Abstract
Rationale: Recombinant fragment of human surfactant protein D (rfhSP-D) has been shown to suppress house dust mite– and Aspergillus fumigatus–induced allergic inflammation in murine models.
Objectives: We sought to elucidate the effect of rfhSP-D on high-affinity IgE receptor– and CD23-mediated, grass pollen–induced allergic inflammatory responses.
Methods: rfhSP-D, containing homotrimeric neck and lectin domains, was expressed in Escherichia coli BL21(λDE3)pLysS cells. Peripheral blood mononuclear cells and sera were obtained from individuals with grass pollen allergy (n = 27). The effect of rfhSP-D on basophil activation and histamine release was measured by flow cytometry. IgE-facilitated allergen binding and presentation were assessed by flow cytometry. T-helper cell type 2 (Th2) cytokines were measured in cell culture supernatants. The effect of rfhSP-D on IgE production by B cells when stimulated with CD40L, IL-4, and IL-21 was also determined.
Measurements and Main Results: rfhSP-D bound to Phleum pratense in a dose- and calcium-dependent manner. Allergen-induced basophil responsiveness and histamine release were inhibited in the presence of rfhSP-D, as measured by CD63, CD203c (P = 0.0086, P = 0.04205), and intracellularly labeled diamine oxidase (P = 0.0003, P = 0.0148). The binding of allergen–IgE complexes to B cells was reduced by 51% (P = 0.002) in the presence of rfhSP-D. This decrease was concomitant with reduction in CD23 expression on B cells (P < 0.001). rfhSP-D suppressed allergen-driven CD27−CD4+CRTh2+ T-cell proliferation (P < 0.01), IL-4, and IL-5 levels (all P < 0.01). Moreover, rfhSP-D inhibited CD40L/IL-4– and IL-21–mediated IgE production (77.12%; P = 0.02) by B cells.
Conclusions: For the first time, to our knowledge, we show that rfhSP-D inhibited allergen-induced basophil responses at a single-cell level and suppressed CD23-mediated facilitated allergen presentation and Th2 cytokine production. In addition, rfhSP-D inhibited IgE synthesis by B cells, which is also a novel observation.
Keywords: innate immunity, recombinant fragment of human surfactant protein D, allergic rhinitis, facilitated allergen presentation, IgE synthesis
At a Glance Commentary
Scientific Knowledge on the Subject
Pulmonary surfactant protein D is a soluble pattern recognition innate immune molecule involved in the clearance of pathogens, apoptotic/necrotic cells, and down-regulation of allergic inflammation. A recombinant fragment of human surfactant protein D (rfhSP-D) has been shown to be involved in pattern recognition of glycoprotein allergens derived from the house dust mite (Dermatophagoides pteronyssinus) and Aspergillus fumigatus and to inhibit histamine release by sensitized basophils in vitro. The effects of rfhSP-D on allergic effector cells and allergen-induced T- and B-cell responses have yet to be evaluated.
What This Study Adds to the Field
For the first time, we demonstrate that rfhSP-D prevents CD23-mediated, IgE-facilitated allergen presentation by B cells to CD4+ T cells and inhibits Th2 proallergic responses. rfhSP-D also inhibits IgE production by B cells. Moreover, the effect of rfhSP-D on allergen-induced basophil activation and histamine release at the single-cell level is reported.
Lung surfactant protein D (SP-D) is a soluble innate immune molecule involved in the recognition and clearance of pathogens, apoptotic/necrotic cells, and modulation of allergic inflammation (1, 2). A recombinant fragment of human surfactant protein D (rfhSP-D), comprising homotrimeric neck and C-type lectin or carbohydrate recognition domain (CRD), has been shown to be as effective as the full-length molecule in suppressing immunological parameters (3, 4) in a murine model of allergic hypersensitivity to Aspergillus fumigatus allergens (5, 6). rfhSP-D can recognize glycoprotein allergens and inhibit histamine release by sensitized basophils in vitro in response to house dust mite (Dermatophagoides pteronyssinus) (6–9) and A. fumigatus allergens. Madan and colleagues demonstrated that therapeutic application of rfhSP-D caused a marked reduction in specific IgE and IgG1 levels, along with peripheral blood eosinophilia and pulmonary infiltration in a BALB/c murine model of allergic bronchopulmonary aspergillosis (10). In addition, rfhSP-D treatment was found to reduce the splenic levels of proallergic T-helper cell type 2 (Th2) cytokines (IL-4 and IL-5) and increase the protective T-helper cell type 1 (Th1) cytokine level (IFN-γ). Although rfhSP-D has been shown to modulate IgE-driven allergic inflammation, the exact mechanisms by which it exerts its immunomodulatory effects remain unclear.
We therefore tested if rfhSP-D binds to grass pollen allergen (Phleum pratense or Phlp) and inhibits histamine release and activation of basophils derived from individuals with grass pollen allergy. We further hypothesized that rfhSP-D can inhibit IgE-facilitated antigen presentation (FAP), which is dependent on the interaction of allergen–IgE complexes with low-affinity IgE receptor (CD23) on the surface of B cells. Moreover, the effect of rfhSP-D on Th2 cells and IgE synthesis from B cells was examined. Some of the results of this study were previously reported in the form of an abstract (11).
Methods
Subjects
Untreated, well-characterized patients with grass pollen allergy (seasonal allergic rhinitis) (n = 27) were recruited (Table 1). All subjects were selected on the basis of moderate to severe seasonal allergic rhinitis and poor symptom control in previous years despite regular medication use. The subjects had a positive skin prick test response (>5-mm wheal) to P. pratense grass pollen extract (Soluprick; ALK-Abelló, Hørsholm, Denmark). The study protocol was approved by the Royal Brompton and Harefield Hospitals NHS Trust Ethics Committee. All subjects provided written informed consent.
Table 1.
Subject Characteristics
| Seasonal Allergic Rhinitis (n = 27) | |
|---|---|
| Sex, M/F, n | 15/12 |
| Age, yr, mean (range) | 29 (23–64) |
| Allergen grass-specific IgE, mean (SD) | 33.9 ± 28.7 |
| Total IgE, mean (SD) | 387.1 ± 362.1 |
| Allergen skin prick test, mm2, mean (SD) | 7 (3.95) |
Distributions of age, sex, specific IgE, and skin prick test are displayed.
