Abstract
Background
We conducted a randomized phase III trial comparing tegafur/uracil (UFT) and Polysaccharide-K (PSK) to surgery alone in curatively resected stage II rectal cancer patients.
Methods
Patients were randomly assigned to receive either UFT and PSK or surgery alone in a 1:1 ratio with a minimization method to balance the treatment allocation. The primary end point of this study was the disease-free survival (DFS). The secondary end point was the overall survival (OS).
Results
From October 2011 to February 2013, 111 patients were registered from 62 institutions. The study was prematurely closed due to poor accrual after reaching 20% of its goal. The patients’ characteristics were similar between the UFT and PSK group and the surgery-alone group. The DFS rate was 76.0% at 3 years and 65.1% at 5 years in the UFT and PSK arm and 84.0% at 3 years and 77.2% at 5 years in the surgery-alone arm. The DFS was slightly worse in the UFT + PSK arm than in the surgery-alone arm, but the difference did not reach statistical significance (log rank p = 0.102). The OS rate was 100% at 3 years and 97.9% at 5 years in the UFT + PSK arm, while that was 100% at 3 years and 93.4% at 5 years in the surgery-alone arm. The OS was similar in the UFT + PSK arm and surgery-alone arm (p = 0.533).
Conclusion
The present study suggests that UFT and PSK are not attractive candidates to advance to the next phase III study because the DFS was slightly worse in the UFT and PSK arm than in the surgery-alone arm.
Keywords: Rectal cancer; PSK; UFT, RFS, OS
Introduction
Colorectal cancer is the third-most commonly diagnosed cancer in males and the second-most commonly diagnosed cancer in females, with an estimated 1.4 million new cases and 693,900 deaths occurring in 2012 [1]. Approximately, one-third of these tumors arise in the rectum [2]. Complete resection is essential for achieving a cure of colorectal cancer. Epidemiological studies have reported that postoperative recurrence of rectal cancer is higher than that of colon cancer [3, 4]. For tumors confined to the rectal wall (including T1 and some T2 tumors), local excision can result in good local control while preserving the anal sphincter. However, most tumors are diagnosed at more advanced stages. The perioperative adjuvant treatment for stage III rectal cancer is internationally accepted as a standard treatment with established efficacy, but the usefulness of such treatment for stage II rectal cancer remains controversial [5, 6]. The major Western guidelines recommend adjuvant chemotherapy for “high-risk stage II” rectal cancer [7].
In Japan, tegafur/uracil (UFT; Taiho Pharmaceutical Co., Tokyo, Japan), an orally administered fluoropyrimidine inhibitor of dihydropyrimidine dehydrogenase that contains tegafur and uracil in a 1:4 molar ratio, recently showed a survival benefit in rectal cancer. Hamaguchi et al. conducted two independent randomized controlled trials in patients with stage III colon and rectal cancer (National Surgical Adjuvant Study of Colorectal Cancer, NSAS-CC trial). They found that postoperative adjuvant chemotherapy with UFT was tolerated and successfully improved the recurrence-free survival (RFS) and overall survival (OS) in patients with stage III rectal cancer. Furthermore, previous studies have shown favorable toxicity of adjuvant treatment with UFT. Therefore, the use of UFT as an adjuvant treatment was deemed an option for stage II rectal cancer [8].
Protein-bound polysaccharide K (KRESTIN, PSK; Kureha Chemical Industry Co., Tokyo, Japan), which is extracted from the mycelia of Coriolus versicolor, has immunomodulatory activities. It induces the production of interleukin (IL)-2 and interferon (IFN), thereby stimulating lymphokine-activated killer cells (LAK) and enhancing natural killer (NK) cells. A randomized, double-blind trial as well as a meta-analysis of those trials for resectable gastric cancers showed that PSK provided borderline significant benefits in improving the disease-free survival (DFS) and OS [9, 10]. The beneficial effects of PSK have been attributed to the activation of leucocyte chemotactic locomotion and phagocytic activity. With respect to colorectal cancers, the efficacy of adding PSK for standard cytotoxic chemotherapy has also been reported, mainly in colon cancers [10–13].
Given the above, to investigate the benefits of UFT plus immunochemotherapy with PSK, we conducted a randomized phase III trial comparing UFT and PSK to surgery alone in curatively resected stage II rectal cancer patients.
