Abstract
Rationale: The clinical significance of diaphragm weakness in critically ill patients is evident: it prolongs ventilator dependency and increases morbidity, duration of hospital stay, and health care costs. The mechanisms underlying diaphragm weakness are unknown, but might include mitochondrial dysfunction and oxidative stress.
Objectives: We hypothesized that weakness of diaphragm muscle fibers in critically ill patients is accompanied by impaired mitochondrial function and structure, and by increased markers of oxidative stress.
Methods: To test these hypotheses, we studied contractile force, mitochondrial function, and mitochondrial structure in diaphragm muscle fibers. Fibers were isolated from diaphragm biopsies of 36 mechanically ventilated critically ill patients and compared with those isolated from biopsies of 27 patients with suspected early-stage lung malignancy (control subjects).
Measurements and Main Results: Diaphragm muscle fibers from critically ill patients displayed significant atrophy and contractile weakness, but lacked impaired mitochondrial respiration and increased levels of oxidative stress markers. Mitochondrial energy status and morphology were not altered, despite a lower content of fusion proteins.
Conclusions: Critically ill patients have manifest diaphragm muscle fiber atrophy and weakness in the absence of mitochondrial dysfunction and oxidative stress. Thus, mitochondrial dysfunction and oxidative stress do not play a causative role in the development of atrophy and contractile weakness of the diaphragm in critically ill patients.
Keywords: mechanical ventilation, critically ill, diaphragm weakness, mitochondrial dysfunction, oxidative stress
At a Glance Commentary
Scientific Knowledge on the Subject
The clinical significance of diaphragm weakness in critically ill patients is evident: it prolongs ventilator dependency and increases morbidity, duration of hospital stay, and healthcare costs. Diaphragm weakness in critically ill patients is caused largely by atrophy and contractile weakness of individual diaphragm muscle fibers. The mechanisms underlying diaphragm muscle fiber atrophy and contractile weakness are unknown, but they might include mitochondrial dysfunction.
What This Study Adds to the Field
We tested the hypothesis that weakness of diaphragm muscle fibers in critically ill patients is accompanied by impaired mitochondrial function and structure and by increased markers of oxidative stress. Our findings show that diaphragm muscle fibers from critically ill patients display significant atrophy and contractile weakness, but lack both dysfunctional mitochondrial respiration and increased levels of oxidative stress markers. Thus, mitochondrial dysfunction and oxidative stress do not play a causative role in the development of atrophy and contractile weakness of the diaphragm in critically ill patients.
Critically ill patients develop weakness of the diaphragm, the main inspiratory muscle, as indicated by a reduced pressure-generating capacity, and a decreased diaphragm thickness and thickening fraction (1–6). Weakness of the diaphragm contributes to prolonged weaning from mechanical ventilation, and increases morbidity and mortality in critically ill patients (7–9).
Diaphragm weakness in mechanically ventilated critically ill patients is largely caused by atrophy and contractile weakness of individual diaphragm muscle fibers (10, 11). Fiber atrophy is accompanied by activation of the ubiquitin–proteasome pathway, indicating a role for proteolytic pathways in diaphragm weakening in critically ill patients (11). The mechanisms that trigger proteolysis in diaphragm muscle fibers of critically ill patients are unknown.
Studies on animal models and on brain-dead organ donors suggest that these mechanisms include oxidative stress, induced by mitochondrial alterations during mechanical ventilation (12–18). Mitochondria adapt to energetic stress by changing their structure and function either through fusion with adjacent mitochondria or through division, also referred to as fission. This process of fusion and fission is referred to as mitochondrial dynamics (19). Fusion allows sharing of mitochondrial DNA (20) and matrix contents (21) for optimal quality and function, whereas impaired fusion leads to fragmentation of the mitochondrial network, mitochondrial dysfunction, and the production of reactive oxygen species (22, 23). Studies suggest that influx of energetic substrates, such as lipid and glucose, exceeds the demand of diaphragm muscle fibers during a sudden drop in substrate utilization rate during mechanical unloading of the diaphragm in brain-dead organ donors and mechanically ventilated rodents (14, 24). This state of metabolic oversupply might promote mitochondrial fission (25) and mitochondrial dysfunction (23), and increase oxidative stress (16).
However, the clinical features of critically ill patients greatly differ from those of controlled ventilated animals and brain-dead organ donors. Therefore, it is currently unknown, yet important to establish, whether mitochondrial dysfunction and oxidative stress develop in the diaphragm of critically ill patients. In the case that mitochondria play a key role in development of diaphragm weakness in these patients, modulation of mitochondrial function might prove an important strategy to attenuate the development of respiratory muscle weakness.