Expression and Purification of rfhSP-D
The rfhSP-D molecule was expressed in Escherichia coli. Details of the methods are provided in the online supplement. The endotoxin level in the protein preparation was determined by using the QCL-1000 Limulus Amebocyte Lysate System (Lonza, Allendale, NJ). The assay was linear over a range of 0.1–1.0 endotoxin units (EU)/ml (10 EU = 1 ng of endotoxin) and found to be less than 1 EU/ml of rfhSP-D. Bis(sulfosuccinimidyl)suberate (BS3) cross-linking reagent (Thermo Fisher Scientific, Loughborough, UK) was used to confirm the trimerization of rfhSP-D.
Binding of rfhSP-D to P. pratense Allergen
A 96-well Nunc MaxiSorp microtiter plate (Thermo Fisher Scientific) was coated overnight with 5 μg/ml Phlp allergen and left overnight at 4°C. The plate was blocked with 1% wt/vol bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 2 hours at room temperature. The microtiter plate was then washed three times with PBS and 0.05% Tween 20, and biotinylated rfhSP-D or BSA (control) was added at varying concentrations in 5 mM CaCl2. After the addition of rfhSP-D, the plate was further incubated for 2 hours at 37°C and then washed as before. Horseradish peroxidase–conjugated streptavidin at 1:1,000 dilution was added to each well and further incubated for 1 hour at 37°C, followed by an additional washing step. Binding of rfhSP-D to Phlp allergen was detected by addition of o-phenylenediamine substrate (Sigma-Aldrich, Dorset, UK), and color was read at 415 nm. Far Western blotting was used to detect the binding of rfhSP-D with P. pratense extract. Details of the methods are described in the Supplementary Methods section in the online supplement.
Ex Vivo Basophil Reactivity and Histamine Release Assay
The effects of rfhSP-D on allergen-induced basophil responsiveness and histamine release at a single-cell level were measured by flow cytometry (12). Briefly, 0, 33, and 100 ng/ml of Phlp was added to heparinized whole blood obtained from patients with grass pollen allergy. These blood samples with or without rfhSP-D (5 μg/ml) were incubated for 15 minutes in a water bath at 37°C. Cells were then immunostained with anti–human CD3, CD303, CD294 (chemoattractant receptor–homologous molecule expressed on Th2 cells [CRTh2]), CD203c, CD63, and CD107a (all from BD Biosciences, San Jose, CA). Erythrocytes from whole blood were lysed with BD lysing solution (BD Biosciences) for 10 minutes at room temperature in the dark. Samples were centrifuged (5 min, 200 × g), and the supernatants were discarded. Cells were fixed and then permeabilized with BD Cytofix/Cytoperm (BD Biosciences). Fluorochrome-labeled diamine oxidase (DAO) (BD Biosciences) was added, and the cells were incubated for 30 minutes at 4°C. Cells were washed and resuspended in 450 μl of ice-cold fixative solution (BD Biosciences) prior to acquisition on a BD FACSCanto II flow cytometer (BD Biosciences). Analyses were performed using BD FACSDiva version 6.1.1 software (BD Biosciences).
IgE-facilitated Allergen Binding Assay
IgE-facilitated allergen binding to B cells was performed as previously described (13, 14). CD23-enriched B cells were treated with 5 μg/ml rfhSP-D before or after allergen–IgE complex formation in the presence of 5 mM CaCl2 for 1 hour. Indicator serum (20 μl) containing a high concentration of grass pollen (P. pratense)-specific IgE greater than 100 kU/L was preincubated with 5 μl of allergen (5 μg/ml) at 37°C for 1 hour to form allergen–IgE complexes. Next, 1 × 105 Epstein-Barr virus–transformed B cells (5 μl) were added to the allergen-IgE mixture and incubated for further 1 hour at 4°C. Cells were then washed, and allergen–IgE complexes bound to B cells were detected using a polyclonal human anti-IgE–labeled antibody (Miltenyi Biotech, Woking, UK). Cells were acquired by flow cytometry (BD FACSCanto II flow cytometer) (see online supplement for further details).
IgE-facilitated Antigen Presentation
CD4+CD25− T cells and B cells were enriched by magnetic isolation from peripheral blood mononuclear cells (PBMCs) obtained from individuals with grass pollen allergy. Sera from subjects with grass pollen allergy (20 μl) were preincubated with 0, 0.1, 1, and 10 μg/ml allergen (5 μl) at 37°C for 1 hour to form allergen–IgE complexes, which were then added to autologous B cells (irradiated at 6,000 rad) and incubated for 18 hours prior to coculture with CD4+CD25− T cells for 6 days. T-cell proliferation was measured by tritiated thymidine (3H-thymidine) incorporation, and cytokine levels were measured in the cell culture supernatants using the commercially available MAGPIX MILLIPLEX kit (EMD Millipore, Watford, UK) (see the Supplementary Methods section in the online supplement). Furthermore, the PBMCs obtained were immunostained with CellTrace Violet dye (Thermo Fisher Scientific) and incubated with grass pollen allergen (0, 1, 5, or 15 μg/ml) for 7 days in the presence or absence of rfhSP-D (5 μg/ml or 10 μg/ml) and BSA (10 μg/ml) at 37°C (5% vol/vol CO2). Cells were surface stained with anti–human CD4, CD25, CD27, and CD294 (CRTh2) antibodies and intracellularly stained with anti–IL-4, anti–IL-5, and anti–IFN-γ antibodies (BD Biosciences).
IgE Secretion Assay
The immunomodulatory effects of rfhSP-D on IgE synthesis by B cells was assessed using PBMCs obtained from well-characterized individuals with grass pollen allergy (n = 10). PBMCs were stimulated with recombinant P. pratense (5 μg/ml) and IL-4 (100 ng/ml) (R&D Systems, Abingdon, UK), CD40L (100 ng/ml) (R&D Systems), and IL-21 (100 ng/ml) (Prospec-Tany TechnoGene, East Brunswick, NJ) in the presence of rfhSP-D and BSA at 5 μg/ml each for 14 days at 37°C. Total IgE levels were quantified in the cell culture supernatants using ImmunoCAP Total IgE fluorescence enzyme immunoassay (Thermo Fisher Scientific).
Statistical Analysis
Within-group comparisons were performed using the Wilcoxon signed-rank test. Between-group comparisons were performed using the Mann-Whitney U test. Correlation coefficients were obtained by Spearman’s method. The statistical software package used was Prism version 6 (GraphPad Software Inc., La Jolla, CA). P values less than 0.05 were considered significant.
