Abstract
Burkitt lymphoma is an aggressive B cell malignancy accounting for 1–2% of all adult lymphomas. Treatment with dose-intensive, multi-agent chemotherapy is effective but associated with considerable toxicity. In this observational study, we compared real-world efficacy, toxicity, and costs of four frequently employed treatment strategies for Burkitt lymphoma: the Lymphome Malins B (LMB), the Berlin-Frankfurt-Münster (BFM), the HOVON, and the CODOX-M/IVAC regimens. We collected data from 147 adult patients treated in eight referral centers. Following central pathology assessment, 105 of these cases were accepted as Burkitt lymphoma, resulting in the following treatment groups: LMB 36 patients, BFM 19 patients, HOVON 29 patients, and CODOX-M/IVAC 21 patients (median age 39 years, range 14–74; mean duration of follow-up 47 months). There was no significant difference between age, sex ratio, disease stage, or percentage HIV-positive patients between the treatment groups. Five-year progression-free survival (69%, p = 0.966) and 5-year overall survival (69%, p = 0.981) were comparable for all treatment groups. Treatment-related toxicity was also comparable with only hepatotoxicity seen more frequently in the CODOX/M-IVAC group (p = 0.004). Costs were determined by the number of rituximab gifts and the number of inpatients days. Overall, CODOX-M/IVAC had the most beneficial profile with regards to costs, treatment duration, and percentage of patients completing planned treatment. We conclude that the four treatment protocols for Burkitt lymphoma yield nearly identical results with regards to efficacy and safety but differ in treatment duration and costs. These differences may help guide future choice of treatment.
Electronic supplementary material
The online version of this article (10.1007/s00277-017-3167-7) contains supplementary material, which is available to authorized users.
Keywords: Burkitt lymphoma, Drug therapy, Survival, Cost analysis
Introduction
Sporadic Burkitt lymphoma (BL) is a rare and highly aggressive B cell malignancy, accounting for 1–2% of all adult lymphomas in Western Europe and North America. In the adult population, BL most often affects young to middle-aged patients with a median age at diagnosis of 35 years [1]. Patients often present with bulky extranodal disease, bone marrow infiltration, and central nervous system involvement [2, 3].
Because of rapid tumor growth, prompt diagnosis and start of treatment are important to optimize outcome [4]. Current treatment strategies for adults have often been adapted from pediatric protocols [2, 3]. All of these protocols aim to deliver dose-intensive, multi-agent chemotherapy with minimization of treatment delays, and maintenance of serum drug concentrations. Examples are the French Lymphome Malins B (LMB) regimen developed by the Société Française d’Oncologie Pédiatrique [5, 6], the German Berlin-Frankfurt-Münster (BFM) regimen developed by the German Multicenter Study Group for Adult ALL (GMALL) [7], the regimen of the Dutch-Belgian Cooperative Trial Group for Hematology Oncology (HOVON) regimen [8], and the CODOX-M/IVAC regimen [9]. The complete response (CR) rates achieved with these regimens range between 72 and 89% [5–11].
Consensus on the optimal first line treatment for adult BL is still lacking. The low incidence of BL has thus far precluded direct comparison between treatment regimens in a randomized prospective clinical trial. Retrospective comparison of published clinical series is hampered by different patient selection criteria and changing histopathological definitions for aggressive B cell lymphoma including BL in successive WHO lymphoma classifications.
In the Netherlands, the LMB, BFM, HOVON, and CODOX-M/IVAC regimens are all in active use with treatment center preference based on historic and regional associations. Each of these regimens consists of a backbone of three to six courses of high-dose chemotherapy. The regimens differ in inclusion of maintenance therapy and autologous stem cell transplantation (SCT) as part of first line treatment, as well as agents used and dosages.
To support a rational choice for a standardized treatment of adult BL, we performed a retrospective observational analysis of real-world efficacy, toxicity, and costs of these four treatment protocols as used in daily clinical practice. In view of the evolving BL definitions over time, a central pathology assessment was included to guarantee meaningful comparisons.
Patients and methods
Patient selection and clinical data collection
All patients treated between 1995 and 2012 with any of the four treatment protocols under study in seven university medical centers and one non-academic tertiary referral hospital in the Netherlands were included in this study. Patients with prior first line treatment other than a maximum of three (R-) CHOP (-like) courses were excluded. Clinical data were collected from the hospital records using a standardized case report form. Adverse events were scored according to the Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Specifically, infectious disease was defined as infections requiring intravenous antibiotic, antifungal, or antiviral intervention, or requiring radiologic or operative intervention; nephrotoxicity was defined as reduction of glomerular filtration rate to < 25% or creatinine increase > 3× baseline; hepatotoxicity was defined as elevation of transaminases > 5.0× upper normal level. Numbers of transfusions were extracted from the local blood bank databases. Treatment response was based on the original radiology reports (CT or PET-CT according to local practice). All treatment centers used the (revised) Cheson response criteria for response evaluation from 1999 onwards [12, 13]. Risk scores reported were the International Prognostic Index (IPI) score [12] and the Mead 2002 BL risk score [9].
Central pathology assessment
Patients treated with the four treatment regimens under study were originally diagnosed with Burkitt lymphoma, small non-cleaved cell lymphoma, atypical Burkitt lymphoma, Burkitt-like lymphoma, B cell lymphoma with features intermediate between Burkitt lymphoma and diffuse large B cell lymphoma (BLU), Burkitt leukemia, mature B cell leukemia, or L3-leukemia according to the WHO lymphoma classification used at the time of diagnosis. Central pathology assessment was performed by two expert hematopathologists in two stages (D.d.J., P.M.K.). First, original pathology reports, including consult reports at the time of diagnosis, were reviewed. A case was accepted as BL if the following criteria were met: small- or medium-sized cells with monotonous morphology, proliferative index (MIB1) > 95%, and immunophenotype consistent with BL with BCL-2 staining negative or weak and CD10 and/or BCL-6 staining positive. A demonstrable MYC-translocation by cytogenetic testing or fluorescence in situ hybridization was considered supportive but not required for selection. Second, in those cases where the available pathology reports were insufficient or incomplete, complete formal review was performed, including additional BCL-2, CD10, and/or BCL-6 staining if not previously done. All cases not meeting the listed BL criteria were excluded from this study.
Treatment regimens
Details on the treatment regimens included in this study are summarized in Table 1. All treatment regimens included rituximab from 2003 to 2004 onwards. Additional information can be found in the supplemental treatment regimen information.
Table 1.
