shown in Fig. 1. All of the target compounds were made from methyl 1H-indazole-3-carboxylate (compound 1) or methyl 1H-indole-3-carboxylate (compound 2). The enantiomers of the target compounds were synthesized by a slightly modified version of a previously described method [3, 10]. Compound 1 or 2 was N-alkylated by alkyl halide under basic conditions and yielded compounds that were deprotected by hydrolysis of the methyl group. The target compounds were synthesized by chlorination of the carboxylic acids by oxalyl chloride and then amidation with the amino acid derivatives. The chemical structures of the compounds dealt with in this study are shown in Fig. 2.
Fig. 1.
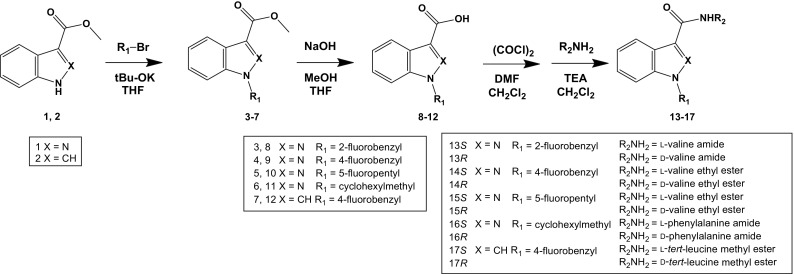
Procedures for synthesizing the enantiomers dealt with in this study. tBu-OK potassium tert-butoxide, THF tetrahydrofuran, DMF N,N-dimethylformamide, (COCl) 2 oxalyl chloride, TEA triethylamine, MeOH methanol
Fig. 2.
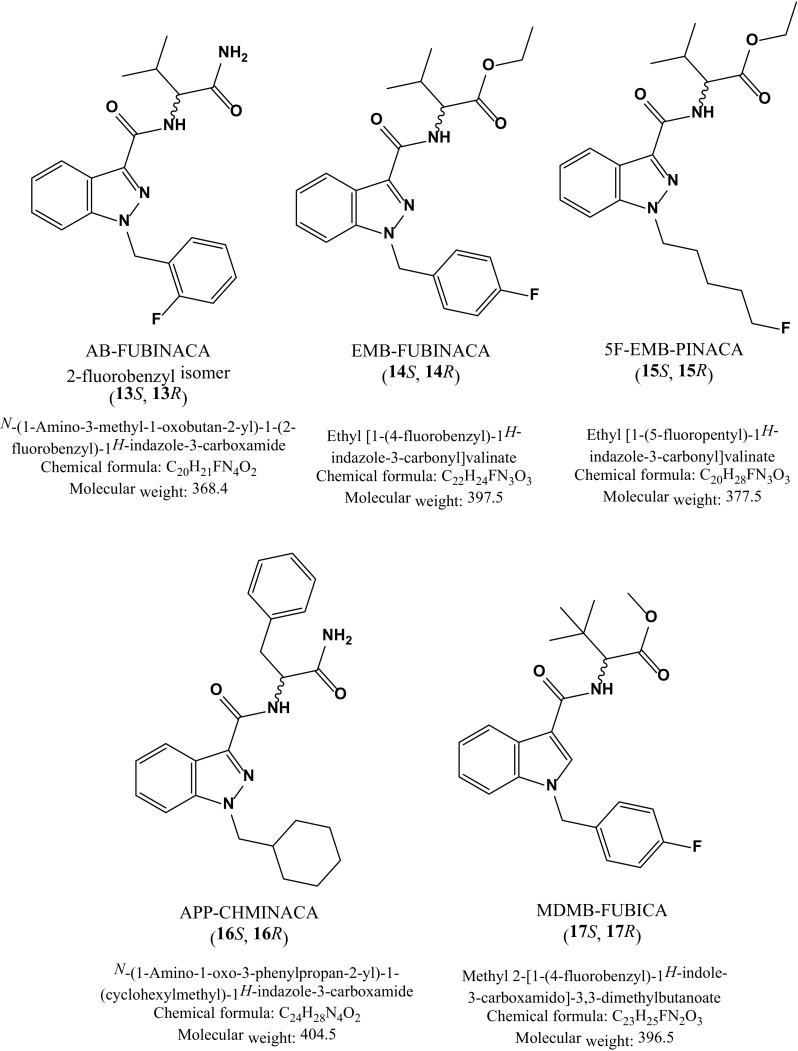
Chemical structures of the target compounds in this study
N-Alkylation of methyl 1H-indazole/indole-3-carboxylate
Compound 5 was synthesized as described previously [3]. Compounds 3, 4, 6, and 7 were prepared in the same manner, except for the starting compounds. To a solution of compound 1 or 2 in tetrahydrofuran (THF), potassium tert-butoxide (t-BuOK) (1.2 equivalent) was added. To the flask containing the solution, alkyl halide (1.5 equivalent) was added and stirred at room temperature for more than 12 h. Ethyl acetate and distilled water were added to the solution, and the organic layer was separated from the aqueous layer. The organic layer was then washed with saturated aqueous NaCl and dried with anhydrous MgSO4. The solution was filtered, and the solvent was removed under a vacuum. The residue was purified by an Isolela Speckt System (Biotage, Tokyo, Japan) on a SNAP Ultra column (Biotage) with ethyl acetate in n-hexane as the mobile phase. The solvent of the obtained fraction was removed under a vacuum to yield compounds 3, 4, 5, 6 or 7.