Results
rfhSP-D Binds to P. pratense Allergen in a Calcium- and Carbohydrate-Dependent Manner
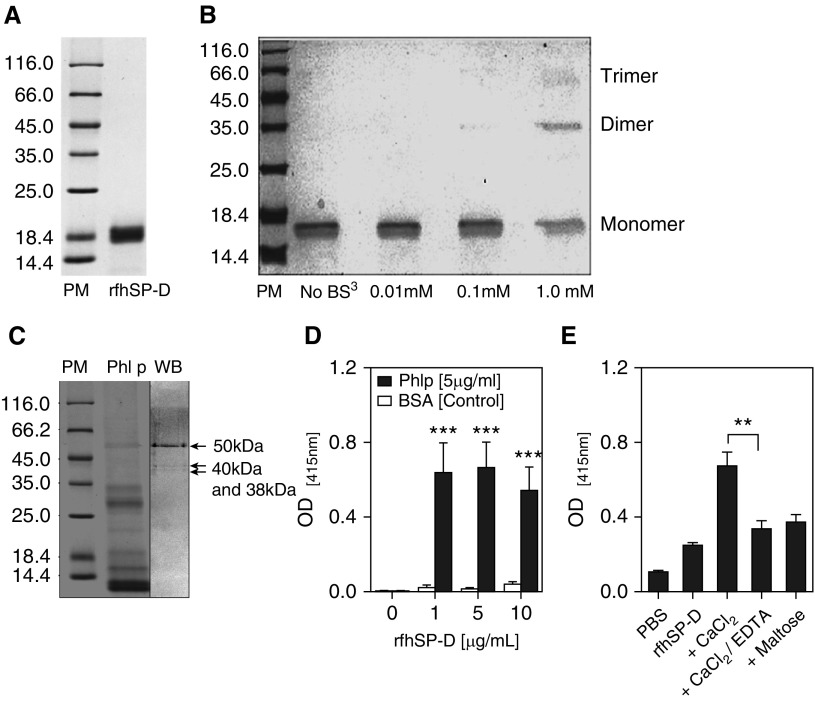
Affinity-purified rfhSP-D–containing homotrimeric neck and CRD regions appeared as an approximately 20 kD band on a 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (Figure 1A). A dose-dependent BS3 cross-linking effect on rfhSP-D trimerization was observed. BS3 induced cross-linking at the concentration of 1 mM, confirming trimerization of rfhSP-D in solution (Figure 1B). rfhSP-D was shown to bind to three grass pollen proteins around the regions of 50, 40, and 38 kD by far Western blot analysis (Figure 1C). Optimal binding occurred at the 5 μg/ml concentration of rfhSP-D (Figure 1D). Moreover, this binding of rfhSP-D to P. pratense allergen was calcium dependent and partly carbohydrate dependent. The interaction was considerably inhibited in the presence of 5 mM ethylenediaminetetraacetic acid (P = 0.002) and 5 mM maltose (Figure 1E).
Figure 1.
Purification of recombinant fragment of human surfactant protein D (rfhSP-D) and characterization of its binding to Phleum pratense (Phl P) extract (A) by 15% vol/vol sodium dodecyl sulfate–polyacrylamide gel electrophoresis showing purified rfhSP-D protein at approximately 20 kD. (B) Trimerization of rfhSP-D was achieved at 1 mM concentration of bis(sulfosuccinimidyl) suberate (BS3) cross-linking agent. (C) Far Western blot (WB) showing that rfhSP-D binds to three P. pratense proteins (50 kD, 40 kD, and 38 kD). Lane 1 = protein marker (PM); lane 2 = P. pratense extract (Phl p); lane 3 = Western blot. (D) rfhSP-D binds to P. pratense extract. (E) The binding of rfhSP-D to P. pratense extract is calcium and carbohydrate dependent and is inhibited by 5 mM ethylenediaminetetraacetic acid (EDTA) and 5 mM maltose. Data are presented as median (interquartile range) and are representative of five to seven independent experiments. **P < 0.01; ***P < 0.001. BSA = bovine serum albumin; OD = optical density; PBS = phosphate-buffered saline.
rfhSP-D Inhibits High-Affinity IgE Receptor–mediated Basophil Activation and Histamine Release
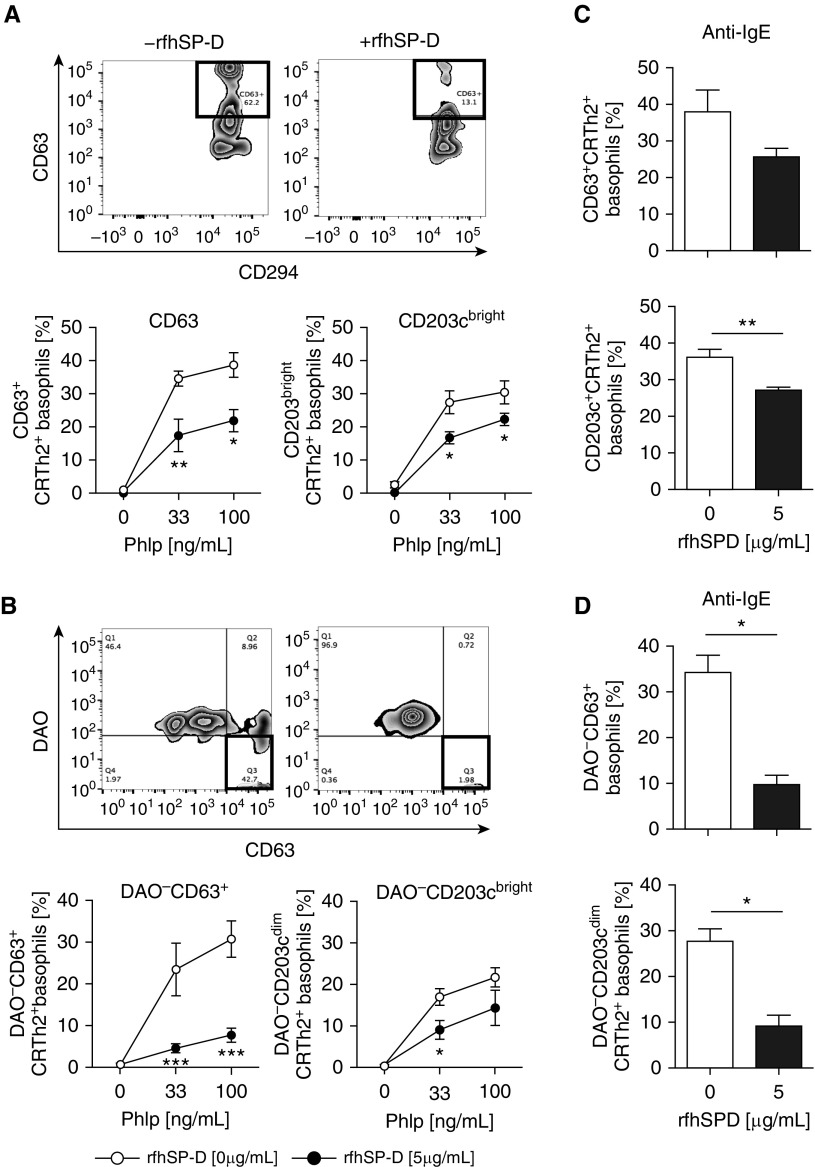
Ex vivo grass pollen–induced basophil responsiveness, as measured by the expression of CD63 and CD203Cbright on CRTh2+ basophils, was inhibited by rfhSP-D (5 μg/ml) (Figure 2A). At the optimal allergen (P. pratense) concentrations (33 ng/ml, 100 ng/ml), the proportion of CD63+CRTh2+ and CD203cbrightCRTh2+ basophils was significantly higher in the absence of rfhSP-D and decreased in the presence of rfhSP-D (5 μg/ml) (P = 0.0086 and P = 0.0205, respectively) (Figure 2A). Fluorochrome-labeled DAO was used to detect intracellular histamine in the presence and absence of rfhSP-D after ex vivo allergen stimulation of basophils. The proportions of DAO−CD63+ and DAO−CD203cbright basophils were significantly increased after ex vivo grass pollen allergen stimulation in a dose-dependent manner. This increase in the proportions of DAO−CD63+ and DAO−CD203cbright basophils was inhibited when basophils were exposed to rfhSP-D (5 μg/ml) at 33 and 100 ng/ml concentrations (P = 0.0003 and P = 0.0148, respectively) (Figure 2B). The proportions of DAO−CD63+ and DAO−CD203cbright basophils were lower after IgE-mediated cross-linking of high-affinity IgE receptor on basophils (Figures 2C and 2D) in the presence of rfhSP-D (5 μg/ml) (P = 0.0262; P = 0.034).