Chemotherapeutic drugs and drug dosages per treatment regimen
| Treatment regimena | LMB [6] | BFM [10] | HOVON [8]b | CODOX-M/IVAC [9]c | ||
|---|---|---|---|---|---|---|
| Agents (no. courses × no. consecutive days) | ||||||
| Alkylating agents | Cyclophosfamide | 6800 (1 × 1 day, 2 × 3 days, and 2 × 2 days) | 3000 (3 × 5 days) | 7000 (3 × 1 day + 1 × 2 days) | 3200 (2 × 5 days) | mg/m2 |
| Ifosfamide | 8000 (2 × 5 days) | 15,000 (2 × 5 days) | mg/m2 | |||
| Melphalan | 140 (1 × 1 day) | mg/m2 | ||||
| Carmustine | 300 (1 × 1 day) | mg/m2 | ||||
| Antimetabolites | Cytarabine | 25,500 (4 × 5 days continuous) | 8600 (2 × 2 days, 2 × 1 day) | 800 (1 × 4 days) | 8000 (2 × 2 days) | mg/m2 |
| Methotrexate | 9000 (4 × 1 day) | 9000 (6 × 1 day) | 13,440 (2 × 1 day) | mg/m2 | ||
| Antitumor antibiotics | Adriamycine | 240 (4 × 1 day) | 200 (2 × 2 days) | 280 (3 × 1 day,1 × 2 days) | 80 (2 × 1 day) | mg/m2 |
| Topoisomerase inhibitors | Etoposide | 2500 (4 × 3 days) | 1000 (2 × 2 days) | 2800 (2 × 4 days) | 600 (2 × 5 days) | mg/m2 |
| Mitoxantrone | 30 (1 × 1 day) | mg/m2 | ||||
| Teniposide | 400 (2 × 2 days) | mg/m2 | ||||
| Mitotic inhibitors | Vincristine | 12 (6 × 1 day) | 8 (4 × 1 day) | 6 (3 × 1 day) | 8 (2 × 1 day) | mg |
| Vindesine | 10 (2 × 1 day) | mg/m2 | ||||
| Corticosteroids | Prednisolone | 1740 (1 × 7 days, 4 × 5 days) | 300 (1 × 5 days) | 1250 (5 × 5 days) | mg/m2 | |
| Dexamethasone | 300 (6 × 5 days) | mg/m2 | ||||
| IT medication | Cytarabine | 240 (8×) | 160 (8×) | 140 (2 × 1) | mg | |
| Methotrexate | 90 (8×) | 60 (8×) | 75 (5×) | 48 (4 × 1) | mg | |
| Dexamethason | 32 (8×) | |||||
| Hydroxycortison | 160 (8×) | |||||
| Monoclonal antibodies | Rituximabd | 1500 (4×) | 3000 (8×) | 1875 (5×) | 2250 (6×) | mg/m2 |
| Total number of courses | 5 (low-int risk) 8 (high risk) e | 6 | 6 | 3 (low risk) 4 (high risk) e | ||
aDoses given are for a patient < 40 years with a body surface area of 2 mg/m2 and a body weight of 75 kg, high risk BL without CNS involvement, and no dose reductions
bPreceded by 3× iR-CHOP from approximately 2005 onwards (included in calculations)
cNo patients included in this study received dose-modified CODOX-M/IVAC
dMedian number of rituximab gifts as administered per treatment regimen from 2003 to 2004 onwards
eLow-intermediate risk LMB, patients without bone marrow or CNS involvement; high risk LMB, all others patients; low risk CODOX-M/IVAC, BL score 0; and high risk CODOX-M/IVAC, BL score >0
Statistical analyses
Baseline characteristics between the different treatment groups were compared using the χ 2 test or Fisher’s exact test for categorical data and the Kruskal-Wallis test for numerical data. Overall survival (OS) and progression-free survival (PFS) were calculated from start of the studied treatment regimen on an intention-to-treat basis and defined as the time to death from any cause (OS), and time to diagnosis of RD or relapse or death from any cause (whichever came first) (PFS). Survival of patients was censored at 5 years from the start of the studied treatment regimen or at the date of last contact, whichever came first. The Kaplan-Meier method was used to estimate OS and PFS, and to calculate 95% confidence intervals (CI). The log-rank test was used to compare survival between subgroups. Treatment-related mortality was calculated in a competing risks model in which lymphoma-related mortality was considered as a competing event. The cumulative incidence of relapse (CIR) was calculated in a competing risks model, considering non-relapse mortality as competing event. End of treatment was taken as starting time and patients with refractory disease were excluded from the analysis. Gray’s test was used to compare CIR between groups. Hazard ratios (HR) were calculated using univariate Cox regression models. Analyses were performed using SPSS, version 20, and R, version 3.3.0, with libraries “cmprsk” and “prodlim.” All reported p values are two-sided with a significance level of α = 0.05.
Cost assessment
For each treatment regimen costs for medication use, erythrocyte and platelet transfusions, inpatients days and autologous graft collection (if applicable) were calculated, for an “average” patient based on the following assumptions: < 40 old, body surface area of 2 m2, weight of 75 kg, high risk BL without CNS involvement, no dose reductions, administration of the median number of rituximab gifts and the mean number of blood product transfusions, and hospital admission for the mean number of days as reported for that treatment regimen. Costs of supportive medication and outpatient evaluations were not taken into account but were assumed to be comparable between all regimens. All costs are reported in Euro and indexed to the year 2015 using the Dutch consumer price index as published on the CBS Statistics Netherlands website [14]. Sources used were the knowledge database of the Royal Dutch Pharmacists Association (z-index) [15] per December 2015 (costs for carmustine and vindesine were obtained from the Leiden University Medical Center Pharmacy), the Dutch guideline for economic evaluations [16], and previous published studies [17–19].