The present study aimed to establish that contractile weakness of the diaphragm in critically ill patients is associated with mitochondrial dysfunction. We hypothesized that weakness of diaphragm muscle fibers of these patients was accompanied by impaired mitochondrial respiration and structure, and increased markers of oxidative stress. To test these hypotheses, we studied diaphragm biopsies of 36 critically ill patients and compared the findings with those obtained from biopsies of 27 patients with suspected early-stage lung malignancy who served as control subjects. Some of the results of this study have been previously reported in the form of an abstract (26).
Methods
Additional details are in the online supplement.
Patients
Diaphragm muscle biopsies were obtained from mechanically ventilated critically ill patients undergoing abdominal or thoracic surgery for clinical reasons (critically ill group). As a control group, diaphragm biopsies were obtained from patients undergoing lung surgery for suspected early-stage lung malignancy. Exclusion criteria are listed in the online supplement. The Medical Ethics Committee of the Vrije Universiteit (VU) University Medical Center approved the protocol. Patients were recruited in the VU University Medical Center, Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital (both in Amsterdam, the Netherlands), and Medisch Spectrum Twente (Enschede, the Netherlands), and written informed consent was obtained from each patient and/or legal representative.
Biopsy Handling
Diaphragm biopsies were handled as described in the online supplement. Because of the limited biopsy size, not all experiments could be performed on all biopsies; see Tables E1.1 and E1.2 in the online supplement.
Contractile Force Measurements
Contractility of single diaphragm muscle fibers was measured as described previously (11). Some of the contractility data have been published previously (11); see Tables E1.1 and E1.2.
Mitochondrial Function
Mitochondrial respiration and succinate dehydrogenase activity were determined as described previously (27, 28).
Electron Microscopy
Micrographs were assessed for mitochondrial abnormalities, lipid droplets, and glycogen deposits and quantified for mitochondrial size, number, and density.
Protein and mRNA Analyses
The content of mitochondrial complexes I–V, markers for mitochondrial biogenesis and dynamics, and oxidative stress were measured by Western blotting, and described previously (29–34). Nuclear factor erythroid 2–related factor 2 (Nrf2) was measured by real-time PCR, as described previously (30).
Bioluminescence
ATP concentration was measured by bioluminescence.
Statistical Analysis
Normally distributed data are represented as the mean ± SEM, and not normally distributed data are shown as median and interquartile range. Data were analyzed with GraphPad Prism version 6.07 (GraphPad Software, San Diego, CA). Differences between groups were considered significant if P < 0.05.
Results
Patient Characteristics
The demographic and clinical characteristics of control patients (n = 27) and critically ill patients (n = 36) are described in Tables 1, 2, and E2. Control and critically ill patients were mechanically ventilated for 1.5 (1.0–2) hours and 76.5 (34–247) hours, respectively (P < 0.0001). Specifications of mechanical ventilation duration and arterial blood gas analysis are shown in Tables E3 and E4.
Table 1.
Characteristics of Control Patients
| Control Patient (No.) | Age (yr) | Sex (M/F) | BMI (kg/m2) | Relevant Medical History | TNM Classification | MV (h) |
|---|---|---|---|---|---|---|
| 1 | 52 | M | 25 | T2DM, HT, COPD GOLD II, ex-smoker | pT3N0M0 | 1.5 |
| 2 | 22 | M | 23 | Smoker | pT1bN0M0 | 2 |
| 3 | 66 | M | 24 | HT, prostate cancer, COPD GOLD I, ex-smoker | pT2aN1M0 | 2 |