Patients and methods
Study design
The JFMC38 trial was a randomized, open-label, multicenter, phase III study. The trial was performed in patients diagnosed with stage II rectal cancer in Japan. Patients were randomly assigned to receive UFT and PSK or surgery alone in a 1:1 ratio with a minimization method to balance the treatment allocation according to the pathological stage (pT3 or pT4), primary tumor location (Ra or Rb), age (< 65 or ≥ 65 years of age), and medical center. The primary end point of this study was the DFS, and the secondary end point was the OS.
Ethics
Study data and informed consent were obtained in accordance with the Declaration of Helsinki and approved by the Ethics Review Board of each institution. All patients were given a written explanation of the study, and they provided their written informed consent before participating.
Inclusion and exclusion criteria
Tumors were staged according to the UICC version 6 [14]. The inclusion criteria were as follows: (1) Stage II, histologically confirmed adenocarcinoma of the rectum; (2) Patients who underwent curative surgery with ≥ D2 lymph node dissection; (3) Negative status for pathological lymph nodes; (4) Performance status of 0–2; (5) Not receiving pre-operative chemotherapy and/or radiation therapy; (6) Adequate hematologic, liver, and coagulation profiles (white blood cell [WBC] count ≥ 3,000/mm3 and ≤ 12,000/mm3, neutrophils count ≥ 1,500/mm3, platelet count ≥ 100,000/mm3, GOT ≤ 100 U/l and GPT ≤ 100 U/l, total bilirubin < 1.5 mg/dl, creatinine < 1.5 mg/dl, and normal ECG); (7) Able to start chemotherapy within 8 weeks after the operation; and (8) Provided consent to participate in this clinical study.
The exclusion criteria were as follows: (1) Unable to ingest anything or with digestive organ stricture; (2) Presence of serious coexisting morbidities; (3) Active synchronous or metachronous malignant disease; (4) Pregnant or lactating; (5) Not suitable for participating in the study for any other reason.
Treatment methods
Control arm: Surgery alone
UFT-PSK arm: UFT was administered orally at 400 mg/m2/day thrice daily after meals for 5 days and followed by 2 days’ rest (one cycle) for 1 year after surgery. PSK was administered orally 3 g/day thrice daily after meals for 1 year after surgery.
Follow-up
Patients were followed up in accordance with the protocol of the present study. Briefly, during protocol treatment, the clinical findings and laboratory data were evaluated every 2 weeks. After completion of the protocol treatment, patients were followed up in accordance with a predefined surveillance schedule until recurrence or death was confirmed for 5 years after surgery. Recurrence was assessed based on computed tomography (CT) scans. These tests were carried out every 4 months during the first 2 years after surgery and once every 6 months from the third year onward.
Statistical analyses
The DFS at 5 years was predicted to be 75% in the surgery-alone arm and 85% in the PSK + UFT arm with assumption of a dropout rate of 5%, recruitment period for 36 months and additional follow-up period for 60 months. We calculated that a total enrollment of 540 patients was needed using a log-rank test, a two-sided alpha of 5%, and a statistical power of 80%.
The background characteristics of the postoperative clinical and pathological parameters between the UFT + PSK arm and surgery-alone arm are shown as percentages for categorical variables and the median with the range for continuous variables. The OS was defined as the period between randomization and any cause of death. The DFS was defined as the period between randomization and the occurrence of recurrence, second cancer, or death, whichever came first. The data for patients who had not experienced an event were censored at the date of the final observation. The OS and DFS curves were calculated using the Kaplan–Meier method, and were compared by the stratified log-rank test (stratification factors: pathological T stage, primary tumor location, and age). A Cox proportional hazards model was used to estimate the adjusted hazard ratio, with adjustment for the same stratification factors from the log-rank test as covariates. In addition, a multivariate Cox proportional hazards model was created using the stratification factors and other factors (sex, tumor diameter, lymph node metastasis, lymphadenectomy, lymphatic invasion, and vascular invasive) as covariates. Other factors were selected based on a priori knowledge. Subgroup analyses based on the stratification factors (pathological T stage, primary tumor location, and age) and other factors (sex, tumor diameter, lymph node metastasis, lymphatic invasion, and vascular invasive) were conducted, and the interaction between the treatment effect and each stratification factor was evaluated for the DFS. A two-sided p < 0.05 was defined as indicating statistical significance. The SAS software program, version 9.4 (SAS Institute Inc., Cary, NC, USA), was used for all statistical analyses. This study was approved by the ethics committee of the Japanese Foundation for Multidisciplinary Treatment of Cancer.