Results
Patient characteristics and chemotherapy regimens
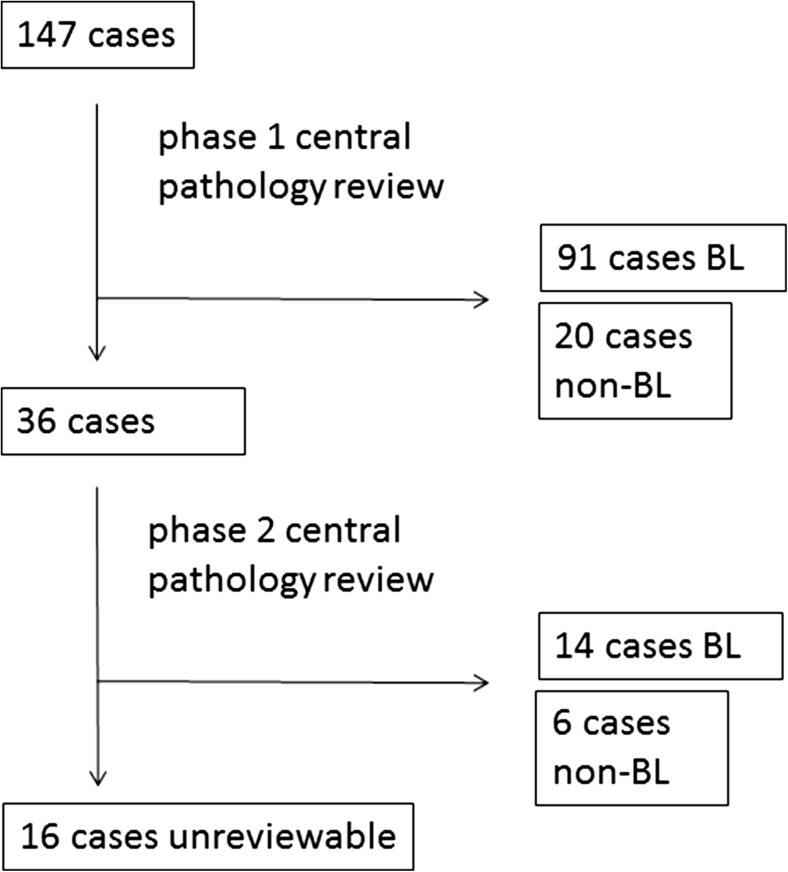
A total of 147 adolescent and adult patients (14–74 years) treated for BL with the LMB, the BFM, the HOVON, or the CODOX-M/IVAC regimen between 1995 and 2012 were identified (Fig. 1). Of these, 91 cases fulfilled the criteria for BL in the first phase of the pathology assessment and 14 additional cases after complete assessment resulting in a total of 105 confirmed BL cases. Twenty-six cases were rejected as non-BL. Sixteen cases were considered unreviewable.
Fig. 1.
Central pathology assessment flow chart
Patient characteristics and chemotherapy regimens
Baseline patient characteristics are listed in Table 2. Thirty-six patients were treated with the LMB regimen, 19 with the BFM regimen, 29 with the HOVON regimen, and 21 with the CODOX-M/IVAC regimen. Of the LMB patients, 11 received the low-intermediate risk schedule (31%). Of the CODOX-M/IVAC patients, five received the low risk schedule consisting of three CODOX-M courses (24%). Rituximab was included in the treatment regimen of 64% (LMB) to 100% (CODOX-M/IVAC) of patients. The treatment groups were similar in most characteristics, with the following exceptions. The HOVON group contained fewer patients with central nervous system (CNS) involvement (p = 0.065), no patients with leukemic BL (n.s.), and fewer patients with high WHO scores (p = 0.002), resulting in slightly better overall IPI scores (p = 0.031). The BFM group contained fewer patients with ≥ 2 extranodal sites (p = 0.037). Lastly, the LMB and HOVON groups had fewer patients treated with rituximab (p = 0.003) than the BFM and CODOX-M/IVAC groups. Since the latter regimens were introduced more recently in the Netherlands, they had a higher percentage of patients included after 2003–2004.
Table 2.
Patient characteristics per treatment regimen
| Treatment regimen | LMB | BFM | HOVONa | CODOX-M/IVAC | All | P value |
|---|---|---|---|---|---|---|
| N | 36 | 19 | 29 | 21 | 105 | |
| Age median (range) | 35 (14–74) | 40 (19–57) | 39 (15–57) | 39 (17–62) | 39 (14–74) | 0.784 |
| < 40 years | 22 (61%) | 9 (47%) | 15 (52%) | 11 (52%) | 57 (54%) | |
| ≥ 40 years | 14 (39%) | 10 (53%) | 14 (48%) | 10 (48%) | 46 (46%) | |
| Sex | 0.098 | |||||
| Male | 27 (75%) | 9 (47%) | 23 (79%) | 14 (67%) | 73 (70%) | |
| Female | 9 (25%) | 10 (53%) | 6 (21%) | 7 (33%) | 32 (30%) | |
| Ann Arbor stage | 0.695 | |||||
| I–II | 9 (25%) | 3 (16%) | 9 (31%) | 5 (24%) | 26 (25%) | |
| III–IV | 27 (75%) | 16 (84%) | 20 (69%) | 16 (76%) | 79 (75%) | |
| Extranodal involvement | ||||||
| Bone marrow | 12 (34%) | 7 (37%) | 7 (24%) | 9 (43%) | 35 (34%) | 0.559 |
| Central nervous system | 8 (22%) | 5 (26%) | 3 (10%) | 9 (43%) | 25 (24%) | 0.065 |
| Gastrointestinal tract | 13 (36%) | 10 (53%) | 14 (48%) | 6 (29%) | 43 (41%) | 0.335 |
| ≥ 2 sites | 19 (54%) | 2 (14%) | 8 (29%) | 9 (43%) | 38 (39%) | 0.037 |
| LDH > upper normal level | 28 (80%) | 14 (78%) | 17 (63%) | 16 (76%) | 75 (74%) | 0.464 |
| WHO performance scoreb | 0.002 | |||||
| 0–1 | 20 (56%) | 6 (55%) | 22 (92%) | 19 (73%) | 67 (73%) | |
| > 1 | 16 (44%) | 5 (45%) | 2 (8%) | 2 (27%) | 25 (27%) | |
| Bulky disease ≥ 10 cm | 9 (27%) | 2 (11%) | 6 (21%) | 7 (33%) | 24 (24%) | 0.371 |
| Peripheral blood blasts ≥ 30% | 1 (3%) | 2 (2%) | 0 | 2 (10%) | 5 (5%) | 0.224 |
| IPI-score c | 0.031 | |||||
| 0–2 | 11 (31%) | 6 (38%) | 17 (65%) | 12 (57%) | 46 (47%) | |
| 3–5 | 25 (69%) | 10 (62%) | 9 (35%) | 9 (43%) | 53 (53%) | |
| BL-risk scorec | 0.874 | |||||
| Low | 4 (11%) | 1 (6%) | 3 (12%) | 3 (14%) | 11 (11%) | |
| High | 32 (89%) | 16 (94%) | 23 (89%) | 18 (86%) | 89 (89%) | |
| HIV positivity | 3 (9%) | 1 (6%) | 6 (21%) | 3 (16%) | 13 (13%) | 0.384 |
| Rituximab in regimen | 23 (64%) | 18 (95%) | 23 (79%) | 21 (100%) | 85 (81%) | 0.003 |
| Lymphoma treatment prior to initiation of studied regimend | 3 (8%) | 5 (26%) | 3 (10%) | 1 (5%) | 12 (11%) | 0.141 |
LDH lactate dehydrogenase, HIV human immunodeficiency virus
aSeven of these patients were included in the original prospective HOVON 27 study [8]
bWHO performance score data missing for 13 patients, resulting in incalculable IPI-scores for 6 patients
cInternational Prognostic Index (IPI) score: age > 60 years, Ann Arbor stage III/IV disease, elevated serum lactate dehydrogenase (LDH), WHO performance score > 1, > 1 extranodal site (1 point for each) [20]; BL risk score: Ann Arbor stage III/IV disease, elevated serum LDH, WHO performance score > 1, and bulky disease (1 point for each) [9]
dMaximum of 3 (R-) CHOP (-like) courses
Seventy-five patients (71%) completed the planned treatment regimen without treatment modifications. Five patients switched to palliative therapy due to progressive disease (two LMB, one BFM, and two HOVON). Twenty patients switched to more intensive therapy or received additional chemotherapy courses due to insufficient response or heightened risk as perceived by the treating physician (11 LMB, 2 BFM, 6 HOVON, and 1 CODOX-M/IVAC). Three patients received fewer courses than planned or switched to less intensive therapies due to toxicity or comorbidity (two LMB, one HOVON). Two patients died early during treatment (one LMB, one CODOX-M/IVAC). For the different treatment regimens, the percentage of patients completing planned treatment modifications was respectively 56% for LMB, 84% for BFM, 69% for HOVON, and 91% for CODOX-M/IVAC (p = 0.020).