| 4 | 58 | F | 28 | None | pT1aN0M0 | 2 |
| 5 | 60 | M | 24 | Lobectomy for T1N0M0 lung tumor, COPD GOLD I, ex-smoker | pT1aN1M0 | 1.5 |
| 6 | 56 | M | 21 | COPD GOLD II, ex-smoker | pT4N2M0 | 2 |
| 7 | 70 | M | 30 | HT, COPD GOLD I, ex-smoker | pT1bN1M0 | 1.5 |
| 8 | 60 | F | 26 | HT | pT2aN0M0 | 0.75 |
| 9 | 59 | F | 28 | Chronic bronchitis, smoker | Benign, cyst | 0.75 |
| 10 | 64 | F | 26 | COPD GOLD II, smoker | pT1bN0M0 | 1.25 |
| 11 | 59 | F | 21 | Breast carcinoma, cervix carcinoma, chronic bronchitis, COPD GOLD II, smoker | Adenocarcinoma CT2BN2M1A | 0.75 |
| 12 | 52 | M | 23 | Smoker, alcohol abuse | pT2N0M0 | 1 |
| 13 | 64 | M | 31 | HT, MI, T2DM, smoker | Benign, bronchiectasis and inflammation | 1 |
| 14 | 73 | F | 21 | Bronchiectasis, allergic bronchopulmonary aspergillosis, COPD GOLD I | Benign, inflammation with central necrosis | 2.5 |
| 15 | 74 | M | 23 | Colon cancer, alcohol abuse, COPD GOLD I, ex-smoker | Chondrosarcoma | 1 |
| 16 | 75 | M | 26 | T2N2C tongue base carcinoma, COPD GOLD I | Benign, inflammation | 0.5 |
| 17 | 55 | F | 27 | Allergic asthma, recurrent pneumonia, smoker | Carcinoid T1bN0 | 1 |
| 18 | 35 | M | 26 | Smoker | Benign, hamartoma | 0.5 |
| 19 | 40 | M | 24 | None | Carcinoid pT1aN0R0 | 1.5 |
| 20 | 72 | M | 28 | AF, non–small cell lung cancer cT3TN0M0, COPD GOLD I, ex-smoker | pT2N0M0 | 1.5 |
| 21 | 68 | F | 29 | HT, T2DM, ex-smoker | pT2N2M0 | 0.75 |
| 22 | 67 | M | 27 | HT, T2DM, renal cell carcinoma, prostate cancer | Metastasis of clear cell renal cell carcinoma | 1.25 |
| 23 | 50 | M | 24 | COPD GOLD I, smoker | Mucinous adeno-carcinoma T1aN0M0 | 1.25 |
| 24 | 58 | F | 23 | Chronic bronchitis, COPD GOLD I, ex-smoker | Adenocarcinoma pT0N0M0 | 2.5 |
| 25 | 61 | M | 33 | Testis seminoma, COPD GOLD II, ex-smoker | pT1aN0M0 | 2 |
| 26 | 69 | M | 33 | HT, ex-smoker | Adenocarcinoma pT3N1M0 | 1.5 |
| 27 | 58 | M | 28 | HT, ex-smoker | pT3N1 | 2.4 |
Definition of abbreviations: AF = atrial fibrillation; BMI = body mass index; COPD = chronic obstructive pulmonary disease (GOLD classification); GOLD = Global Initiative for Chronic Obstructive Lung Disease; HT = hypertension; MI = myocardial infarction; MV = mechanical ventilation; T2DM = type 2 diabetes mellitus; TNM = classification of tumors based on tumor size (T), lymph nodes involved (N), and metastases (M).
Table 2.
Characteristics of Critically Ill Patients
| Critically Ill Patient (No.) | Age (yr) | Sex (M/F) | BMI (kg/m2) | Relevant Medical History | Reason for Admission to ICU | Surgery in Which Biopsy Was Obtained | MV (h) | Septic | Died in ICU | APACHE II Score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | F | 18 | COPD GOLD I | Respiratory failure after VATS lobectomy | Rethoracotomy: lobectomy necrotic middle lobe | 31 | Y | N | 22 |
| 2 | 67 | M | 28 | Concentric LV hypertrophy with decreased LVEF | Hemorrhagic shock due to retroperitoneal hematoma | Third relaparotomy: closure abdomen | 574 | N | N | 38 |
| 3 | 67 | F | 19 | CVA | Abdominal sepsis due to intestinal perforation | Relaparotomy: drainage abscess | 28 | Y | Y | 18 |
| 4 | 53 | M | 30 | None | Hemorrhagic shock after thoracic endovascular aortic repair | Rethoracotomy: evacuating hemothorax | 315 | N | N | 29 |
| 5 | 47 | F | 22 | None | Severe trauma | Relaparotomy: gauze removal | 40 | N | N | 35 |
| 6 | 67 | F | 23 | HT, CVA, hypothyroidism, smoker | Abdominal sepsis due to intestinal necrosis by mesenteric ischemia | Relaparotomy: drainage abscess | 337 | Y | Y | 28 |
| 7 | 25 | F | 33 | None | Severe trauma | Second relaparotomy: closure abdomen | 219 | Y | N | 45 |
| 8 | 66 | M | 29 | HT | Abdominal sepsis due to perforated appendicitis | Relaparotomy: rinsing abdominal cavity | 28 | Y | N | 11 |