Results
Recruitment and patients’ characteristics
From October 2011 to February 2013, 200 institutions collaborated with the JFMC-38 study, and 111 patients were registered from 62 institutions. The study was prematurely closed due to poor accrual after reaching 20% of its goal. At the time of the analysis in June 2017, 7 patients had died, and 29 had experienced the primary event. Due to this low number of observed primary events, the power for the formal statistical analysis was limited.
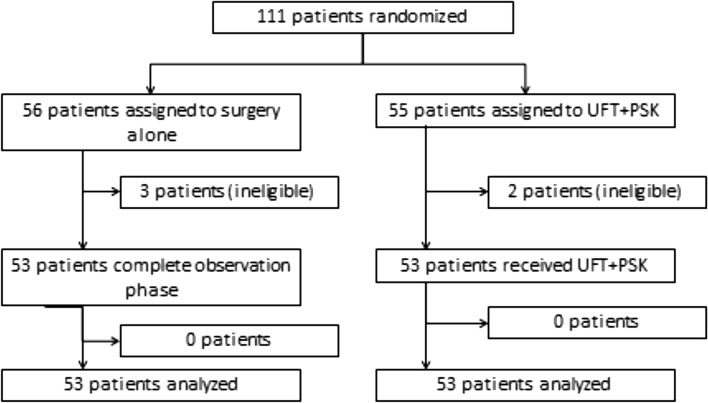
Among 111 patients, 55 were allocated to the UFT + PSK arm and 56 to the surgery-alone arm. Figure 1 shows the consort diagram of the present study. After randomization, 5 patients (2 in the UFT + PSK arm and 3 in the control arm) were found to be ineligible. Primary analyses were based on data from all randomly assigned patients, excluding those who were ineligible. The two groups were well balanced with regard to the baseline clinical characteristics and pathological findings (Table 1). The median duration of follow-up was 5.3 years.
Fig. 1.
Consort diagram of the present study
Table 1.
Patient characteristics
| Characteristics | Total (n = 106) | Surgery-alone group (n = 53) | UFT + PSK group (n = 53) |
|---|---|---|---|
| No. of patients (%) | No. of patients (%) | No. of patients (%) | |
| Age | |||
| < 65 years | 51 (48.1) | 25 (47.2) | 26 (49.1) |
| > 65 years | 55 (51.9) | 28 (52.8) | 27 (50.9) |
| ECOG performance status | |||
| 0 | 99 (93.4) | 47 (88.7) | 52 (98.1) |
| 1–2 | 7 (6.6) | 6 (11.3) | 1 (1.9) |
| Tumor location | |||
| Ra | 56 (52.8) | 29 (54.7) | 27 (50.9) |
| Rb | 49 (46.3) | 23 (43.4) | 26 (49.1) |
| Missing | 1 (0.9) | 1 (1.9) | 0 (0) |
| Tumor diameter (mm) | |||
| Median | 51.5 | 52.5 | 50 |
| Range | 19–140 | 25–110 | 19–140 |
| Pathological T factor | |||
| T3 | 95 (89.6) | 45 (84.9) | 50 (94.3) |
| T4 | 10 (9.5) | 7 (13.2) | 3 (5.7) |
| Missing | 1 (0.9) | 1 (1.9) | 0 (0) |
| Lymphatic invasion | |||
| Negative | 56 (52.8) | 26 (49.1) | 30 (56.6) |
| Positive | 49 (46.3) | 26 (49.1) | 23 (43.4) |
| Missing | 1 (0.9) | 1 (1.9) | 0 (0) |
| Vascular invasion | |||
| Negative | 39 (36.8) | 15 (28.3) | 24 (45.3) |
| Positive | 66 (62.3) | 37 (69.8) | 29 (54.7) |
| Missing | 1 (0.9) | 1 (1.9) | 0 (0) |
| Histological type | |||
| Differentiated | 102 (96.4) | 51 (96.2) | 51 (96.2) |
| Undifferentiated | 4 (3.6) | 2 (3.8) | 2 (3.8) |
ECOG Eastern Cooperative Oncology Group
Safety and feasibility
Table 2 show the adverse events reported in the present study. The most commonly reported adverse events of any grade in the UFT + PSK arm were elevated serum bilirubin nausea, vomiting, and fatigue. The adverse events of grade 3 or 4 that were more frequent in the UFT + PSK arm were elevated aspartate aminotransferase and alanine aminotransferase.