Response rates and survival
Clinical outcomes related to chemotherapy regimens are shown in Table 3. Median follow-up of all patients was 47 months (range 4–172 months). Duration of follow-up was variable between treatment groups (p = 0.002) due to CODOX-M/IVAC having been introduced more recently (maximum follow-up duration 75 months). To minimize potential effects of late non-relapse mortality in the other three treatment groups (maximum follow-up duration 147–172 months), survival was censored at 5 years.
Table 3.
Outcomes per treatment regimen
| Treatment regimen | LMB | BFM | HOVON | CODOX-M/IVAC | All | P value |
|---|---|---|---|---|---|---|
| N | 36 | 19 | 29 | 21 | 105 | |
| Response rates | ||||||
| Complete response | 22 (65%) | 10 (53%) | 20 (69%) | 15 (75%) | 67 (66%) | 0.501 |
| Partial response | 5 (15%) | 4 (21%) | 3 (10%) | 2 (10%) | 14 (14%) | 0.705 |
| Refractory disease | 7 (21%) | 5 (26%) | 6 (21%) | 3 (15%) | 21 (21%) | 0.858 |
| Not evaluable | 2 (6%) | 0 | 0 | 1 (5%) | 3 (3%) | |
| Relapse rates | ||||||
| Relapse (at 1 year after end of treatment, corrected for competing events) | 7% (95% CI 0–17%) | 0% (95% CI 0–0%) | 9% (95% CI 0–21%) | 12% (95% CI 0–28%) | 7% (95% CI 2–13%) | 0.612 |
| 5-year survival rates | ||||||
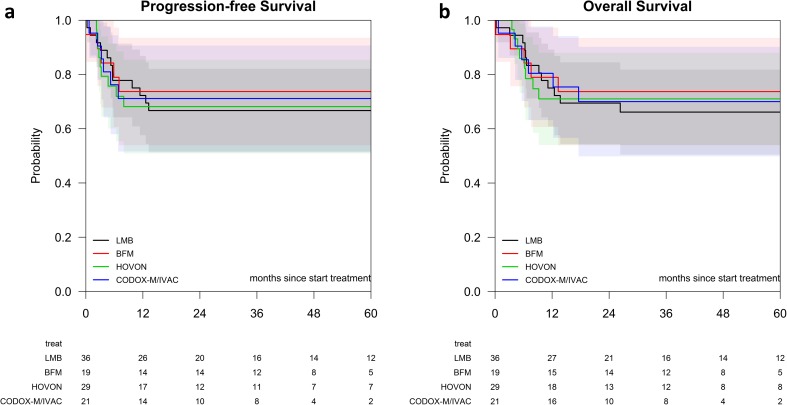
| Progression-free survival | 67% (95% CI 53–80%) | 74% (95% CI 57–91%) | 68% (95% CI 53–83%) | 71% (95% CI 54–88%) | 69% (95% CI 60–78%) | 0.966 |
| Overall survival | 66% (95% CI 53–80%) | 74% (95% CI 54–93%) | 71% (95% CI 54–88%) | 70% (95% CI 50–90%) | 69% (95% CI 60–78%) | 0.981 |
Of the 67 patients who achieved CR (Table 3), four patients relapsed (6%) and eventually died of disease progression. Two of these patients had been treated with the LMB regimen and two with the HOVON regimen. One patient in CR received intensification therapy including an allogeneic SCT directly following the treatment regimen because of extensive CNS involvement at presentation but died due to treatment-related complications. All other patients with initial complete responses were alive at the end of follow-up (93%).
There were no significant differences between the treatment regimens with respect to progression-free survival and overall survival, response rates, and relapse rates (Fig. 2 and Table 3). Because advanced patient age has been associated with poorer outcome in BL and the different treatment regiments might impact older patients differently, we stratified for patient age < 40 years versus patient age ≥ 40 years. No significantly different survival rates emerged between the treatment regimens for the different age groups (p = 0.991 for 5-year OS of patients < 40 years, p = 0.845 for 5-year OS of patients ≥ 40 years). Likewise, no different survival rates were detected following stratification for low (0–2) versus high (3–5) IPI scores (p = 0.885 for 5-year OS of patients with low IPI scores, p = 0.841 for 5-year OS of patients with high IPI scores). We could make no calculations for low versus high BL scores due to insufficient events in the low BL score group. Prognostic factors and survival rates for the various risk groups are listed in the Supplementary Material.
Fig. 2.
a Progression-free survival of BL patients treated with the LMB, BFM, HOVON, or CODOX-M/IVAC regimens. b Overall survival of BL patients treated with the LMB, BFM, HOVON, or CODOX-M/IVAC regimens. LMB, black line; BFM, red line; HOVON, green line; and CODOX-M/IVAC, blue line
Toxicity and treatment-related mortality
Transfusion requirements and CTCAE grade III–IV infectious disease, nephrotoxicity, or hepatotoxicity occurred in all four treatment groups. The affected percentage of patients was as follows for the LMB, BFM, HOVON, and CODOX-M/IVAC regimens, respectively: transfusion requirement 97, 95, 88, and 100% (p = 0.386); infectious disease 71, 63, 52, and 86% (p = 0.095); nephrotoxicity 11, 5.3, 4, and 7% (p = 0.672); hepatotoxicity 29, 26, 4, and 53% (p = 0.004). No significant difference in transfusion requirement or the occurrence of these toxicities was detected between patients < 40 years and patients ≥ 40 years.