Figure 2.
Recombinant fragment of human surfactant protein D (rfhSP-D) suppresses grass pollen allergen–driven, chemoattractant receptor–homologous molecule expressed on T-helper cell type 2–positive (CRTh2+) basophil activation and histamine release. (A) Representative fluorescence-activated cell sorting analysis plots of CD63+CRTh2+ basophils inhibited by rfhSP-D. CD63+CRTh2+ and CD203cbrightCRTh2+ basophils from patients with seasonal allergic rhinitis (n = 9) stimulated with Phleum pratense (PhlP) were suppressed in the presence of rfhSP-D (5 μg/ml). (B) Representative fluorescence-activated cell sorting analysis plot showing histamine suppression by diamine oxidase (DAO) in the presence of rfhSP-D using intracellularly labeled DAO. DAO−CD63+ and DAO−CD203cbright histamine release was suppressed by 5 μg/ml of rfhSP-D. (C and D) rfhSP-D suppressed basophil activation and histamine release, as measured by intracellularly labeled DAO stimulated with anti-IgE (100 ng/ml). Data are expressed as median (interquartile range). *P < 0.05; **P < 0.01; ***P < 0.001.
rfhSP-D Inhibits Binding of Allergen–IgE Complexes to B Cells
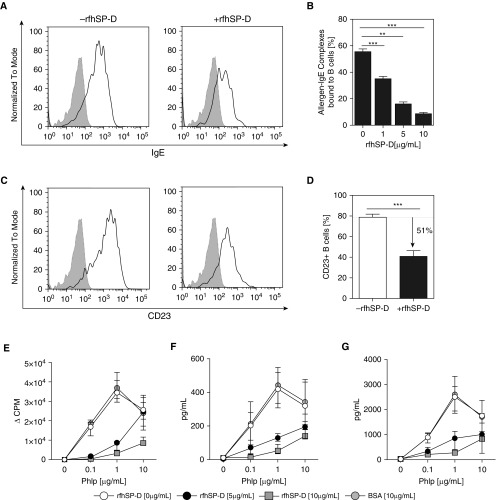
Using an in vitro functional assay of FAP (13, 15), we assessed whether rfhSP-D would inhibit cooperative binding of allergen–IgE complexes to CD23 on the surface of B cells (Figures 3A and 3C). Allergen–IgE complexes binding to B cells were decreased in a dose-dependent manner in the presence of rfhSP-D and were optimal at 10 μg/ml (P = 0.0001) (Figure 3B). This reduction in allergen–IgE binding to B cells coincided with the reduction of CD23 expression on B cells when cells were pretreated with rfhSP-D (Spearman’s rank correlation, r = −0.383; P < 0.001) (Figure 3D). However, rfhSP-D did not have an effect on preformed allergen–IgE complexes binding to CD23 on the surface of B cells (see Figure E2 and Supplementary Methods section in the online supplement).
Figure 3.
Inhibition of IgE-facilitated allergen binding and presentation by recombinant fragment of human surfactant protein D (rfhSP-D). The effect of rfhSP-D on cooperative binding of allergen–IgE complexes to CD23+ B cells was assessed in patients with grass pollen allergy (n = 10). Sera were incubated with 1 μg/ml Phleum pratense (PhlP) in the presence of rfhSP-D (5 and 10 μg/ml) and bovine serum albumin (BSA) (10 μg/ml). (A) Representative fluorescence-activated cell sorting analysis plot illustrating inhibition of allergen–IgE complex binding. (B) Dose-dependent inhibition of allergen–IgE complex binding to B cells. (C) Representative fluorescence-activated cell sorting analysis plot illustrating inhibition of CD23 binding. (D) Binding of allergen–IgE complexes to CD23+ B cells was reduced by 51%. rfhSP-D suppresses (E) CD4+CD25− T-cell proliferation, (F) IL-4+CD4+CD25− T cells, and (G) IL-5+CD4+CD25− T cells (n = 9). Data are expressed as median (interquartile range). The Mann-Whitney U test was used for between-group analyses, and the Wilcoxon signed-rank test was used for within-group analysis. **P < 0.01; ***P < 0.001. CPM = counts per minute.