Table 2.
Relevant adverse events
| Adverse event | Surgery alone group | UFT + PSK group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade1 | Grade2 | Grade3 | Grade4 | Grade 3 or 4 | Grade1 | Grade2 | Grade3 | Grade4 | Grade 3or 4 | |
| Number of patients | (%) | Number of patients | (%) | |||||||
| Hematological | ||||||||||
| Leukopenia | 1 (1.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (9.4) | 2 (3.8) | 0 (0) | 0 (0) | 0 (0) |
| Neutropenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (13.2) | 0 (0) | 0 (0) | 0 (0) |
| Anemia | 9 (17.0) | 2 (3.8) | 0 (0) | 0 (0) | 0 (0) | 7 (13.2) | 3 (5.7) | 1 (1.9) | 0 (0) | 1 (1.9) |
| Thrombocytopenia | 3 (5.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (7.5) | 1 (1.9) | 0 (0) | 0 (0) | 0 (0) |
| No hematological | ||||||||||
| Elevated ALT | 8 (15.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (15.1) | 2 (3.8) | 2 (3.8) | 0 (0) | 2 (3.8) |
| Elevated AST | 5 (9.4) | 0 (0) | 1 (1.9) | 0 (0) | 1 (1.9) | 7 (13.2) | 1 (1.9) | 2 (3.8) | 0 (0) | 2 (3.8) |
| Elevated serum Bil | 2 (3.8) | 1 (1.9) | 0 (0) | 0 (0) | 0 (0) | 10 (18.9) | 6 (11.3) | 0 (0) | 0 (0) | 0 (0) |
| Elevated creatine | 3 (5.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.9) | 3 (5.7) | 0 (0) | 0 (0) | 0 (0) |
| Stomatitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.9) | 2 (3.8) | 0 (0) | 0 (0) | 0 (0) |
| Nausea/vomiting | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (17.0) | 2 (3.8) | 1 (1.9) | 0 (0) | 1 (1.9) |
| Diarrhea | 1 (1.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (17.0) | 2 (3.8) | 1 (1.9) | 0 (0) | 1 (1.9) |
| Rash | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (5.7) | 3 (5.7) | 0 (0) | 0 (0) | 0 (0) |
| Pigmentation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (5.7) | 1 (1.9) | 0 (0) | 0 (0) | 0 (0) |
| Anorexia | 1 (1.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (15.1) | 2 (3.8) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 1 (1.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (15.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Among the 53 patients in the safety population who received UFT + PSK, the relative dose intensity (RDI) of UFT was > 90% in 37 patients (69.8%), 75–90% in 6 patients (11.3%), 50–75% in 5 patients (9.4%), and < 50% in 3 patients (5.7%), and data were missing in 2 patients (3.8%). The RDI of PSK was > 90% in 39 patients (73.6%), 75–90% in 5 patients (9.4%), 50–75% in 4 patients (7.6%), and < 50% in 3 patients (5.7%), and data were missing in 2 patients (3.8%). The reasons for withdrawal of treatment in the UFT + PSK arm included refusal of the patient to continue treatment because of adverse events or other factors in nine patients, the detection of metastasis or relapse in six, and other reasons in six.