| 9 | 69 | M | 22 | None | Esophageal rupture | Thoracotomy: esophageal repair | 17 | Y | N | 27 |
| 10 | 51 | M | 29 | Hypothyroidism | Severe trauma | Fourth relaparotomy: closure abdomen | 85 | N | N | 30 |
| 11 | 46 | F | 19 | Asthma, smoker | IHCA due to hemorrhagic shock of abdominal origin | Third relaparotomy: gauze removal | 61 | N | Y | 37 |
| 12 | 52 | M | 29 | None | Severe trauma | Relaparotomy: closure abdomen | 287 | Y | N | 20 |
| 13 | 81 | F | 24 | HT | Severe trauma | Thoracotomy: reconstruction of two ribs | 43 | N | Y | 19 |
| 14 | 77 | F | 28 | T2DM, AF | Abdominal sepsis due to cecal perforation | Second relaparotomy: rinsing abdominal cavity | 32 | Y | Y | 19 |
| 15 | 72 | M | 20 | T2DM, colon carcinoma, chronic bronchitis, COPD GOLD II | Abdominal sepsis due to duodenal perforation | Relaparotomy: cholecystectomy | 20 | Y | Y | 19 |
| 16 | 79 | F | 23 | Breast carcinoma, L4–L5 paraplegia | Abdominal sepsis due to gastro-intestinal perforations | Third relaparotomy: colostomy | 48 | Y | Y | 22 |
| 17 | 74 | M | 25 | HT, AF | Respiratory failure due to bilateral pneumonia | Relaparotomy: evacuation empyema | 275 | Y | Y | 19 |
| 18 | 83 | M | 24 | HT, rectum carcinoma | Abdominal sepsis due to intestinal perforation | Relaparotomy: hemicolectomy | 49 | Y | Y | 35 |
| 19 | 68 | M | 24 | Prostate carcinoma, COPD GOLD II, smoker | IHCA after Bentall procedure because of aortic dissection | Thoracotomy: fixation rib fractures | 350 | N | N | NA |
| 20 | 68 | F | 27 | HT, T2DM, CVA, smoker | Respiratory failure due trachea deviation by struma | CABG and hemistrumectomy | 90 | N | Y | 18 |
| 21 | 69 | F | 34 | HT, T2DM, chronic kidney disease, smoker | Heart failure after aortic valve replacement | Relaparotomy: drainage ascites | 255 | N | Y | 16 |
| 22 | 22 | F | 20 | None | Severe trauma | Second relaparotomy: closure abdomen | 203 | N | N | 31 |
| 23 | 72 | M | 26 | HT, chronic kidney disease, secondary hyperparathyroidism | OHCA with traumatic CPR | Second relaparotomy: removal gauze | 61 | N | N | 42 |
| 24 | 55 | F | 21 | Lower leg amputation, right kidney infarction, milt infarction | Abdominal sepsis due to perforations by intestinal ischemia | Relaparotomy: closure abdomen | 69 | Y | N | 19 |
| 25 | 78 | M | 18 | HT, PAD, stenosis AMS, ex-smoker | Postoperative care after endovascular treatment because of mesenteric ischemia | Third relaparotomy: closure abdomen | 122 | Y | N | 27 |
| 26 | 75 | M | 22 | HT, chronic coronary artery disease, chronic kidney disease, smoker | Postoperative care after thoracoabdominal aortic aneurysm repair | Fourth relaparotomy: Hartmann procedure | 290 | Y | Y | 22 |
| 27 | 67 | F | 29 | HT, hypothyroidism, chronic sensory idiopathic axonal polyneuropathy | Postoperative care after thoracoabdominal aortic aneurysm repair | Relaparotomy: stent replacement | 20 | Y | Y | 30 |
| 28 | 54 | M | 26 | None | Postoperative care after ruptured abdominal aortic aneurysm repair | Fourth relaparotomy: sigmoid resection with placement colostomy | 50 | N | N | 40 |
| 29 | 21 | M | 33 | None | Severe trauma | Relaparotomy: cecal and transcolon suture | 222 | Y | N | 26 |
| 30 | 66 | F | 21 | None | Severe trauma with intestinal damage | Fourth relaparotomy: rinsing abdominal cavity | 134 | Y | N | 24 |
| 31 | 55 | M | 28 | HT, nephrectomy | Intestinal ischemia after nephrectomy | Relaparotomy: resection part of duodenum and jejunum | 21 | Y | N | 19 |
| 32 | 61 | M | 29 | HT, T2DM | Postoperative care after ruptured abdominal aortic aneurysm repair | Third relaparotomy: closure abdomen | 298 | Y | N | 27 |
| 33 | 69 | M | 29 | HT, hypercholesterolemia, PAD, ex-smoker | IHCA after myocardial infarction | Laparotomy: resection necrotic ileum | 225 | N | N | 30 |