CD23-mediated and IgE-facilitated Allergen Presentation by B Cells to T Cells Is Inhibited by rfhSP-D
To determine whether rfhSP-D could inhibit IgE-facilitated allergen presentation and CD4+CD25− T-effector cell activation, autologous B cells were preincubated with 0, 0.1, 1, or 10 μg/ml grass pollen allergen, IgE-containing serum, and rfhSP-D (0, 5, or 10 μg/ml). CD4+CD25− T-effector cells proliferated in an allergen dose–dependent manner (Figure 3E). rfhSP-D inhibited T-effector cell proliferation at 5 ng/ml (P = 0.0002) and 10 ng/ml (P = 0.007). Similarly, IL-4+CD4+CD25− (P = 0.007; P = 0.002) and IL-5+CD4+CD25− (P = 0.0033; P = 0.0003) T cells proliferated in an allergen dose–dependent manner (Figures 3F and 3G).
rfhSP-D Inhibits Grass Pollen–driven Th2-Cell Responses and Promotes Th1 Responses
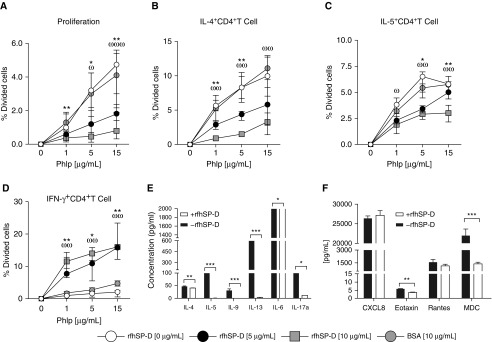
PBMCs obtained from individuals with grass pollen allergy and seasonal allergic rhinitis were stimulated with P. pratense extract (0, 1, 5, and 15 μg/ml) and exposed to varying concentrations of rfhSP-D (0, 5, and 10 μg/ml) for 6 days. rfhSP-D inhibited allergen-driven CD4+CD27−CRTh2+ T-cell proliferation in a dose-dependent manner when cells were stimulated with 1 μg/ml (5 μg/ml rfhSP-D, P < 0.0006; 10 μg/ml rfhSP-D, P < 0.0006), 5 μg/ml (5 μg/ml rfhSP-D, not significant; 10 μg/ml rfhSP-D, P < 0.048), and 15 μg/ml (5 μg/ml rfhSP-D, P < 0.0006; 10 μg/ml rfhSP-D, P < 0.0006) of P. pratense (Figure 4A and Table E1). In addition to T-cell proliferation, an allergen dose–dependent increase in the proportion of IL-4– and IL-5–producing CD4+CD27−CRTh2+ T cells was also observed. IL-4+ and IL-5+CD4+CD27−CRTh2+ T cells were significantly increased at 1 μg/ml (P < 0.007), 5 μg/ml (P < 0.007), and 15 μg/ml (P = 0.007) compared with 0 μg/ml of P. pratense. This increase in the proportion of IL-4+ and IL-5+CD4+CD27−CRTh2+ T cells was significantly reduced by rfhSP-D in a dose-dependent manner (Figures 4B and 4C). Conversely, rfhSP-D induced allergen-driven IFN-γ+CD4+CD27−CRTh2+ T-cell proliferation when stimulated with 1 μg/ml (5 μg/ml rfhSP-D, P = 0.031; 10 μg/ml rfhSP-D, P < 0.007), 5 μg/ml (5 μg/ml rfhSP-D, P < 0.007; 10 μg/ml rfhSP-D, P < 0.007), and 15 μg/ml (5 μg/ml rfhSP-D, P < 0.007; 10 μg/ml rfhSP-D, P < 0.007) of P. pratense (Figure 4D).
Figure 4.
Recombinant fragment of human surfactant protein D (rfhSP-D) suppresses Phleum pratense (PhlP)-stimulated, T-cell–proliferative responses. Peripheral blood mononuclear cells from patients with grass pollen allergy (n = 9) were stimulated with P. pratense (0, 1, 5, and 15 μg/ml) and then exposed to 0, 5, or 10 μg/ml of rfhSP-D or to 10 μg/ml bovine serum albumin (BSA) as a control. CD4+ T-cell proliferation was measured by flow cytometry using CellTrace Violet–immunostained CD27−CD4+CRTh2+ T cells. (A–C) rfhSP-D suppresses CD4+ T-cell proliferation (A); IL-4+CD4+ T cells (B); and IL-5+CD4+ T cells (C) in the presence of Phlp at 1, 5, or 15 μg/ml (n = 9) in a dose-dependent manner. (D) Production of IFN-γ+CD4+ T cells was increased in the presence of 5 and 10 μg/ml rfhSP-D. Data are expressed as median (interquartile range). In A–D, the Mann-Whitney U test was used for between-group analysis, and the Wilcoxon signed-rank test was used for within-group analysis. *P < 0.05 and **P < 0.01, 0 versus 5 μg/ml rhfSPD; ωP < 0.05, ωωP < 0.01, and ωωωP < 0.001, 0 versus 10 μg/ml rhfSPD. (E and F) Cell culture supernatant was collected, and secreted cytokines and chemokines were measured. All data are shown as mean ± SEM. In E and F, P values were determined by Wilcoxon signed-rank test. *P < 0.05; **P < 0.01; ***P < 0.001. MDC = macrophage-derived chemokine; RANTES = regulated upon activation, normal T-cell expressed and secreted.
rfhSP-D Modulates P. pratense–driven Th2 Responses
We also studied the effect of rfhSP-D on P. pretense–driven T-cell proliferation via 3H-thymidine incorporation assay. Pretreatment of PBMCs with rfhSP-D resulted in approximately 94% (P < 0.0001) and 93% (P < 0.0001) suppression of allergen-driven T-cell proliferation when 5 μg/ml and 10 μg/ml rfhSP-D, respectively, were used (see Figure E1). The ability of rfhSP-D to inhibit allergen-driven proallergic Th2 cytokine responses was also assessed using multiplex cytokine analysis. rfhSP-D (5 μg/ml) suppressed IL-4 (13.41%; P = 0.0019), IL-5 (99.31%; P < 0.0001), IL-9 (99.82%; P < 0.0001), IL-13 (99.48%; P < 0.0001), IL-6 (64.70%; P = 0.0286), and IL-17a (89.74%; P = 0.0286) levels (Figure 4E). rfhSP-D also suppressed eotaxin (36.33%; P < 0.0001) and macrophage-derived chemokine (93.78%; P < 0.0001) levels, whereas no effect of rfhSP-D was observed on the secretion levels of CXCL8 (P = 0.7808) and regulated upon activation, normal T-cell expressed and secreted (RANTES) (P = 0.2150) (Figure 4F).