Survival analyses
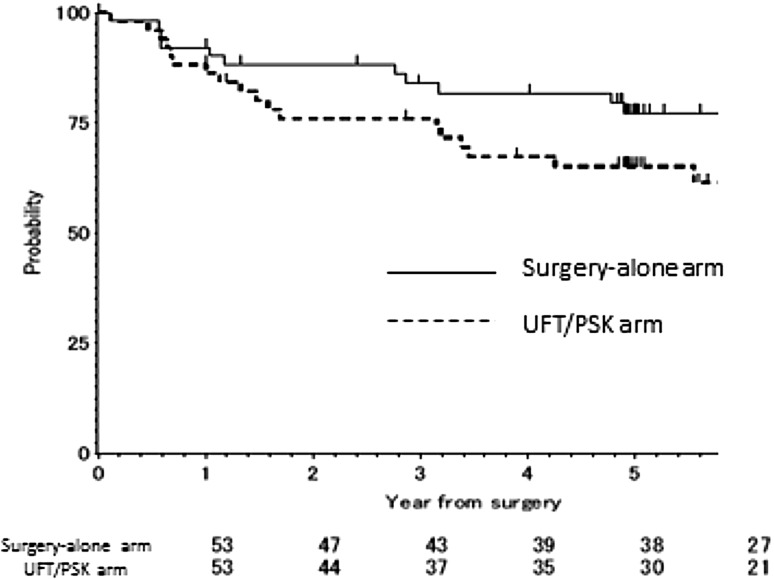
The DFS rate was 76.0% at 3 years and 65.1% at 5 years in the UFT + PSK arm and 84.0% at 3 years and 77.2% at 5 years in the surgery-alone arm. The DFS was slightly worse in the UFT + PSK arm than in the surgery-alone arm, but the difference did not reach statistical difference (log rank p = 0.102). The DFS curves are shown in Fig. 2. The hazard ratio for DFS was 1.94 (95% confidence interval [CI], 0.90 to 4.18; p value = 0.092). In the multivariate Cox proportional hazards model, the adjusted hazard ratio for DFS was 2.45 (95% CI, 0.99 to 6.03; p value = 0.052). In the subgroup analysis, UFT + PSK was statistically inferior to the control arm (hazard ratio = 2.85; 95% CI, 1.01 to 8.03; p value = 0.048), but there were no statistically significant interactions between the treatment effect and either subgroup. Table 3 showed the site of first relapse according to treatment group.
Fig. 2.
A comparison of the recurrence-free survival between the UFT/PSK arm and surgery-alone arm
Table 3.
Site of first relapse, according to treatment group
| Total number of relapse | Total (n = 29) |
Surgery alone group (n = 11) |
UFT + PSK group (n = 18) |
|---|---|---|---|
| (%) | (%) | (%) | |
| Local | 8 (27.6) | 3 (27.2) | 5 (27.8) |
| Lymph node | 2 (6.9) | 1 (9.1) | 1 (5.6) |
| Hematogenous | |||
| Lung | 5 (17.2) | 1 (9.1) | 4 (22.2) |
| Liver | 4 (13.8) | 1 (9.1) | 3 (16.7) |
| Others | 10 (34.5) | 5 (45.5) | 5 (27.8) |
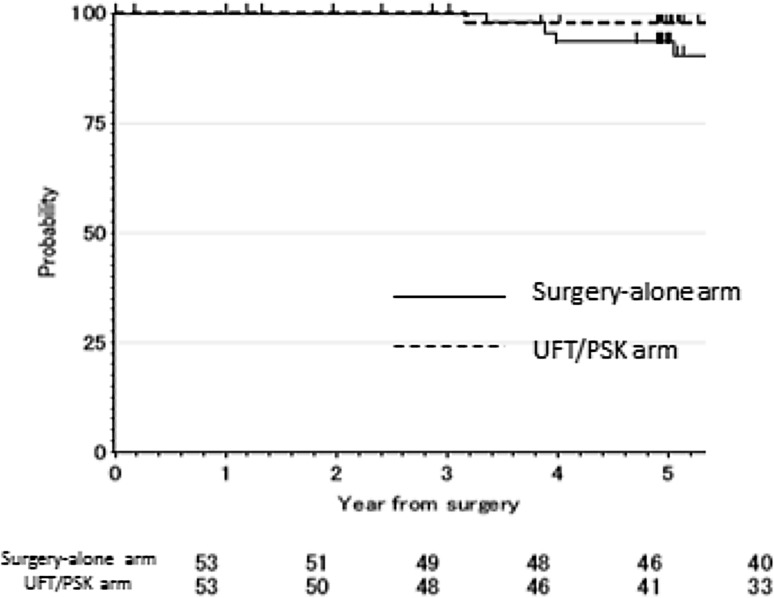
The OS rate was 100% at 3 years and 97.9% at 5 years in the UFT + PSK arm and 100% at 3 years and 93.4% at 5 years in the surgery-alone arm. The OS was similar in the UFT + PSK arm and surgery-alone arm (p = 0.533). The OS curves are shown in Fig. 3. The hazard ratio for the OS was 1.82 (95% CI, 0.38 to 8.74; p value = 0.454).
Fig. 3.