Hydrolysis of methyl N-alkyl-1H-indazole-3-carboxylate
Synthesis of compound 10 was performed as described previously [3]. Compounds 8, 9, and 11 were synthesized similarly, except for the starting compounds. To a flask containing compounds 3, 4, or 6, THF, ethanol, and 1 M NaOH(aq) (approximately 2.5 mL/1 mmol of the material compounds) were added and stirred at room temperature for more than 16 h. After the reaction, the organic solvents were removed under a vacuum. Ethyl acetate and 20% Na2CO3 in H2O were added to the solution and stirred, and the solution was allowed to separate. The organic layer was extracted by 20% Na2CO3 in H2O again, and the aqueous layers were combined. The organic layer was extracted twice with 1 M NaOH in H2O. The aqueous layers were combined and neutralized with concentrated HCl, extracted with ethyl acetate twice, and then the organic layer was washed twice with brine. Anhydrous MgSO4 was added to the organic layer and removed under a vacuum to yield compounds 8, 9, or 11.
Hydrolysis of methyl N-alkyl-1H-indole-3-carboxylate
Compound 12 was synthesized by the hydrolysis of compound 7. To a flask containing compound 7, THF, ethanol, methanol, and 10 M NaOH(aq) (approximately 2 mL/1 mmol of compound 7) were added and stirred at room temperature for 2.5 h. After the reaction, the organic solvents were removed under a vacuum. Ethyl acetate was added to the solution and mixed well, and the solution was allowed to separate. The organic layer was extracted twice with 1 M NaOH(aq), and the aqueous layers were then combined. The aqueous layer was neutralized with HCl and extracted with ethyl acetate twice. The organic layer was then washed twice with brine. Subsequently, anhydrous MgSO4 was added to the combined organic layer and removed under a vacuum to yield compound 12.
(S)-N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-(2-fluorobenzyl)-1H-indazole-3-carboxamide (13S)
To a flask containing compound 8 in dichloromethane (20 mL/1 mmol of compound 8), N,N-dimethylformamide (20 μL/1 mmol) and oxalyl chloride (2.5 equivalent) were added and stirred at room temperature for 30 min. The solvent was then removed under a vacuum to obtain 1-(2-fluorobenzyl)-1H-indazole-3-carbonyl chloride as a hazy oily residue. The residue was dissolved in dichloromethane (10 mL), and l-valine amide hydrochloride (1.1 equivalent) was added. Triethylamine (1 mmol) was added to the mixture and then the resulting solution was stirred at room temperature for 90 min. The solvent was removed under reduced pressure and dissolved in ethyl acetate. The solvent was separated with water and washed with 0.1 M HCl, saturated NaHCO3, and saturated NaCl. Drying with MgSO4 and removal of the solvent afforded compound 13 S as a fibrous white solid.
1H NMR (DMSO-d 6): δ 8.19 (1H, dd, J = 8.0, 1.0 Hz), 7.77 (1H, d, J = 8.5 Hz), 7.69 (1H, d, J = 9.0 Hz), 7.65 (1H, s), 7.48 (1H, t-like), 7.43–7.34 (1H, m), 7.30 (1H, dd, J = 8.0, 7.0 Hz), 7.26–7.18 (2H, m), 7.17–7.09 (2H, m), 5.84 (2H, s), 4.42 (1H, dd, J = 9.0, 6.0 Hz), 2.09 (1H, m), 0.94 (3H, d, J = 7.0 Hz), 0.89 (3H, d, J = 7.0 Hz). 13C NMR (DMSO-d 6): δ 172.5, 161.1, 159.9 (d, J = 246 Hz), 140.8, 137.2, 130.1 (d, J = 8 Hz), 129.7 (d, J = 4 Hz), 127.0, 124.7 (d, J = 4 Hz), 123.5 (d, J = 14 Hz), 122.7, 122.1, 121.8, 115.5 (d, J = 21 Hz), 110.4, 56.8, 46.5 (d, J = 3 Hz), 31.2, 19.3, 17.9.
(R)-N-(1-Amino-3-methyl-1-oxobutan-2-yl)-1-(2-fluorobenzyl)-1H-indazole-3-carboxamide (13R)
Subjecting compound 8 to a procedure similar to that for 13 S, but substituting d-valine amide hydrochloride for l-valine amide, yielded 13 R as a fibrous white solid.
1H NMR (DMSO-d 6): δ 8.20 (1H, d, J = 8.0 Hz), 7.77 (1H, d, J = 8.5 Hz), 7.71 (1H, d, J = 9.0 Hz), 7.66 (1H, s), 7.48 (1H, t-like), 7.42–7.34 (1H, m), 7.30 (1H, t-like), 7.28–7.20 (2H, m), 7.18–7.10 (2H, m), 5.84 (2H, s), 4.42 (1H, dd, J = 9.0, 6.0 Hz), 2.10 (1H, m), 0.94 (3H, d, J = 7.0 Hz), 0.89 (3H, d, J = 7.0 Hz). 13C NMR (DMSO-d 6): δ 172.5, 161.2, 159.9 (d, J = 247 Hz), 140.8, 137.2, 130.2 (d, J = 9 Hz), 129.8 (d, J = 4 Hz), 127.0, 124.7 (d, J = 3 Hz), 123.6 (d, J = 15 Hz), 122.7, 122.1, 121.8, 115.6 (d, J = 21 Hz), 110.5, 56.8, 46.5 (d, J = 3 Hz), 31.2, 19.4, 18.0.