| 34 | 71 | M | 28 | HT, aortic valve sclerosis | Abdominal sepsis after hemicolectomy | fifth relaparotomy: resection sigmoid and part of small intestine, replacement colostomy | 84 | Y | N | 31 |
| 35 | 60 | F | 20 | HT, CVA, COPD GOLD II, smoker | Postoperative care after endovascular treatment because of mesenteric ischemia | Third relaparotomy: resection terminal ilium | 42 | N | N | 17 |
| 36 | 34 | M | 28 | None | Severe trauma | Relaparotomy: closure abdomen | 18 | N | N | 16 |
Definition of abbreviations: AF = atrial fibrillation; AMS = arteria mesenterica superior; APACHE = Acute Physiology and Chronic Health Evaluation; BMI = body mass index; CABG = coronary artery bypass grafting; COPD = chronic obstructive pulmonary disease; CPR = cardiopulmonary resuscitation; CVA = cerebrovascular accident; GOLD = Global Initiative for Obstructive Lung Disease; HT = hypertension; ICU = intensive care unit; IHCA = in-hospital cardiac arrest; LV = left ventricle; LVEF = left ventricular ejection fraction; MV = mechanical ventilation; N = no; NA = not available; OHCA = out-of-hospital cardiac arrest; PAD = peripheral artery disease; T2DM = type 2 diabetes mellitus; VATS = video-assisted thoracoscopic surgery; Y = yes.
Atrophy and Contractile Weakness of Diaphragm Muscle Fibers
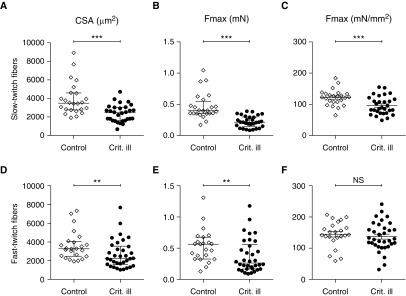
To investigate whether diaphragm muscle fibers from critically ill patients show atrophy and contractile weakness, we measured the cross-sectional area and maximal absolute force of individual diaphragm muscle fibers. Compared with control patients, the cross-sectional area in critically ill patients was significantly smaller in both the slow-twitch fibers (3,490 [2,763–4,595] vs. 2,461 [1,608–3,037] μm2; P < 0.0001) (Figure 1A) and fast-twitch fibers (3,275 [2,473–4,064] vs. 2,235 [1,570–3,550] μm2; P = 0.008) (Figure 1D). Moreover, maximal absolute force was lower in both slow-twitch fibers (0.40 [0.35–0.55] vs. 0.21 [0.14–0.30] mN; P < 0.0001) (Figure 1B) and fast-twitch fibers (0.56 [0.33–0.68] vs. 0.26 [0.17–0.56] mN; P = 0.01) (Figure 1E) of critically ill patients versus control patients. Next, we examined whether this reduction in force was proportional to the reduction in cross-sectional area by normalizing maximal force to cross-sectional area. This examination revealed that in critically ill patients normalized maximal force was significantly reduced in slow-twitch fibers (122 ± 5 vs. 96 ± 5 mN/mm2; P = 0.001) (Figure 1C) but not in fast-twitch fibers (145 ± 9 vs. 137 ± 8 mN/mm2; P = 0.52) (Figure 1F). Taken together, these results indicate that critically ill patients develop significant atrophy resulting in contractile weakness of the diaphragm, exacerbated by a reduction in the intrinsic force-generating capacity of slow-twitch fibers.
Figure 1.
Atrophy and contractile weakness of diaphragm muscle fibers in critically ill patients. (A and D) The cross-sectional area of diaphragm muscle fibers was significantly smaller for both slow- and fast-twitch fibers in critically ill patients than in control patients, indicating atrophy. (B and E) Maximal absolute force was lower in both slow- and fast-twitch fibers from critically ill patients, indicating contractile weakness. (C and F) Maximal normalized force, that is, maximal absolute force normalized to fiber cross-sectional area, was lower in slow-twitch but not in fast-twitch diaphragm muscle fibers from critically ill patients. Each data point represents the mean of all slow-twitch or fast-twitch muscle fibers measured from a diaphragm biopsy of one patient. Data in A, B, D, and E are presented as median and interquartile range, and data in C and F are presented as mean ± SEM. Crit. ill = critically ill; CSA = cross-sectional area; Fmax = maximal force; NS = not significant. **P ≤ 0.01, ***P ≤ 0.001.