Treatment-related deaths were rare: two patients died due to sepsis during the first course of chemotherapy) (one CODOX-M/IVAC, one LMB), five patients died from treatment-related complications from intensification therapy, including the three patients who received an allogeneic SCT. Two patients died during follow-up from unknown causes. Treatment-related mortality corrected for competing events was 5% (95% CI, 1–9%) at 1 year and 6% (95% CI, 1–10%) at 2 years after start of treatment.
Treatment of partial response, refractory disease, and disease relapse
Treatment strategies for partial response, refractory disease, and disease relapse were highly variable and dependent on patient condition, extent of disease, and earlier treatment. Of the 14 patients with PR, 9 patients received additional therapy. Four of these patients received local radiation therapy only, none of whom relapsed. The other five patients received additional chemotherapy, followed in three cases by autologous SCT. All of these patients achieved prolonged remissions. Of the untreated patients, two relapsed and one died of unknown causes. The other two patients remained in remission. Overall, 5-year OS of PR patients was 79% (median follow up 51 months, range 18–172 months) as opposed to 92% 5-year OS of CR patients. Of the 22 patients with RD, 21 died due to disease progression, one patient achieved remission after an intensive chemotherapy schedule.
All patients who relapsed eventually died, either due to disease progression or to complications of therapy. Median duration of disease-free survival in patients who relapsed was 3.5 months to first relapse. All relapses but one occurred within the first 9 months. In total, 23 of the 31 patients that were no longer alive at the end of follow-up died due to progressive disease.
Duration and costs of treatment
For all patients who completed high-risk protocol treatment without treatment schedule modifications, we calculated total duration of treatment and number of inpatient days as well as treatment costs (Table 4). The CODOX-M/IVAC regimen had the overall shortest duration of treatment (95 days versus 149–231 days for the other regimens). The HOVON regimen had the lowest number of inpatient days (63 days versus 93–134 days for the other regimens). The low risk variants of CODOX-M/IVAC and LMB (not shown in table) had median treatment duration of 78 days (range 68–85) and 102 days (range 90–159) and median inpatient treatment of 58 days (range 45–62) and 51 days (range 38–66), respectively.
Table 4.
Duration and cost of treatment per treatment regimen
| Treatment regimen | LMBa | BFM | HOVONb | CODOX-M/IVAC | P value |
|---|---|---|---|---|---|
| N | 13 | 16 | 18 | 15 | |
| Median number of treatment days (range) | |||||
| Duration of treatment according to protocol | 217 | 168 | 105 | 84 | |
| Observed duration of treatment | 231 (193–319) | 171 (146–226) | 149 (121–215) | 95 (80–155) | < 0.001 |
| Planned number of inpatient days according to protocol | 129 | 147 | 66 | 84 | |
| Observed number of inpatient days | 102 (70–148) | 134 (100–169) | 63 (53–109) | 93 (75–130) | < 0.001 |
| Treatment costs in € | |||||
| Medication costs excluding rituximab | 5940 | 7702 | 11,272 | 9323 | |
| Rituximab | 7282 | 14,564 | 9103 | 10,923 | |
| Blood product transfusions | 14,964 | 5349 | 4838 | 16,204 | |
| Diagnostic procedures | 18,627 | 27,941 | 13,971 | 18,627 | |
| Inpatient days | 64,063 | 86,320 | 46,959 | 55,991 | |
| Autologous graft mobilization and harvestc | – | – | 5251 | – | |
| Total costs in € | 110,876 | 141,877 | 91,394 | 111,068 | |
aData shown for high risk protocol
bIncluding 3× RiCHOP
cCosts for daycare, medication, laboratory activities, and apheresis procedure related to mobilization/harvesting of autologous graft
For treatment costs, the number of inpatient days and the number of rituximab gifts were the most important determinants. The number of rituximab gifts was not defined in the original treatment protocols. In practice, most treatment centers chose to administer 1–2 rituximab gifts per chemotherapy course in varying schedules. In the treatment regimens evaluated in this study, the number of rituximab gifts varied from four (LMB regimen) to eight (BFM regimen). The HOVON regimen was associated with the lowest total costs mainly due to the lowest number of inpatient days (91,394 €). The LMB regimen was associated with the lowest drug costs mainly due to the lowest number of rituximab administrations (13,222 € drug cost). The BFM protocol carried the highest costs as it comprised both the highest number of inpatient days as well as the highest cumulative rituximab dose (141,877 €).
Discussion
In the absence of a standard first line treatment of adult BL, we studied “real-world” efficacy, toxicity, and costs of four BL regimens frequently used in the Netherlands: LMB, BFM, HOVON, and CODOX-M/IVAC. Our aim was to support an evidence-based choice for a first line BL treatment regimen. Central pathology assessment was performed to ensure inclusion only of BL cases selected according to 2008 WHO classification criteria. These criteria remain essentially unchanged in the upcoming WHO 2016 classification [21], so that our study population remains a good approximation also of the WHO 2016 BL population.
Having validated the diagnosis, we found patient selection in the four treatment groups to be mostly similar in terms of age, known risk factors, and composite indices. The HOVON treatment group contained relatively fewer high-risk patients because patients with CNS disease and leukemic BL were excluded from the HOVON27 trial, which included patients up to 2003 [8], and were also not routinely treated with this regimen in the years that followed. In the HOVON and LMB treatment groups, fewer patients received rituximab as a higher percentage of patients started treatment prior to 2003–2004. Despite these differences, we found PFS and OS rates to be comparable between the four treatment regimens. Addition of rituximab did not significantly affect OS but did show a possible trend toward improved survival especially in the older patient groups (Supplemental Material on prognostic factors). Our data do not allow us to draw conclusions on the optimal number of rituximab gifts.
Next to efficacy, the toxicity spectrum could serve as an important parameter for optimal treatment choice. Overall, the treatment regimens seem to be comparably safe. Only hepatotoxicity was significantly different between the treatment regimens and highest for the CODOX-M/IVAC regimen. The relevance of this finding is unclear as dose adjustment for hepatotoxicity was reported for only one patient during first line treatment (LMB regimen). Since our analysis was limited to toxicities that could be quantifiably extracted from patient records, mucositis was not evaluated despite being a frequent cause of morbidity in patients undergoing intensive chemotherapy. In prospective studies (Table 5), incidence of mucositis was reported as 12–14% for LMB [6], 29% for BFM [10], 39% for HOVON [8], and 50% for CODOX-M/IVAC [9]. While these data imply that the CODOX-M/IVAC regimen may be more strongly associated with mucositis than the other treatment regimens, this did not reflect in a diminished percentage of patients completing treatment.
Table 5.