Ethyl [1-(4-fluorobenzyl)-1H-indazole-3-carbonyl]-l-valinate (14S)
Subjecting compound 9 to a procedure similar to that for 13 S, but substituting l-valine ethyl ester hydrochloride for l-valine amide, yielded 14 S as a clear oily residue.
1H NMR (DMSO-d 6): δ 8.15 (1H, d, J = 8.5 Hz), 8.12 (1H, d, J = 8.0 Hz), 7.80 (1H, d, J = 8.5 Hz), 7.47 (1H, ddd-like), 7.37–7.32 (2H, m), 7.30 (1H, t-like), 7.20–7.14 (2H, m), 5.78 (2H, s), 4.45 (1H, dd, J = 8.0, 7.0 Hz), 4.22–4.12 (2H, m), 2.30–2.22 (1H, m), 1.22 (3H, t, J = 7.0 Hz), 0.97 (6H, t-like). 13C NMR (DMSO-d 6): δ 171.5, 161.9, 161.6 (d, J = 244 Hz), 140.5, 136.9, 133.0 (d, J = 3 Hz), 129.5 (d, J = 8 Hz), 127.0, 122.8, 122.4, 121.7, 115.5 (d, J = 21 Hz), 110.6, 60.6, 57.4, 51.6, 29.9, 19.0, 18.7, 14.1.
Ethyl [1-(4-fluorobenzyl)-1H-indazole-3-carbonyl]-d-valinate (14R)
Subjecting compound 9 to a procedure similar to that for 13 S, but substituting d-valine ethyl ester hydrochloride for l-valine amide, yielded 14 R as a clear oily residue.
1H NMR (DMSO-d 6): δ 8.15 (1H, d, J = 8.5 Hz), 8.12 (1H, d, J = 8.5 Hz), 7.80 (1H, d, J = 8.5 Hz), 7.46 (1H, ddd-like), 7.37–7.32 (2H, m), 7.29 (1H, t-like), 7.20–7.14 (2H, m), 5.78 (2H, s), 4.45 (1H, dd, J = 8.5, 6.5 Hz), 4.22–4.12 (2H, m), 2.30–2.22 (1H, m), 1.22 (3H, t, J = 7.0 Hz), 0.97 (6H, t-like). 13C NMR (DMSO-d 6): δ 171.5, 161.8, 161.6 (d, J = 244 Hz), 140.5, 136.9, 133.0 (d, J = 3 Hz), 129.5 (d, J = 8 Hz), 127.0, 122.8, 122.4, 121.7, 115.5 (d, J = 21 Hz), 110.6, 60.6, 57.4, 51.6, 29.9, 19.0, 18.7, 14.1.
Ethyl [1-(5-fluoropentyl)-1H-indazole-3-carbonyl]-l-valinate (15S)
Subjecting compound 10 to a procedure similar to that for 13 S, but substituting l-valine ethyl ester hydrochloride for l-valine amide, yielded 15 S as a clear oily residue.
1H NMR (DMSO-d 6): δ 8.14 (1H, d, J = 8.0 Hz), 7.99 (1H, d, J = 8.5 Hz), 7.81 (1H, d, J = 8.5 Hz), 7.47 (1H, ddd-like), 7.29 (1H, t-like), 4.54 (2H, t, J = 7.0 Hz), 4.47 (1H, t, J = 6.0 Hz), 4.42 (1H, dd, J = 8.5, 6.5 Hz), 4.35 (1H, t, J = 6.0 Hz), 4.22–4.12 (2H, m), 2.31–2.21 (1H, m), 1.97–1.88 (2H, m), 1.75–1.61 (2H, m), 1.41–1.31 (2H, m), 1.22 (3H, t, J = 7.0 Hz), 0.98 (3H, d, J = 4.5 Hz), 0.96 (3H, d, J = 4.0 Hz). 13C NMR (DMSO-d 6): δ 171.5, 161.9, 140.6, 136.2, 126.7, 122.6, 122.1, 121.6, 110.5, 83.6 (d, J = 162 Hz), 60.6, 57.2, 48.6, 30.0, 29.3 (d, J = 19 Hz), 29.0, 22.0 (d, J = 6 Hz), 19.0, 18.6, 14.1.
Ethyl [1-(5-fluoropentyl)-1H-indazole-3-carbonyl]-d-valinate (15R)
Subjecting compound 10 to a procedure similar to that for 13 S, but substituting d-valine ethyl ester hydrochloride for l-valine amide, yielded 15 R as a clear oily residue.