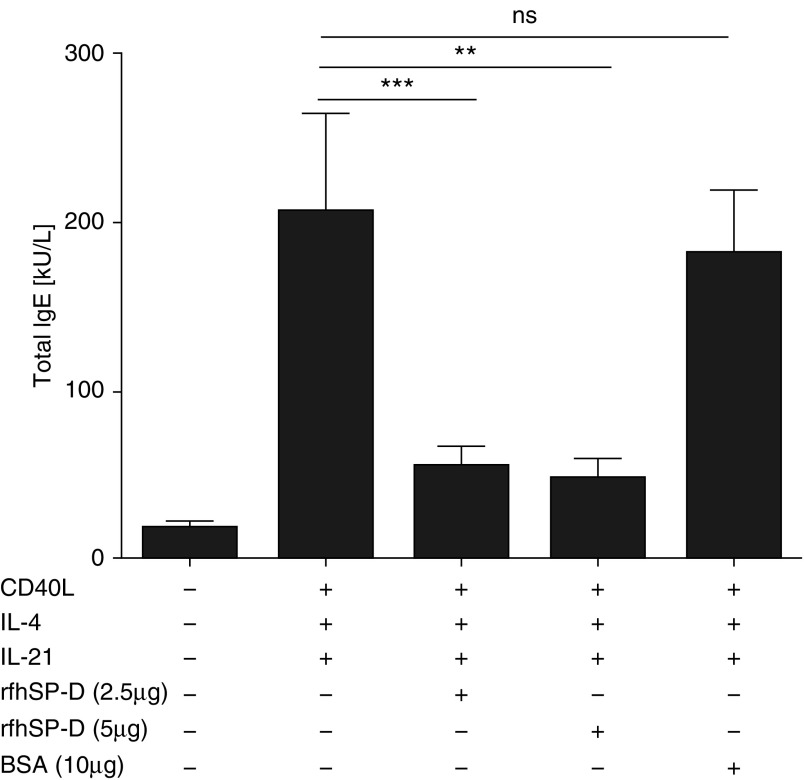
Effect of rfhSP-D on IgE Production by B Cells
The immunomodulatory effect of rfhSP-D on IgE synthesis was determined by stimulating PBMCs obtained from individuals with grass pollen allergy in the presence of CD40L, IL-4, and IL-21. CD40L and IL-4 induced IgE production, whereas IL-21 further enhanced IgE synthesis from B cells in PBMC cultures (60.97%: P = 0.3104). Remarkably, rfhSP-D inhibited CD40L/IL-4– and IL-21–induced total IgE production (77.12%; P = 0.0195) (Figure 5).
Figure 5.
Recombinant fragment of human surfactant protein D (rfhSP-D) inhibits IgE production from B cells in peripheral blood mononuclear cell (PBMC) culture. PBMCs obtained from individuals with allergy (n = 10) were stimulated with grass pollen allergen in the presence of IL-4, CD40L, and IL-21 for 14 days. Total IgE production from B cells was measured in the cell culture supernatants by ImmunoCAP assay in the presence of rfhSP-D and bovine serum albumin (BSA) (5 μg/ml). All data are shown as median (interquartile range). P values were determined by Wilcoxon sign-rank test. **P < 0.01; ***P < 0.001. ns = not significant.
Discussion
In this study, we have shown, for the first time to our knowledge, that rfhSP-D inhibits grass pollen allergen–induced basophil activation and suppresses histamine release at a single-cell level. Furthermore, rfhSP-D prevents CD23-mediated, IgE-facilitated allergen presentation by B cells to CD4+ T cells and inhibits proallergic Th2 immune responses. Furthermore, rfhSP-D inhibits IgE production by B cells in vitro when stimulated with CD40L, IL-4, and IL-21.
rfhSP-D has been shown to have therapeutic effects in murine models of allergy. These effects include lowering of IgE levels, suppression of peripheral and pulmonary eosinophilia, and Th2-to-Th1 cytokine polarization (6, 16, 17). However, this effect has been shown only in mice and not in humans (6). rfhSP-D has previously been shown to have various immunomodulatory properties (2, 4, 16–18). However, the underlying mechanisms by which rfhSP-D suppresses allergic inflammatory response have not been fully determined. In vitro studies showed that rfhSP-D bound to P. pratense allergen in a dose-, calcium-, and carbohydrate-dependent manner. Far Western blot analysis revealed that rfhSP-D bound three proteins in the P. pratense extract that were approximately 50 kD, 40 kD, and 38 kD in size. The interaction of rfhSP-D with the carbohydrate residues on the P. pratense allergens via CRDs is consistent with previous reports (19–21).
rfhSP-D was able to inhibit ex vivo allergen-induced basophil activation, as measured by CD63 and CD203c expression. We demonstrated suppression of histamine release at the single-cell level using a novel method incorporating fluorochrome-labeled DAO (12). In the mid-1990s, an enzyme-affinity-gold method based on the affinity of DAO for its substrate, histamine, was used to localize intracellular histamine in mast cells (22). Subsequently, a DAO colloidal gold–based technique was also used to localize histamine within basophils (23). We used a multiparametric gating strategy to measure intracellularly labeled DAO at the single-cell level. This multiparametric combined labeling of DAO and CD markers provides information on not only activation status at the single-cell level but also functional, allergen-specific basophil readout. Using a novel approach, we combined detection of two basophil surface markers as well as intracellular DAO. We show that rfhSP-D inhibits allergen-induced basophil activation and suppresses histamine release.