Preserved Mitochondrial Respiration in Diaphragm Muscle Fibers
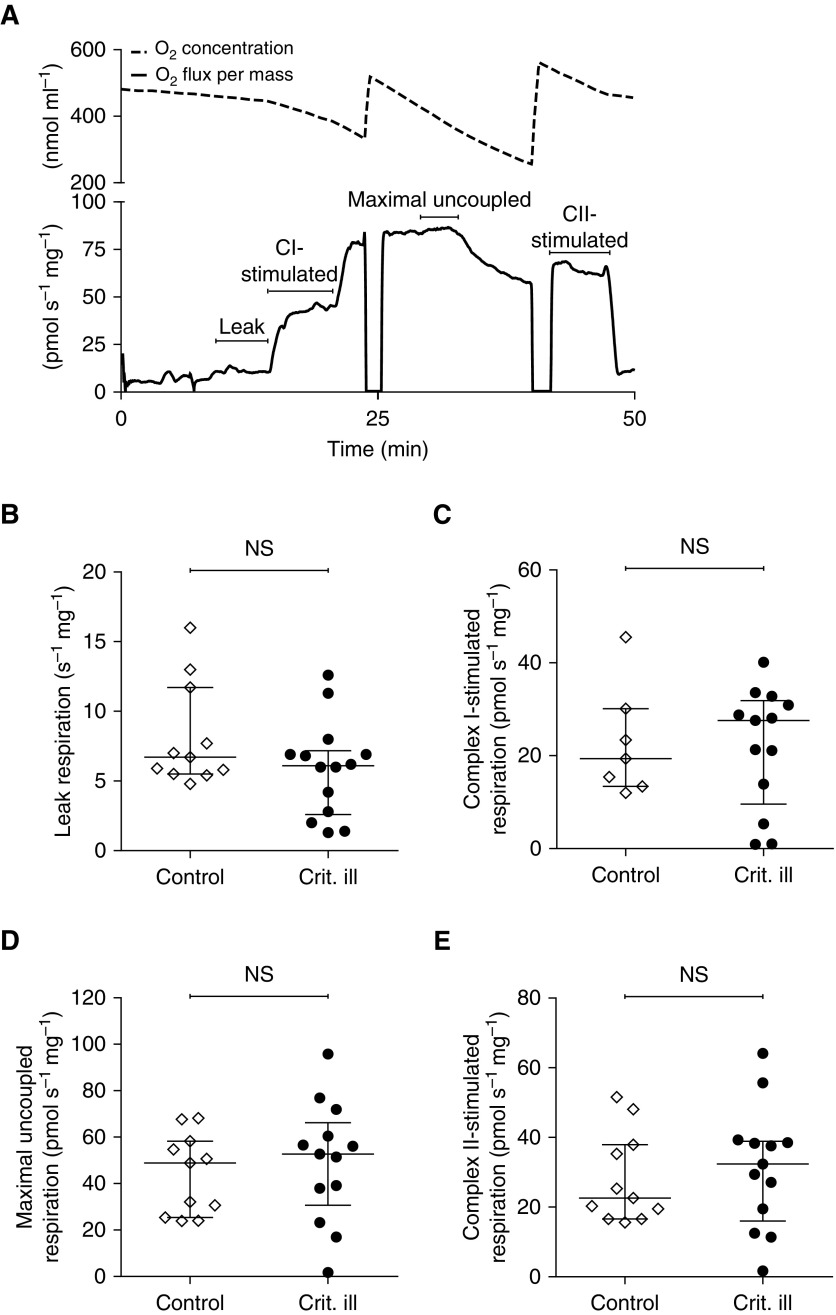
To examine whether atrophy and contractile weakness of diaphragm fibers of critically ill patients were associated with mitochondrial dysfunction, we measured mitochondrial respiration in the diaphragm biopsies. Figure 2A shows a typical example of the respirometry recordings. Leak respiration, reflecting proton leak, was not different in critically ill than in control patients (6.7 [5.5–11.7] vs. 6.1 [2.6–7.2] pmol s–1 mg–1; P = 0.31) (Figure 2B). Also, maximal complex I–stimulated respiration (19.4 [13.4–30.1] vs. 27.6 [9.6–31.9] pmol s–1 mg–1; P = 0.78) (Figure 2C), maximal uncoupled respiration (48.9 [25.4–58.3] vs. 52.7 [30.6–66.2] pmol s–1 mg–1; P = 0.56) (Figure 2D), and uncoupled complex II–stimulated respiration (22.6 [16.6–37.9] vs. 32.4 [16.0–38.9] pmol s–1 mg–1; P = 0.58) (Figure 2E) did not differ between control and critically ill patients, respectively. Post-hoc analysis revealed that mitochondrial respiration was also not different in nonseptic versus septic critically ill patients, patients with a low versus high Acute Physiology and Chronic Health Evaluation (APACHE) II score, and survivors versus nonsurvivors (see Figure E1).