1H NMR (DMSO-d 6): δ 8.14 (1H, d, J = 7.5 Hz), 7.99 (1H, d, J = 8.0 Hz), 7.81 (1H, d, J = 8.5 Hz), 7.47 (1H, ddd-like), 7.29 (1H, t-like), 4.53 (2H, t, J = 7.0 Hz), 4.47 (1H, t, J = 6.0 Hz), 4.43 (1H, dd, J = 8.5, 6.5 Hz), 4.35 (1H, t, J = 6.0 Hz), 4.24–4.10 (2H, m), 2.31–2.20 (1H, m), 1.97–1.88 (2H, m), 1.75–1.61 (2H, m), 1.41–1.31 (2H, m), 1.22 (3H, t, J = 7.0 Hz), 0.98 (3H, d, J = 4.0 Hz), 0.96 (3H, d, J = 4.5 Hz). 13C NMR (DMSO-d 6): δ 171.5, 161.9, 140.6, 136.2, 126.7, 122.6, 122.1, 121.6, 110.5, 83.6 (d, J = 162 Hz), 60.6, 57.2, 48.6, 30.0, 29.3 (d, J = 19 Hz), 29.0, 22.0 (d, J = 6 Hz), 19.0, 18.6, 14.1.
(S)-N-(1-Amino-1-oxo-3-phenylpropan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide (16S)
Subjecting 11 to a procedure similar to that for 13 S, but substituting l-phenylalanine amide hydrochloride for l-valine amide yielded 16 S as a crystalline white solid.
1H NMR (DMSO-d 6): δ 8.11 (1H, d, J = 8.0 Hz), 7.90 (1H, d, J = 8.0 Hz), 7.76 (1H, d, J = 8.5 Hz), 7.67 (1H, s), 7.45–7.41 (1H, m), 7.26–7.14 (7H, m), 4.78–4.72 (1H, m), 4.37–4.26 (2H, m), 3.19–3.05 (2H, m), 1.99–1.87 (1H, m), 1.64–1.60 (3H, m), 1.47 (2H, t-like), 1.23–1.10 (3H, m), 1.07–0.97 (2H, m). 13C NMR (DMSO-d 6): δ 172.6, 161.3, 141.1, 137.6, 136.4, 129.3, 128.0, 126.5, 126.3, 122.4, 121.8, 121.6, 110.6, 54.5, 53.2, 38.4, 37.7, 30.0, 25.8, 25.2, 25.1.
(R)-N-(1-Amino-1-oxo-3-phenylpropan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide (16R)
Subjecting compound 11 to a procedure similar to that for 16 S, but substituting d-phenylalanine amide hydrochloride for l-phenylalanine amide hydrochloride, yielded 16 R as a crystalline white solid.
1H NMR (DMSO-d 6): δ 8.11 (1H, d, J = 8.0 Hz), 7.90 (1H, d, J = 8.0 Hz), 7.76 (1H, d, J = 8.5 Hz), 7.66 (1H, s), 7.45–7.41 (1H, m), 7.26–7.14 (7H, m), 4.78–4.72 (1H, m), 4.37–4.26 (2H, m), 3.19–3.05 (2H, m), 1.97–1.88 (1H, m), 1.64–1.60 (3H, m), 1.47 (2H, t-like), 1.24–1.10 (3H, m), 1.07–0.97 (2H, m). 13C NMR (DMSO-d 6): δ 172.6, 161.3, 141.1, 137.6, 136.4, 129.3, 128.0, 126.5, 126.3, 122.3, 121.8, 121.6, 110.6, 54.5, 53.2, 38.4, 37.7, 30.0, 25.8, 25.2, 25.1.
Methyl (S)-2-[1-(4-fluorobenzyl)-1H-indole-3-carboxamido]-3,3-dimethylbutanoate (17S)
Subjecting 12 to a procedure similar to that for 13 S, but substituting l-tert-leucine methyl ester hydrochloride for l-valine amide yielded 17 S as a white powder.
1H NMR (DMSO-d 6): δ 8.50 (1H, s), 8.11 (1H, d, J = 8.0 Hz), 7.71 (1H, d, J = 8.5 Hz), 7.52 (1H, d, J = 8.5 Hz), 7.34–7.31 (2H, m), 7.20–7.11 (4H, m, overlapped), 5.47 (2H, s), 4.50 (1H, d, J = 8.5 Hz), 3.66 (3H, s), 1.04 (9H, s). 13C NMR (DMSO-d 6): δ 172.0, 164.3, 161.6 (d, J = 244 Hz), 136.0, 133.8 (d, J = 3 Hz), 132.1, 129.2 (d, J = 8 Hz), 126.9, 122.2, 121.3, 120.9, 115.5 (d, J = 22 Hz), 110.6, 109.3, 60.0, 51.4, 48.8, 33.8, 26.8.
Methyl (R)-2-[1-(4-fluorobenzyl)-1H-indole-3-carboxamido]-3,3-dimethylbutanoate (17R)
Subjecting compound 12 to a procedure similar to that for 17 S, but substituting d-tert-leucine methyl ester hydrochloride for l-tert-leucine methyl ester hydrochloride, yielded 17 R as a white powder.