Summary of literature on studied treatment regimens
| Study | Protocol | N (enrollment) | Median age | Rituximab included | CR (%) | OS | NRM (%, years) |
|---|---|---|---|---|---|---|---|
| Soussain et al. [5] | LMB retrospective | 65 (1984–1991) | 26 | No | 89 | 74% (3 years) | 12 (1) |
| Diviné et al. [6] | LMB prospective | 72 (1996–2001) | 33 | No | 72 | 70% (2 years) | 4 (1) |
| Choi et al. [22] | LMB retrospective | 38 (1998–2008) | 47 | No | 74 | 68% (1 year) | 11 (1) |
| Hoelzer et al. [7] | BFM prospective | 35 L3-ALL only (1991-nr) | 36 | No | 74 | 51% (4 years) | 6 (4) |
| Tauro et al. [23] | BFM retrospective | 46 (nr-nr) | 32 | No | 87 | 80% (2 years) | Nr |
| Pohlen et al. [24] | BFM retrospective | 28 | Nr | 84 | Nr | 7 (5) | |
| Intermesoli et al. [25] | BFM retrospective | 105 (2002–2010) | 47 | Yes | 79 | 67% (3 years) | 18 (3) |
| Hoelzer [10] | BFM prospective | 263 (2002–2011) | 42 | Yes | 88 | 70% (5 years) | 5 (5) |
| van Imhoff et al. [8] | HOVON27 prospective | 27 (1994–2003) | 36 | No | 81 | 81% (5 years) | 0 (5) |
| Mead et al. [9] | CODOX-M/IVAC prospective | 52 (1995–1999) | 35 | No | 89 | 73% (2 years) | 13 (2) |
| Lacasce et al. [26] | dmCODOX -M/IVAC prospective | 14 (nr) | 47 | No | 86 | 64% (2 years) | 0 (2) |
| Mead et al. [11] | dmCODOX-M/IVAC prospective | 53 (2002–2005) | 37 | No | 74 | 67% (2 years) | 8 (2) |
| Maruyama et al. [27] | CODOX-M/IVAC retrospective | 15 | 39 | 87 | 87% (5 years) | 0 (5) | |
| Barnes et al. [28] | dmCODOX-M/IVAC retrospective | 80 (1992–2009) | 46 | No (40), yes (40) | 88 | 71% (3 years) | 8 (3) |
| Wästerlid et al. [29] | BFM, CODOX-M/IVAC | 71, 32 | 40, 42 | Nr, nr | 82, 69% | Nr, nr |
CR complete response following protocol treatment, OS overall survival, NRM non-relapse mortality, Nr not reported
As the final important parameter to guide medical decision-making, we evaluated the actual costs of the various treatment regimens and the treatment durations. Treatment duration is an important determinant from a comprehensive health economics point of view as it affects the time period a patient is impaired at work and at home. Dominant drivers of treatment cost were length of in-hospital stay (€ 640 per day) and the cumulative rituximab dose (€ 1821 per gift) for medication costs. The HOVON regimen was associated with the shortest in-hospital stay and the lowest medication costs. CODOX-M/IVAC was associated with the shortest total treatment duration and the second shortest duration of in-hospital stay and medication costs. Medication costs for all regimens are likely to decrease in the future as biosimilars of rituximab become available.
With efficacy and safety comparable between the four treatment regimens and health economics favoring the HOVON and CODOX-M/IVAC regimens, we weighed the advantages and disadvantages of these two regimens with regard to the choice for a standard first line BL therapy. Although the HOVON treatment group contained less high risk patients, the CODOX-M/IVAC regimen performed equally well and was completed by a significantly higher percentage of patients without significant treatment modifications. The CODOX-M/IVAC regimen has the further advantage of a low risk protocol variant with reduced doses of alkylating agents. Since these drugs are most commonly associated with chemotherapy-induced infertility, this is of relevance for the low risk population that mostly comprises young patients [30]. Based on these considerations, the CODOX-M/IVAC regimen seems the most rational choice for a standard first line BL therapy.
There are limitations to our study due to its retrospective observational nature. One important aspect pertains to possible treatment center-related differences that might affect outcome. The LMB-, HOVON-, and CODOX-M/IVAC regimens were each practiced in two or three hospitals lessening the impact of center-specific policies, but the BFM regimen was practiced in one center only. The survival rates we found are nevertheless comparable to those published in prospective studies of the individual treatment regimens and a population-based study comparing BFM, CODOX/M-IVAC, hyper-CVAD, and CHOP/CHOEP regimens (Table 5), externally validating our results. Another limitation results from the changing standards for response evaluation from 1995 to 2012, initially involving CT and later PET-CT imaging. In our study, two patients with PR did not receive intensification therapy but nevertheless remained in remission and may in fact have had a (unconfirmed) CR. Unfortunately, we could not perform a central radiology review, but even with the current standard of care, response evaluation in BL is known to be difficult. In a study of 27 BL patients with post-treatment PET-CT, positive predictive value was only 20% (negative predictive value was 100%) [31]. A study in pediatric Burkitt patients likewise showed a tendency for false positives due to acute inflammation and tumor necrosis [32]. In our study, in one PR patient, an extirpation was performed of a single residual mass that remained PET-positive despite intensification therapy, revealing an absence of vital BL tissue. This was not, however, routinely done. We believe in selected cases it may be prudent to strive for pathological confirmation of positive PET/CT results following end of treatment to avoid unnecessary intensification.
An issue highlighted by our study, is the current lack of effective treatment for refractory or relapsed patients. Of the 22 patients in our study that did not have a complete or partial response, 21 died, as well as all patients that relapsed. Escalation to autologous SCT is the best documented treatment option [33, 34]. A 2013 study from the Center for International Blood and Marrow Transplant Research reported on 241 patients receiving an autologous or allogeneic SCT for BL in second or subsequent CR [34]. The 5-year OS was shown to be 31% following autologous SCT and 20% following allogeneic SCT. Patients not in CR at the time of transplant had 5-year OS of 22 and 12%, respectively. In our patient cohort, three patients received allogeneic SCT as intensification therapy or following relapse but all patients died due to treatment-related complications. Autologous SCT did seem to be effective, but only for patients with chemosensitive disease and would be our treatment of choice for patients with PR or relapsed BL. Patients with refractory BL may be better served with novel therapies targeting contributing pathways in an experimental trial setting.
Recently, DA-EPOCH-R was proposed as a novel, highly promising first line treatment regimen [35]. With this dose-adjusted low intensity regimen, 87% freedom of disease progression was reported in 77 patients at a median follow-up of 25 months with relatively limited side effects [36]. Since this treatment can be administered in the outpatient setting, it is attractive from a health economics point of view. Based on the advantages with respect to cost and treatment duration, our data have led to the choice of the CODOX-M/IVAC regimen as the standard arm in the multinational randomized prospective HOVON127 trial that is designed to assess the possible superiority of DA-EPOCH-R (EU Clinical Trials register, EudraCT 2013-004394-27). Also, our data have led at least two treatment centers in the Netherlands to adopt CODOX-M/IVAC as BL treatment of choice.