Figure 2.
Maintained mitochondrial respiration in diaphragm muscle fibers. (A) Example of experimental recording of mitochondrial respiration in diaphragm muscle fibers. (Top) Oxygen concentration, which was maintained above 300 μM to avoid limitations in oxygen supply. (Bottom) Oxygen consumption per milligram wet weight muscle mass. Leak respiration is a measure of oxygen flux compensating for proton leakage from the intermembranous space through the inner membrane to the matrix. Maximal complex I–stimulated respiration is measured after addition of glutamate, malate and pyruvate, and ADP. Maximal uncoupled respiration is measured after adding succinate and FCCP [carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone] to dissipate the mitochondrial membrane potential. Uncoupled complex II–stimulated respiration is the oxygen consumption of complex II by blocking complex I with rotenone. No differences were observed in (B) leak respiration, (C) complex I–stimulated respiration, (D) maximal uncoupled respiration, and (E) uncoupled complex II–stimulated respiration between critically ill patients and control patients. Each data point represents the mean of two mitochondrial respiration measurements in a diaphragm biopsy of one patient. Data in B–E are presented as median and interquartile range. CI = complex I; CII = complex II; Crit. ill = critically ill; NS = not significant.
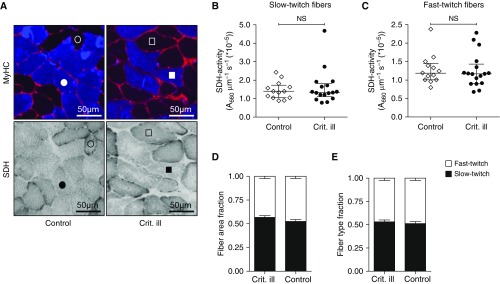
To assess mitochondrial complex II function at the single-cell level, we measured succinate dehydrogenase (SDH) activity in cryosections of the diaphragm biopsies. Figure 3A shows representative examples of cryosections stained for SDH activity. Compared with control patients, the SDH activity in both slow-twitch fibers (1.4 [1.1–1.7] × 10−5 vs. 1.3 [1.1–1.8] × 10−5 A660 μm−1 s–1; P = 0.96) (Figure 3B) and fast-twitch fibers (1.2 [1.0–1.5] × 10−5 vs. 1.2 [0.9–1.4] × 10−5 A660 μm−1 s–1; P = 0.64) (Figure 3C) of critically ill patients was not different. These findings are in line with the respirometry results, and suggest that mitochondrial capacity and function are preserved in diaphragm fibers from critically ill patients.
Figure 3.
Maintained succinate dehydrogenase (SDH) activity in diaphragm muscle fibers. (A) Typical examples of diaphragm cryosections stained for fast-myosin heavy chain (MyHC, top) and for SDH activity (bottom). Blue, fast-MyHC; red, wheat germ agglutinin, indicating cell membranes. The circles represent corresponding slow-twitch (open) and fast-twitch (solid) fibers in control patients, and the squares represent corresponding slow-twitch (open) and fast-twitch (solid) fibers in critically ill patients. (B and C) SDH activity of diaphragm muscle fibers was comparable in both slow- and fast-twitch fibers in critically ill patients compared with control patients. Each data point represents the mean SDH activity of all slow-twitch or fast-twitch fibers measured in cryosections of a diaphragm biopsy of one patient. Data are presented as median and interquartile range. (D and E) The area of the cryosections covered by slow- and fast-twitch fibers (fiber area fraction) and the distribution of slow- and fast-twitch fibers (fiber type fraction) were preserved, suggesting that maintained respiration in critically ill patients was not caused by a fiber type shift toward slow-twitch muscle fibers. Data are presented as mean ± SEM. Crit. ill = critically ill; NS = not significant.