1H NMR (DMSO-d 6): δ 8.50 (1H, s), 8.11 (1H, d, J = 7.0 Hz), 7.71 (1H, d, J = 8.5 Hz), 7.53 (1H, d, J = 8.0 Hz), 7.34–7.31 (2H, m), 7.20–7.13 (4H, m, overlapped), 5.47 (2H, s), 4.50 (1H, d, J = 8.5 Hz), 3.66 (3H, s), 1.04 (9H, s). 13C NMR (DMSO-d 6): δ 172.0, 164.3, 161.6 (d, J = 244 Hz), 136.0, 133.7 (d, J = 3 Hz), 132.1, 129.2 (d, J = 8 Hz), 126.9, 122.2, 121.2, 120.9, 115.5 (d, J = 21 Hz), 110.6, 109.3, 60.0, 51.4, 48.7, 33.8, 26.8.
In vitro assays to evaluate the CB1/CB2 receptor activities
For the evaluation of CB1/CB2 cannabinoid receptor activity, [35S]GTPγS binding assays were performed. These assays were performed at ADME and Tox. Research Institute, Sekisui Medical Co., Ltd. (Tokai-mura, Ibaraki, Japan). The assay conditions were as described previously [11], except for the tested concentration levels of the compounds ranging from 1 × 10−11 to 1 × 10−4 M. Agonistic activities (EC50 value: concentration showing 50% response) of the test compound to the cannabinoid receptors CB1 and CB2 were measured.
Results and discussion