The immunomodulatory effect of rfhSP-D on FAP was also examined using an FAB assay. This assay was used to examine allergen–IgE complexes binding to CD23-enriched B cells that were pretreated with 5 μg/ml of rfhSP-D. Sera obtained from well-characterized patients with grass pollen allergy and seasonal allergic rhinitis were used as a source of specific IgE (24). This assay represents an in vitro model of facilitated allergen presentation where allergen–IgE complexes are incubated with a B-cell line. The complexes bound to CD23 on the surface of B cells are then detected by flow cytometry. Although the readout from this assay does not directly reveal the antigen-presenting capacity of B cells to T cells, this assay has been shown to serve as a representative of this process (25). rfhSP-D suppressed the cooperative binding of allergen–IgE complexes to B cells by up to 51% when CD23-enriched B cells were pretreated with 10 μg/ml rfhSP-D. This is an interesting and novel finding because it has previously been shown that the serum level of soluble CD23 correlates with allergic seasonal symptoms (26, 27). Additional studies also suggest the involvement of CD23 in IgE regulation (28). Moreover, when preformed complexes were exposed to rfhSP-D, the binding of allergen–IgE complexes to CD23 on the surface of B cells was unaffected. This finding suggests that rfhSP-D does block IgE sites that are required for binding to CD23. This is therefore, to our knowledge, the first study that establishes a link between rfhSP-D and CD23, suggesting that interference with FAP by rfhSP-D is dependent on the interaction between rfhSP-D and CD23 (low-affinity IgE receptor). A reduction in CD23 expression will inhibit FAP and hence the allergen-induced Th2 cytokine response. This interaction between rfhSP-D and CD23 requires further characterization to better understand how rfhSP-D can play a role in IgE regulation. It appears that rfhSP-D may prevent the worsening of allergic symptoms that occurs through CD23/IgE-mediated antigen presentation by B cells (29).
A link between an increased allergen-specific IgE level found in the serum of atopic patients and a pronounced allergen-driven T-cell proliferation has also been established in vitro (30). Thus, we examined the effect of rfhSP-D on the antigen presentation and proliferation of CD4+ T cells because the results from the FAB assay can correlate with reduction in T-lymphocyte proliferation (28). We compared the proliferation of untreated P. pretense–stimulated PBMCs with that of those pretreated with rfhSP-D prior to allergen stimulation. We used PBMCs obtained from 10 well-characterized atopic patients who were highly sensitized to P. pratense allergen. Pretreatment of PBMCs with rfhSP-D showed suppression of allergen-induced T-cell proliferation at 5 μg/ml and 10 μg/ml concentrations of rfhSP-D. The antiproliferative effect of rfhSP-D on P. pratense–stimulated PBMCs in this study further confirms results of an earlier study (9, 31) where the inhibitory effect of rfhSP-D was shown on D. pteronyssinus allergen–stimulated lymphocyte proliferation.
The ability of rfhSP-D to inhibit allergen-driven proinflammatory Th2 cytokine and chemokine production was also examined. rfhSP-D inhibited the production of proinflammatory Th2 cytokines such as IL-4, IL-5, IL-9, and IL-13, in addition to suppressing IL-6 and IL-17a. The effect of rfhSP-D on allergy-related chemokines was also examined because chemokines facilitate infiltration at the site of inflammation (32). rfhSP-D was found to suppress the production of eotaxin and macrophage-derived chemokine; however, no effect of rfhSP-D was observed in the case of CXCL8 and RANTES levels, whereas IFN-γ production was increased in presence of both 5 μg/ml and 10 μg/ml rfhSP-D. Thus, rfhSP-D caused inhibition of chemokine production and up-regulation of Th1 cytokine production, which would lead to decreased cellular infiltration.
A novel function of rfhSP-D in the present study is its clear suppressive effect on IgE synthesis. This was shown by coincubating the PBMCs isolated from well-characterized atopic individuals with rfhSP-D for 14 days in the presence of B-cell switch factors IL-4, CD40L, and IL-21. IL-21 was used in this assay because it has previously been shown to enhance IL-4–mediated IgE production by isolated human B cells (22). These data lend further support to our hypothesis that rfhSP-D can modulate allergic inflammation by its ability to suppress IgE biosynthesis. The mechanism of these effects needs to be further explored by assessing whether rfhSP-D can interact with CD21 as well as membrane-bound IgE.
In summary, we have shown that rfhSP-D can interfere with FAP, which represents a novel mechanism by which rfhSP-D suppresses proinflammatory Th2 lymphocyte–driven allergic inflammation and IgE production, and enhances secretion of Th1 cytokine production. However, further clinical studies are required to establish the potential of rfhSP-D as a novel immunomodulator for suppressing the allergic inflammatory response.
Footnotes
Funded by Royal Brompton Hospital charity research funds.
Author Contributions: A.S.Q. and I.S.: performed the majority of the key experiments; A.A.P., J.A.L., R.P., and F.A.: performed crucial supporting experiments; U.K., M.H.S., and S.R.D.: provided crucial reagents and contributed to experimental design; M.H.S.: led the project and wrote the manuscript together with I.S., A.S.Q., and U.K.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201701-0225OC on September 15, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Nayak A, Dodagatta-Marri E, Tsolaki AG, Kishore U. An insight into the diverse roles of surfactant proteins, SP-A and SP-D in innate and adaptive immunity. Front Immunol. 2012;3:131. doi: 10.3389/fimmu.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohnishi H, Yokoyama A, Kondo K, Hamada H, Abe M, Nishimura K, Hiwada K, Kohno N. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165:378–381. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 4.Winkler C, Bahlmann O, Viereck J, Knudsen L, Wedekind D, Hoymann HG, Madsen J, Thum T, Hohlfeld JM, Ochs M. Impact of a Met(11)Thr single nucleotide polymorphism of surfactant protein D on allergic airway inflammation in a murine asthma model. Exp Lung Res. 2014;40:154–163. doi: 10.3109/01902148.2014.891062. [DOI] [PubMed] [Google Scholar]
- 5.Madan T, Reid KB, Singh M, Sarma PU, Kishore U. Susceptibility of mice genetically deficient in the surfactant protein (SP)-A or SP-D gene to pulmonary hypersensitivity induced by antigens and allergens of Aspergillus fumigatus. J Immunol. 2005;174:6943–6954. doi: 10.4049/jimmunol.174.11.6943. [DOI] [PubMed] [Google Scholar]