In summary, our study demonstrates high cure rates for BL in a real-world setting. Given the lack of major differences in outcome and toxicity, health economic aspects may guide the choice of treatment.
Electronic supplementary material
(DOCX 145 kb).
(DOCX 5443 kb).
Acknowledgements
We would like to thank Els Willemse, Elise van Orsouw, and Ineke Dumaij, datamanagers of the Antoni van Leeuwenhoek Hospital, Radboud University Medical Center Nijmegen, and Reinier de Graaf Hospital, respectively, and Patty Jansen, pathologist in the Leiden University Medical Center for their efforts and assistance.
Compliance with ethical standards
Approval for the data collection was obtained from the Ethical Review Board of the Leiden University Medical Center (reference number P13.006). Informed consent was not required for this type of study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Key message
Four different first-line treatment strategies for adult Burkitt lymphoma were compared for real-world outcome, toxicity, and costs in a multicenter observational study. All treatment strategies were associated with high cure rates and were comparable in safety. Considerable differences, however, existed in treatment duration and costs which may guide future treatment choice.
D. de Jong and H. Veelken shared last authors.
Electronic supplementary material
The online version of this article (10.1007/s00277-017-3167-7) contains supplementary material, which is available to authorized users.
References
- 1.Boerma EG, van Imhoff GW, Appel IM, Veeger NJ, Kluin PM, Kluin-Nelemans JC. Gender and age-related differences in Burkitt lymphoma—epidemiological and clinical data from The Netherlands. Eur J Cancer. 2004;40(18):2781–2787. doi: 10.1016/j.ejca.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004;104(10):3009–3020. doi: 10.1182/blood-2004-02-0405. [DOI] [PubMed] [Google Scholar]
- 3.Linch DC. Burkitt lymphoma in adults. Br J Haematol. 2012;156(6):693–703. doi: 10.1111/j.1365-2141.2011.08877.x. [DOI] [PubMed] [Google Scholar]
- 4.Kasamon YL, Swinnen LJ. Treatment advances in adult Burkitt lymphoma and leukemia. Curr Opin Oncol. 2004;16(5):429–435. doi: 10.1097/00001622-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Soussain C, Patte C, Ostronoff M, Delmer A, Rigalhuguet F, Cambier N, Leprise PY, Francois S, Conymakhoul P, Harousseau JL, Janvier M, Chauvenet L, Witz F, Pico J. Small noncleaved cell lymphoma and leukemia in adults-a retrospective study of 65 adults treated with the LMB pediatric protocols. Blood. 1995;85(3):664–674. [PubMed] [Google Scholar]
- 6.Divine M, Casassus P, Koscielny S, Bosq J, Sebban C, Le Maignan C, Stamattoulas A, Dupriez B, Raphael M, Pico JL, Ribrag V, Goelams G. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16(12):1928–1935. doi: 10.1093/annonc/mdi403. [DOI] [PubMed] [Google Scholar]
- 7.Hoelzer D, Ludwig WD, Thiel E, Gassmann W, Loffler H, Fonatsch C, Rieder H, Heil G, Heinze B, Arnold R, Hossfeld D, Buchner T, Koch P, Freund M, Hiddemann W, Maschmeyer G, Heyll A, Aul C, Faak T, Kuse R, Ittel TH, Gramatzki M, Diedrich H, Kolbe K, Fuhr HG, Fischer K, SchadeckGressel C, Weiss A, Strohscheer I, Metzner B, Fabry U, Gokbuget N, Volkers B, Messerer D, Uberla K. Improved outcome in adult B-cell acute lymphoblastic leukemia. Blood. 1996;87(2):495–508. [PubMed] [Google Scholar]
- 8.van Imhoff GW, van der Holt B, MacKenzie MA, Ossenkoppele GJ, Wijermans PW, Kramer MHH, van’t Veer MB, Schouten HC, Kooy MV, van Oers MHJ, Raemaekers JMM, Sonneveld P, Meulendijks LAMH, Kluin PM, Kluin-Nelemans HC, Verdonck LF. Short intensive sequential therapy followed by autologous stem cell transplantation in adult Burkitt, Burkitt-like and lymphoblastic lymphoma. Leukemia. 2005;19(6):945–952. doi: 10.1038/sj.leu.2403733. [DOI] [PubMed] [Google Scholar]
- 9.Mead GM, Sydes MR, Walewski J, Grigg A, Hatton CS, Norbert P, Guarnaccia C, Lewis MS, McKendrick J, Stenning SP, Wright D, Collaborators UL. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13(8):1264–1274. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 10.Hoelzer D, Walewski J, Dohner H, Viardot A, Hiddemann W, Spiekermann K, Serve H, Duhrsen U, Huttmann A, Thiel E, Dengler J, Kneba M, Schaich M, Schmidt-Wolf IG, Beck J, Hertenstein B, Reichle A, Domanska-Czyz K, Fietkau R, Horst HA, Rieder H, Schwartz S, Burmeister T, Gokbuget N, German Multicenter Study Group for Adult Acute Lymphoblastic L Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood. 2014;124(26):3870–3879. doi: 10.1182/blood-2014-03-563627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead GM, Barrans SL, Qian WD, Walewski J, Radford JA, Wolf M, Clawson SM, Stenning SP, Yule CL, Jack AS, Cli UNCRIL, Lymphoma AL A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial) Blood. 2008;112(6):2248–2260. doi: 10.1182/blood-2008-03-145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on L Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 14.CBS Statistics Netherlands Consumer prices (2016) http://statline.cbs.nl/StatWeb/publication/?VW=T&DM=SLEN&PA=71311eng&LA=EN
- 15.Royal Dutch Pharmacists Association Knowledge Bank. https://www.knmp.nl/producten-en-diensten/knmp-kennisbank
- 16.Hakkaart-van Roijen L, van der Linden N, Bouwamans C, Kanters T, Swan Tan S (2015) Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Bijlage 1. https://www.zorginstituutnederland.nl/binaries/zinl/documenten/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg/Richtlijn+voor+het+uitvoeren+van+economische+evaluaties+in+de+gezondheidszorg+%28verdiepingsmodules%29.pdf