In this study, we synthesized both (S)- and (R)-enantiomers of synthetic cannabinoids to evaluate their pharmacological properties. All target compounds were reported in an EMCDDA-Europol joint report to be NPS sold and recreationally used in Europe [12, 13]. NMR data for the enantiomers were similar, as shown in the section on materials and methods above, and the results are in good agreement with the values expected for their structures.
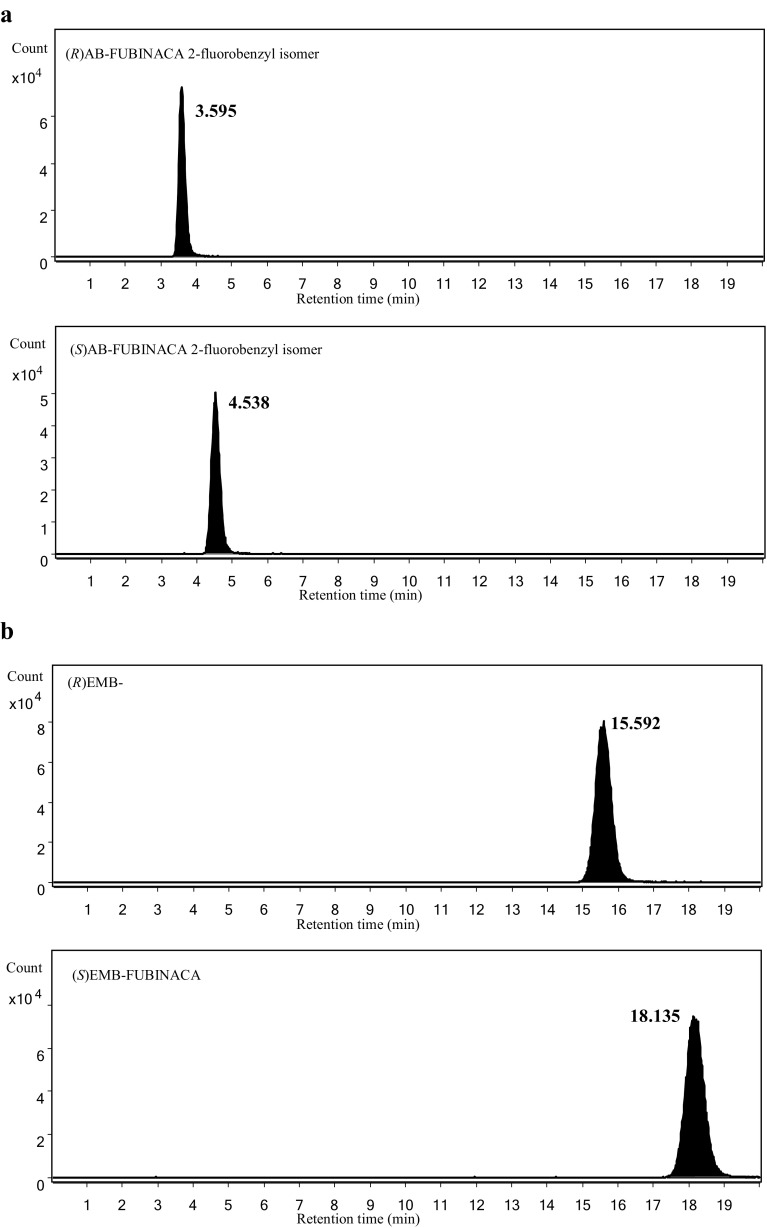
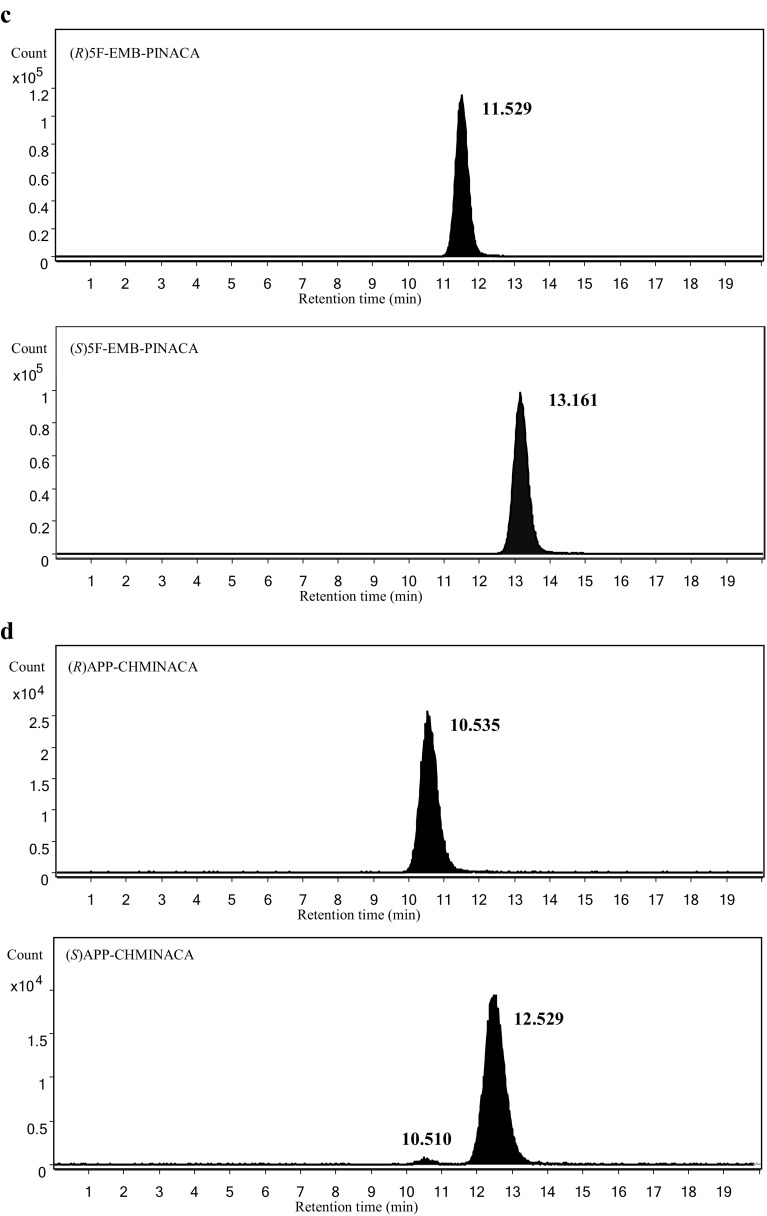
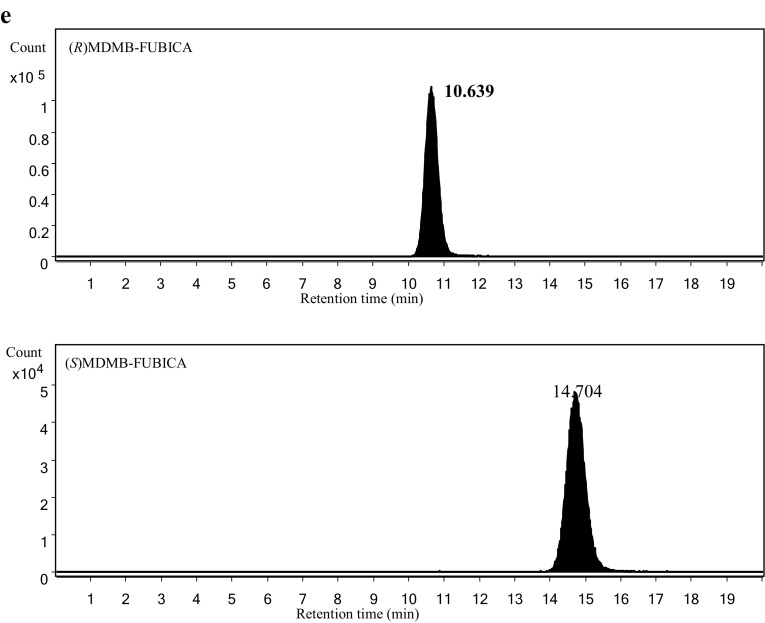
Figure 3 shows the extracted ion chromatograms for the enantiomers of the target compounds (1 μg/mL solution for both enantiomers) obtained by LC–HR-MS using the method that we developed previously [3]. By the previously developed method, all of the enantiomers of the tested compounds were separated, and no enantio-impurities were found in their chromatograms, except for (S) APP-CHMINACA. Approximately less than 2% of (R) APP-CHMINACA was confirmed in the extracted ion chromatogram of (S) APP-CHMINACA as shown in Fig. 3. However, because (R)-enantiomer was much less effective than (S)-enantiomer to both CB1 and CB2 receptors, it is suspected that the effect of contaminated (R)-enantiomer on the EC50 value was almost negligible.
Fig. 3.