- 6.Singh M, Madan T, Waters P, Parida SK, Sarma PU, Kishore U. Protective effects of a recombinant fragment of human surfactant protein D in a murine model of pulmonary hypersensitivity induced by dust mite allergens. Immunol Lett. 2003;86:299–307. doi: 10.1016/s0165-2478(03)00033-6. [DOI] [PubMed] [Google Scholar]
- 7.Strong P, Townsend P, Mackay R, Reid KB, Clark HW. A recombinant fragment of human SP-D reduces allergic responses in mice sensitized to house dust mite allergens. Clin Exp Immunol. 2003;134:181–187. doi: 10.1046/j.1365-2249.2003.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crouch EC. Structure, biologic properties, and expression of surfactant protein D (SP-D) Biochim Biophys Acta. 1998;1408:278–289. doi: 10.1016/s0925-4439(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang JY, Shieh CC, You PF, Lei HY, Reid KB. Inhibitory effect of pulmonary surfactant proteins A and D on allergen-induced lymphocyte proliferation and histamine release in children with asthma. Am J Respir Crit Care Med. 1998;158:510–518. doi: 10.1164/ajrccm.158.2.9709111. [DOI] [PubMed] [Google Scholar]
- 10.Madan T, Kishore U, Singh M, Strong P, Clark H, Hussain EM, Reid KB, Sarma PU. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. 2001;107:467–475. doi: 10.1172/JCI10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh I, Qaseem AS, Pathan AA, Durham SR, Kishore U, Shamji MH. Surfactant protein D (SP-D): a novel therapeutic target for suppressing grass pollen-induced Th2 and B responses in seasonal allergic rhinitis [abstract] Clin Exp Allergy. 2016;46:1630. [Google Scholar]
- 12.Shamji MH, Layhadi JA, Scadding GW, Cheung DK, Calderon MA, Turka LA, Phippard D, Durham SR. Basophil expression of diamine oxidase: a novel biomarker of allergen immunotherapy response. J Allergy Clin Immunol. 2015;135:913–921.e9. doi: 10.1016/j.jaci.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, Larché M, Durham SR, Francis JN. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317:71–79. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, Jacobson MR, Kimber I, Till SJ, Durham SR. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–516.e5. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 15.Shamji MH, Francis JN, Wurtzen PA, Lund K, Durham SR, Till SJ. Cell-free detection of allergen-IgE cross-linking with immobilized phase CD23: inhibition by blocking antibody responses after immunotherapy. J Allergy Clin Immunol. 2013;132:1003–1005.e4. doi: 10.1016/j.jaci.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qaseem AS, Sonar S, Mahajan L, Madan T, Sorensen GL, Shamji MH, Kishore U. Linking surfactant protein SP-D and IL-13: implications in asthma and allergy. Mol Immunol. 2013;54:98–107. doi: 10.1016/j.molimm.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 18.White ES, Xia M, Murray S, Dyal R, Flaherty CM, Flaherty KR, Moore BB, Cheng L, Doyle TJ, Villalba J, et al. Plasma surfactant protein-D, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2016;194:1242–1251. doi: 10.1164/rccm.201505-0862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishor U, Madan T, Sarma PU, Singh M, Urban BC, Reid KB. Protective roles of pulmonary surfactant proteins, SP-A and SP-D, against lung allergy and infection caused by Aspergillus fumigatus. Immunobiology. 2002;205:610–618. doi: 10.1078/0171-2985-00158. [DOI] [PubMed] [Google Scholar]
- 20.Wang JY, Kishore U, Lim BL, Strong P, Reid KB. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin Exp Immunol. 1996;106:367–373. doi: 10.1046/j.1365-2249.1996.d01-838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madan T, Kishore U, Shah A, Eggleton P, Strong P, Wang JY, Aggrawal SS, Sarma PU, Reid KB. Lung surfactant proteins A and D can inhibit specific IgE binding to the allergens of Aspergillus fumigatus and block allergen-induced histamine release from human basophils. Clin Exp Immunol. 1997;110:241–249. doi: 10.1111/j.1365-2249.1997.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood N, Bourque K, Donaldson DD, Collins M, Vercelli D, Goldman SJ, Kasaian MT. IL-21 effects on human IgE production in response to IL-4 or IL-13. Cell Immunol. 2004;231:133–145. doi: 10.1016/j.cellimm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 23.MacGlashan D., Jr Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010;40:1365–1377. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honda Y, Kuroki Y, Matsuura E, Nagae H, Takahashi H, Akino T, Abe S. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am J Respir Crit Care Med. 1995;152:1860–1866. doi: 10.1164/ajrccm.152.6.8520747. [DOI] [PubMed] [Google Scholar]
- 25.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–922. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 26.Boccafogli A, Vicentini L, Lambertini D, Scolozzi R. Soluble CD23 is increased in allergy. Allergy. 1997;52:357–358. doi: 10.1111/j.1398-9995.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 27.Haczku A, Takeda K, Hamelmann E, Loader J, Joetham A, Redai I, Irvin CG, Lee JJ, Kikutani H, Conrad D, et al. CD23 exhibits negative regulatory effects on allergic sensitization and airway hyperresponsiveness. Am J Respir Crit Care Med. 2000;161:952–960. doi: 10.1164/ajrccm.161.3.9905046. [DOI] [PubMed] [Google Scholar]
- 28.Francis JN. The facilitated antigen binding (FAB) assay - a protocol to measure allergen-specific inhibitory antibody activity. Methods Mol Med. 2008;138:255–261. doi: 10.1007/978-1-59745-366-0_21. [DOI] [PubMed] [Google Scholar]
- 29.Mudde GC, Bheekha R, Bruijnzeel-Koomen CA. Consequences of IgE/CD23-mediated antigen presentation in allergy. Immunol Today. 1995;16:380–383. doi: 10.1016/0167-5699(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 30.van der Heijden FL, van Neerven RJ, Kapsenberg ML. Relationship between facilitated allergen presentation and the presence of allergen-specific IgE in serum of atopic patients. Clin Exp Immunol. 1995;99:289–293. doi: 10.1111/j.1365-2249.1995.tb05547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K, Miyahara N, Rha YH, Taube C, Yang ES, Joetham A, Kodama T, Balhorn AM, Dakhama A, Duez C, et al. Surfactant protein D regulates airway function and allergic inflammation through modulation of macrophage function. Am J Respir Crit Care Med. 2003;168:783–789. doi: 10.1164/rccm.200304-548OC. [DOI] [PubMed] [Google Scholar]
- 32.Moser B, Willimann K. Chemokines: role in inflammation and immune surveillance. Ann Rheum Dis. 2004;63(Suppl 2):ii84–ii89. doi: 10.1136/ard.2004.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]