A comparison of the overall survival between the UFT/PSK arm and surgery-alone arm
Discussion
The present randomized phase III study aimed to compare the outcomes of UFT and PSK to those of surgery alone in curatively resected stage II rectal cancer patients. The primary purpose of this study was to confirm the efficacy of UFT and PSK in improving the DFS. The results showed that UFT and PSK did not improve the DFS compared with surgery alone in 20% of the planned sample size. Thus, confirmative results could not be obtained from this study. However, the present findings do suggest that UFT and PSK were not attractive candidates for advancing to a test arm in the next phase III study, as the hazard ratio for the DFS was 1.94 against surgery alone.
First, we want to discuss why the patient accrual was so poor. Some patients avoided registering for the present clinical trial due to the adverse events of UFT. UFT induces both hematological and non-hematological toxicities, such as anorexia, fatigue, and nausea and affects the quality of life of patients. Therefore, even though the physicians explained the details and importance of the clinical trial to the patients, many avoided registering. In addition, some physicians avoided registering patients for the present clinical trial because they wanted to prescribe another adjuvant chemotherapy regimen. The major Western guidelines recommend adjuvant chemotherapy, such as oxaliplatin-based chemotherapy, for “high-risk stage II” rectal cancer [7]. Therefore, even though patients were members of the target population, physicians might have avoided encouraging their registration in the present clinical trial for “high-risk stage II” rectal cancer. As the study was terminated due to poor accrual, the results failed to show the survival superiority of the test arm.
Second, we want to discuss why the DFS of PSK and UFT was not sufficient to support the application of this regimen as an adjuvant treatment for stage II rectal cancer. Given the results of previous studies such as NSAS-CC, we selected UFT as the adjuvant chemotherapy agent for the present study [8]. Non-specific immunomodulators have been considered particularly promising anticancer agents when combined with chemotherapy or chemoradiotherapy for curatively resected gastrointestinal cancers in an adjuvant setting [9, 12], and PSK, OK-432, and Lentinan are now widely used in Japan. With regard to locally advanced gastric cancers, a number of immunochemotherapy clinical trials combined mainly with oral fluorinated pyrimidine anticancer agents have been conducted. A borderline significant effect was demonstrated in randomized clinical trials as well as in a meta-analysis for PSK and OK-432 [9, 10, 15, 16, 15, 16], although no significant effect of Lentinan was proven for advanced or metastatic gastric cancers in a recently published study [17]. Although PSK has shown somewhat favorable outcomes for colon cancer patients in adjuvant settings, initial studies for those agents failed to confirm their significant advantage, and they were eventually excluded from use in community practice in early development. However, the primary end point was not met in the present study.
Some discrepancies were observed in the findings for the DFS and OS in our study. Although the difference did not reach significance, the DFS was slightly worse in the UFT + PSK arm than in the surgery-alone arm. In contrast, the OS rates were similar in the UFT + PSK arm and surgery-alone arm. This discrepancy might be due to treatments after recurrence, especially chemotherapy, prolonging the survival of these patients. Indeed, chemotherapy for recurrence certainly prolongs the OS [18, 19].
In conclusion, the findings from the present study suggest that UFT + PSK was not attractive candidates to advance to the next phase III study because the DFS was slightly worse in the UFT and PSK arm than in the surgery-alone arm.
Acknowledgements
This study was supported, in part, by the nonprofit organization Epidemiological and Clinical Research Information Network (ECRIN).
Compliance with ethical standards
Conflict of interest
Satoshi Morita has received remuneration entity from Taiho and Daiichi-Sankyo. The remaining authors declare that they have no conflict of interest.