Extracted ion chromatograms of a AB-FUBINACA 2-fluorobenzyl isomer at m/z 369.1721 (Δ = ±0.5 mDa), b EMB-FUBINACA at m/z 398.1874 (Δ = ±0.5 mDa), c 5F-EMB-FUBINACA at m/z 378.2187 (Δ = ±0.5 mDa), d APP-CHMINACA at m/z 405.2285 (Δ = ±0.5 mDa), and e MDMB-FUBICA at m/z 397.1922 (Δ = ±0.5 mDa), as a function of enantiomers, obtained by liquid chromatography–high-resolution mass spectrometry
Table 1 shows the results of the [35S]GTPγS binding assays performed in this study. Each compound functioned as both a CB1 and a CB2 receptor agonist, except for (R) MDMB-FUBICA. The EC50 values of (S)-enantiomers for the CB1 receptors were at least one-fifth of or less than those of (R)-enantiomers, which indicates that (S)-enantiomers are more potent CB1 receptor agonists than (R)-enantiomers. The (S)-enantiomer of APP-CHMINACA could activate CB1 at a concentration 134 times lower than that for the (R)-enantiomer. For MDMB-FUBICA, the CB1 receptor activity was not confirmed for the (R)-enantiomer even at the maximum tested concentration, but the EC50 of the (S)-enantiomer was 9.72 × 10−9 M; the difference in the EC50 levels between the enantiomers of MDMB-FUBICA to activate the CB1 receptor was more than 10,000-fold, which indicates that the activities for the CB1 receptor are markedly different depending on the chirality of the compounds. In contrast, we could not observe a clear relevance between EC50 values for the CB2 receptor and chirality of the compounds. (R) APP-CHMINACA and (R) MDMB-FUBICA lacked the potential to be CB1 receptor agonists, but both of their EC50 values for the CB2 receptor were less than 10−6 M; all of the target compounds were strong agonists for the CB2 receptor, irrespective of their chirality.
Table 1.
Activities of enantiomers of each compound at human CB1 and CB2 receptors
| Compound name | Human CB1 receptor | Human CB2 receptor | Selective indexb (CB1/CB2) | ||
|---|---|---|---|---|---|
| EC50 (M) | S/R ratioa | EC50 (M) | S/R ratioa | ||
| (S)AB-FUBINACA 2-fluorobenzyl isomer | 2.92 × 10−9 | 0.21 | 2.44 × 10−9 | 3.9 | 1.20 |
| (R)AB-FUBINACA 2-fluorobenzyl isomer | 1.41 × 10−8 | 6.27 × 10−10 | 22.5 | ||
| (S)APP-CHMINACA | 2.51 × 10−7 | 0.0074 | 8.09 × 10−9 | 0.017 | 31.0 |
| (R)APP-CHMINACA | 3.37 × 10−5 | 4.90 × 10−7 | 68.8 | ||
| (S)EMB-FUBINACA | 4.58 × 10−10 | 0.015 | 2.14 × 10−9 | 2.5 | 0.214 |
| (R)EMB-FUBINACA | 3.07 × 10−8 | 8.63 × 10−10 | 35.6 | ||
| (S)5F-EMB-PINACA | 4.96 × 10−9 | 0.14 | 6.91 × 10−9 | 4.1 | 0.718 |
| (R)5F-EMB-PINACA | 3.59 × 10−8 | 1.68 × 10−9 | 21.4 | ||
| (S)MDMB-FUBICA | 9.72 × 10−9 | <0.0001 | 1.07 × 10−9 | 0.35 | 9.08 |
| (R)MDMB-FUBICA | >1.00 × 10−4 | 3.10 × 10−9 | >30,000 | ||
| CP55940 | 1.28 × 10−9 | 1.54 × 10−10 | 8.3 | ||
a S/R ratio is the EC50 value ratio of an (S)-enantiomer to the corresponding (R)-enantiomer
bSelective index is EC50 ratio of the CB1 to CB2
As for the CB1/CB2 selectivity, (R)-enantiomers tended to be CB2-selective and were at least 20-fold more active than for CB1 for all of the tested compounds. Interestingly, (R) MDMB-FUBICA functioned only on the CB2 receptor as an agonist, while showing no CB1 activity as mentioned above, although (S) MDMB-FUBICA could activate both of these receptors at nanomolar levels. In several preclinical studies, compounds such as JWH-133 and HU308 were used as CB2-selective ligands [14]. More recently, the synthesis and evaluation of novel CB2-selective agonists have been repeatedly reported. In these pharmacological or medicinal chemistry studies seeking CB2 agonists, the most selective compound showed a selective index of CB1/CB2 of only 100- to 1000-fold. In this study, (R) MDMB-FUBICA was shown to have potency to activate the CB2 receptor signal with an EC50 value of 3.1 × 10−9 M, but had no [35S]GTPγS binding activity for the CB1 receptor, even at 1.0 × 10−4 M, which corresponds to a selective index of more than 3.2 × 104-fold.
The metabolism of MDMB-FUBICA enantiomers has not been reported yet; thus it remains unclear whether (R) MDMB-FUBICA can mimic the function of the CB2 receptor agonist when applied to an animal model to evaluate the effect on the central nervous system. Recently, it was reported that the (S)-enantiomer was mainly detected in herbal products containing chiral carboxamide-type synthetic cannabinoids [3, 15]. The cannabimimetic activities of each enantiomer presented in this study may be helpful to precisely recognize forensic cases associated with these synthetic cannabinoids when evaluated with the quantitative data of a specimen analyzed after enantiomeric separation.