- 17.Leunis A, Blommestein HM, Huijgens PC, Blijlevens NM, Jongen-Lavrencic M, Uyl-de Groot CA. The costs of initial treatment for patients with acute myeloid leukemia in the Netherlands. Leuk Res. 2013;37(3):245–250. doi: 10.1016/j.leukres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Gaultney JG, Franken MG, Tan SS, Redekop WK, Huijgens PC, Sonneveld P, Uyl-de Groot CA. Real-world health care costs of relapsed/refractory multiple myeloma during the era of novel cancer agents. J Clin Pharm Ther. 2013;38(1):41–47. doi: 10.1111/jcpt.12020. [DOI] [PubMed] [Google Scholar]
- 19.Blommestein HM, Verelst SG, Huijgens PC, Blijlevens NM, Cornelissen JJ, Uyl-de Groot CA. Real-world costs of autologous and allogeneic stem cell transplantations for haematological diseases: a multicentre study. Ann Hematol. 2012;91(12):1945–1952. doi: 10.1007/s00277-012-1530-2. [DOI] [PubMed] [Google Scholar]
- 20.(1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. The N Engl J Med 329 (14):987–994. doi:10.1056/NEJM199309303291402 [DOI] [PubMed]
- 21.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi MK, Jun HJ, Lee SY, Kim KH, Lim DH, Kim K, Ko YH, Kim WS, Kim SJ. Treatment outcome of adult patients with Burkitt lymphoma: results using the LMB protocol in Korea. Ann Hematol. 2009;88(11):1099–1106. doi: 10.1007/s00277-009-0729-3. [DOI] [PubMed] [Google Scholar]
- 23.Tauro S, Cochrane L, Lauritzsen GF, Baker L, Delabie J, Roberts C, Mahendra P, Holte H. Dose-intensified treatment of Burkitt lymphoma and B-cell lymphoma unclassifiable, (with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma) in young adults (< 50 years): a comparison of two adapted BFM protocols. Am J Hematol. 2010;85(4):261–263. doi: 10.1002/ajh.21648. [DOI] [PubMed] [Google Scholar]
- 24.Pohlen M, Gerth HU, Liersch R, Koschmieder S, Mesters RM, Kessler T, Appelmann I, Muller-Tidow C, Berdel WE. Efficacy and toxicity of a rituximab and methotrexate based regimen (GMALL B-ALL/NHL 2002 protocol) in Burkitt’s and primary mediastinal large B-cell lymphoma. Am J Hematol. 2011;86(12):E61–E64. doi: 10.1002/ajh.22165. [DOI] [PubMed] [Google Scholar]
- 25.Intermesoli T, Rambaldi A, Rossi G, Delaini F, Romani C, Pogliani EM, Pagani C, Angelucci E, Terruzzi E, Levis A, Cassibba V, Mattei D, Gianfaldoni G, Scattolin AM, Di Bona E, Oldani E, Parolini M, Gokbuget N, Bassan R. High cure rates in Burkitt lymphoma and leukemia: a Northern Italy Leukemia Group study of the German short intensive rituximab-chemotherapy program. Haematologica. 2013;98(11):1718–1725. doi: 10.3324/haematol.2013.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacasce A, Howard O, Lib S, Fisher D, Weng A, Neuberg D, Shipp M. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45(4):761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 27.Maruyama D, Watanabe T, Maeshima AM, Nomoto J, Taniguchi H, Azuma T, Mori M, Munakata W, Kim SW, Kobayashi Y, Matsuno Y, Tobinai K. Modified cyclophosphamide, vincristine, doxorubicin, and methotrexate (CODOX-M)/ifosfamide, etoposide, and cytarabine (IVAC) therapy with or without rituximab in Japanese adult patients with Burkitt lymphoma (BL) and B cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and BL. Int J Hematol. 2010;92(5):732–743. doi: 10.1007/s12185-010-0728-0. [DOI] [PubMed] [Google Scholar]
- 28.Barnes JA, LaCasce AS, Feng Y, Toomey CE, Neuberg D, Michaelson JS, Hochberg EP, Abramson JS. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol. 2011;22(8):1859–1864. doi: 10.1093/annonc/mdq677. [DOI] [PubMed] [Google Scholar]
- 29.Wasterlid T, Brown PN, Hagberg O, Hagberg H, Pedersen LM, D'Amore F, Jerkeman M. Impact of chemotherapy regimen and rituximab in adult Burkitt lymphoma: a retrospective population-based study from the Nordic Lymphoma Group. Ann Oncol. 2013;24(7):1879–1886. doi: 10.1093/annonc/mdt058. [DOI] [PubMed] [Google Scholar]
- 30.Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, Vance A, Ginsberg JP. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97(1):134–140. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrillo-Cruz E, Marin-Oyaga VA, Sole Rodriguez M, Borrego-Dorado I, de la Cruz VF, Quiroga Cantero E, Manzanares Perez M, Capote FJ, Ramirez Sanchez MJ, Espigado Tocino I, Perez-Vega H, Vazquez-Albertino R, Perez-Simon JA. Role of 18F-FDG-PET/CT in the management of Burkitt lymphoma. Eur J Haematol. 2015;94(1):23–30. doi: 10.1111/ejh.12284. [DOI] [PubMed] [Google Scholar]
- 32.Riad R, Omar W, Sidhom I, Zamzam M, Zaky I, Hafez M, Abdel-Dayem HM. False-positive F-18 FDG uptake in PET/CT studies in pediatric patients with abdominal Burkitt’s lymphoma. Nucl Med Commun. 2010;31(3):232–238. doi: 10.1097/MNM.0b013e328334fc14. [DOI] [PubMed] [Google Scholar]
- 33.Peniket AJ, de Elvira MCR, Taghipour G, Cordonnier C, Gluckman E, de Witte T, Santini G, Blaise D, Greinix H, Ferrant A, Cornelissen J, Schmitz N, Goldstone AH, Registry EL. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transpl. 2003;31(8):667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 34.Maramattom LV, Hari PN, Burns LJ, Carreras J, Arcese W, Cairo MS, Costa LJ, Fenske TS, Lill M, Freytes CO, Gale RP, Gross TG, Hale GA, Hamadani M, Holmberg LA, Hsu JW, Inwards DJ, Lazarus HM, Marks DI, Maloney DG, Maziarz RT, Montoto S, Rizzieri DA, Wirk B, Gajewski JL. Autologous and allogeneic transplantation for Burkitt lymphoma outcomes and changes in utilization: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2013;19(2):173–179. doi: 10.1016/j.bbmt.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, Widemann B, Staudt LM, Jaffe ES, Little RF, Wilson WH. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915–1925. doi: 10.1056/NEJMoa1308392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunleavy Kea (2015) Risk-adapted therapy in adults with Burkitt lymphoma: preliminary report of a multicenter prospective phase II study of DA-EPOCH-R. Blood (ASH 2015) 126 (342)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 145 kb).
(DOCX 5443 kb).