Research involving human participants
Ethical approval, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Toru Aoyama contributed equally to the Kiyotaka Okuno.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Tveit K, ESMO Guidelines Task Force ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of rectal cancer. Ann Oncol. 2005;16(Suppl 1):i20–i21. doi: 10.1093/annonc/mdi827. [DOI] [PubMed] [Google Scholar]
- 3.Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26:696–701. doi: 10.1093/annonc/mdu560. [DOI] [PubMed] [Google Scholar]
- 4.Folkesson J, Birgisson H, Pahlman L, et al. Swedish rectal cancer trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 5.Kim MB, Hong TS, Wo JY. Treatment of stage II-III rectal cancer patients. Curr Oncol Rep. 2014;16:362. doi: 10.1007/s11912-013-0362-0. [DOI] [PubMed] [Google Scholar]
- 6.Oki E, Ando K, Kasagi Y, Zaitsu Y, Sugiyama M, Nakashima Y, Sonoda H, Ohgaki K, Saeki H, Maehara Y. Recent advances in multidisciplinary approach for rectal cancer. Int J Clin Oncol. 2015;20:641–649. doi: 10.1007/s10147-015-0858-8. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Care Network. Clinical Practice Guidelines in Oncology (NCCNGuidelines®).http://www.nccn.org/professionals/physician_gls/f_guidelines
- 8.Hamaguchi T, Shirao K, Moriya Y, Yoshida S, Kodaira S, Ohashi Y, NSAS-CC Group Final results of randomized trials by the National Surgical Adjuvant Study of Colorectal Cancer (NSAS-CC) Cancer Chemother Pharmacol. 2011;67:587–596. doi: 10.1007/s00280-010-1358-1. [DOI] [PubMed] [Google Scholar]
- 9.Nakazato H, Koike A, Saji S, Ogawa N, Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Study Group of Immunochemotherapy with PSK for Gastric Cancer. Lancet. 1994;343:1122–1126. doi: 10.1016/S0140-6736(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto J, Morita S, Oba K, Matsui T, Kobayashi M, Nakazato H, Ohashi Y, Meta-Analysis Group of the Japanese Society for Cancer of the Colon Rectum Efficacy of adjuvant immunochemotherapy with polysaccharide K for patients with curatively resected colorectal cancer: a meta-analysis of centrally randomized controlled clinical trials. Cancer Immunol Immunother. 2006;55:404–411. doi: 10.1007/s00262-005-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohwada S, Kawate S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, Takahashi T, Nakamura S, Kawashima Y, Nakajima T, Morishita Y, Gunma Oncology Study Group (GOSG) Adjuvant therapy with protein-bound polysaccharide K and tegafur uracil in patients with stage II or III colorectal cancer: randomized, controlled trial. Dis Colon Rectum. 2003;46:1060–1068. doi: 10.1007/s10350-004-7281-y. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Nakazato H, Koike A, Takagi H, Saji S, Baba S, Mai M, Sakamoto J, Ohashi Y, Study Group of Immunochemotherapy with PSK for Colon Cancer Long-term effect of 5-fluorouracil enhanced by intermittent administration of polysaccharide K after curative resection of colon cancer. A randomized controlled trial for 7-year follow-up. Int J Colorectal Dis. 2004;19:157–164. doi: 10.1007/s00384-003-0532-x. [DOI] [PubMed] [Google Scholar]
- 13.Ohwada S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, Takahashi T, Nakamura S, Kakinuma S, Iwazaki S, Ishikawa H, Kawate S, Nakajima T, Morishita Y. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. Br J Cancer. 2004;90:1003–1010. doi: 10.1038/sj.bjc.6601619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobin LH, Wittekind CH, editors. TNM classification of malignant tumors. 6. New York: Wiley; 2002. [Google Scholar]
- 15.Maehara Y, Okuyama T, Kakeji Y, Baba H, Furusawa M, Sugimachi K. Postoperative immunochemotherapy including streptococcal lysate OK-432 is effective for patients with gastric cancer and serosal invasion. Am J Surg. 1994;168:36–40. doi: 10.1016/S0002-9610(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 16.Oba MS, Teramukai S, Ohashi Y, Ogawa K, Maehara Y, Sakamoto J. The efficacy of adjuvant immunochemotherapy with OK-432 after curative resection of gastric cancer: an individual patient data meta-analysis of randomized controlled trials. Gastric Cancer. 2016;19:616–624. doi: 10.1007/s10120-015-0489-9. [DOI] [PubMed] [Google Scholar]
- 17.Yoshino S, Nishikawa K, Morita S, Takahashi T, Sakata K, Nagao J, Nemoto H, Murakami N, Matsuda T, Hasegawa H, Shimizu R, Yoshikawa T, Osanai H, Imano M, Naitoh H, Tanaka A, Tajiri T, Gochi A, Suzuki M, Sakamoto J, Saji S, Oka M. Randomised phase III study of S-1 alone versus S-1 plus lentinan for unresectable or recurrent gastric cancer (JFMC36-0701) Eur J Cancer. 2016;65:164–171. doi: 10.1016/j.ejca.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in longterm survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2001;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]