Conclusions
The activities of the enantiomers of five synthetic cannabinoids (AB-FUBINACA 2-fluorobenzyl isomer, APP-CHMINACA, 5F-EMB-PINACA, EMB-FUBINACA, and MDMB-FUBICA) were evaluated by the [35S]GTPγS binding assay. We also confirmed that these enantiomers could be clearly differentiated by chiral-LC–MS. These findings should contribute to better understanding of the forensic cases associated with these synthetic cannabinoids.
Acknowledgements
This study was partly supported by JSPS KAKENHI Grant No. 15K08834.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Zaitsu K, Hayashi Y, Suzuki K, Nakayama H, Hattori N, Takahara R, Kusano M, Tsuchihashi H, Ishii A. Metabolome disruption of the rat cerebrum induced by the acute toxic effects of the synthetic cannabinoid MAM-2201. Life Sci. 2015;137:49–55. doi: 10.1016/j.lfs.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid-related illnesses and deaths. N Engl J Med. 2015;373:103–107. doi: 10.1056/NEJMp1505328. [DOI] [PubMed] [Google Scholar]
- 3.Doi T, Asada A, Takeda A, Tagami T, Katagi M, Kamata H, Sawabe Y. Enantioseparation of the carboxamide-type synthetic cannabinoids N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(5-fluoropentyl)-1H-indazole-3-carboxamide and methyl [1-(5-fluoropentyl)-1H-indazole-3-carbonyl]-valinate in illicit herbal products. J Chromatogr A. 2016;1473:83–89. doi: 10.1016/j.chroma.2016.10.049. [DOI] [PubMed] [Google Scholar]
- 4.Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxymethamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci USA. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calligaro DO, Eldefrawi ME. High affinity stereospecific binding of [3H] cocaine in striatum and its relationship to the dopamine transporter. Membr Biochem. 1987;7:87–106. doi: 10.3109/09687688709039986. [DOI] [PubMed] [Google Scholar]
- 7.Glennon RA, Young R, Martin BR, Dal Cason TA. Methcathione (“cat”): an enantiomeric potency comparison. Pharmacol Biochem Behav. 1995;50:601–606. doi: 10.1016/0091-3057(94)00348-3. [DOI] [PubMed] [Google Scholar]
- 8.Buchler IP, Hayes MJ, Hegde SG, Hockerman SL, Jones DE, Kortum SW, Rico JG, TenBrink RE, Wu KK (2009) Indazole derivatives. US Patent WO2009106980A2
- 9.Banister SD, Moir M, Stuart J, Kevin RC, Wood KE, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Glass M, Connor M, McGregor IS, Kassiou M. Pharmacology of indole and indazole synthetic cannabinoid designer drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem Neurosci. 2015;6:1546–1559. doi: 10.1021/acschemneuro.5b00112. [DOI] [PubMed] [Google Scholar]
- 10.Asada A, Doi T, Tagami T, Takeda A, Sawabe Y. Isomeric discrimination of synthetic cannabinoids by GC–EI-MS: 1-adamantyl and 2-adamantyl isomers of N-adamantyl carboxamides. Drug Test Anal. 2017;9:378–388. doi: 10.1002/dta.2124. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima J, Takahashi M, Uemura N, Seto T, Fukaya H, Suzuki J, Yoshida M, Kusano M, Nakayama H, Zaitsu K. Identification of N,N-bis (1-pentylindol-3-yl-carboxy) naphthylamine (BiPICANA) found in an herbal blend product in the Tokyo metropolitan area and its cannabimimetic effects evaluated by in vitro [35S]GTPγS binding assays. Forensic Toxicol. 2015;33:84–92. doi: 10.1007/s11419-014-0253-6. [DOI] [Google Scholar]
- 12.EMCDDA (2015) EMCDDA–Europol 2014 Annual Report on the implementation of Council Decision 2005/387/JHA. Implementation reports. http://www.emcdda.europa.eu/system/files/publications/1018/TDAN15001ENN.pdf. Accessed May 2017
- 13.EMCDDA (2016) EMCDDA–Europol 2015 Annual Report on the implementation of Council Decision 2005/387/JHA. Implementation reports. http://www.emcdda.europa.eu/system/files/publications/2880/TDAS16001ENN.pdf. Accessed May 2017
- 14.Aghazadeh Tabrizi M, Baraldi PG, Borea PA, Varani K. Medicinal chemistry, pharmacology, and potential therapeutic benefits of cannabinoid CB2 receptor agonists. Chem Rev. 2016;116:519–560. doi: 10.1021/acs.chemrev.5b00411. [DOI] [PubMed] [Google Scholar]
- 15.Andernach L, Pusch S, Weber C, Schollmeyer D, Münster-Müller S, Pütz M, Opatz T. Absolute configuration of the synthetic cannabinoid MDMB-CHMICA with its chemical characteristics in illegal products. Forensic Toxicol. 2016;34:344–352. doi: 10.1007/s11419-016-0321-1. [DOI] [Google Scholar]