To verify that the preserved mitochondrial respiration was not a reflection of a fiber type switch toward slow-twitch muscle fibers (which typically have a higher mitochondrial density) in critically ill patients, we measured the fiber area fraction and fiber type fraction in diaphragm cryosections. Compared with control patients, the fiber area fraction (fast/slow-twitch, 48/52 vs. 44/56%; P = 0.24) (Figure 3D) and fiber type fraction (fast/slow-twitch, 49/51 vs. 47/53%; P = 0.59) (Figure 2E) in critically ill patients were not different.
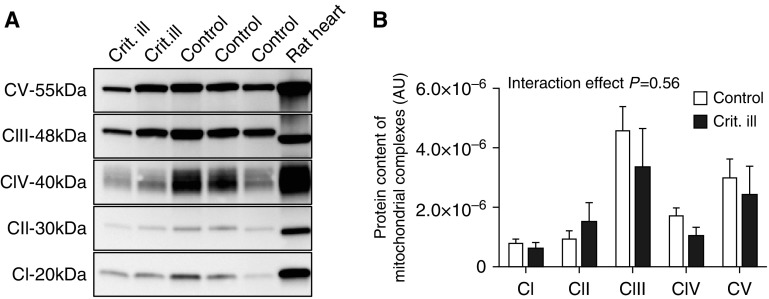
Furthermore, the protein contents of mitochondrial complex I–V in the diaphragm biopsies were determined by Western blotting (see Figure 4A and Figure E3 for typical examples). The protein content of the complexes was not significantly different in critically ill patients compared to control subjects (P = 0.56; Figure 4B), a finding that is in line with the maintained mitochondrial respiration in diaphragm fibers of critically ill patients.
Figure 4.
Preserved content of mitochondrial complexes in diaphragm muscle fibers. (A) Typical example of a Western blot with MitoProfile antibody cocktail of the five complexes. Lanes 1 and 2, diaphragm homogenates of critically ill patients; lane 3, rat heart homogenate (loading control); lanes 4 and 5, diaphragm homogenates of control patients. (B) Quantification of protein content of mitochondrial complexes. Protein content was not lower in diaphragm muscle fibers of critically ill patients (analysis of variance, interaction effect P = 0.56). Data are presented as mean ± SEM. AU = arbitrary units; CI = complex I; CII = complex II; CIII = complex III; CIV = complex IV; Crit. ill = critically ill; CV = complex V.
No Major Alterations in Mitochondrial Structure in Diaphragm Muscle Fibers
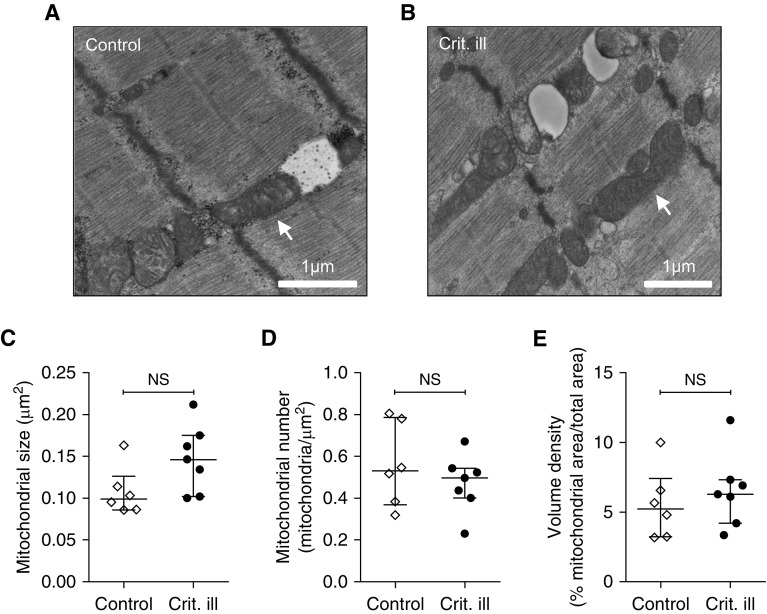
We obtained electron micrographs from six control and seven critically ill patients. Mild abnormalities of mitochondrial shape and internal complexity were observed in three of six control subjects versus six of seven critically ill patients (P = 0.27). Misalignment of mitochondria was observed in zero of six control subjects versus three of seven of the critically ill patients (P = 0.19; Figures 5A and 5B). Furthermore, compared with control patients, mitochondria of critically ill patients did not significantly differ with respect to size (0.10 [0.09–0.013] vs. 0.15 [10–18] μm2; P = 0.097) (Figure 5C), number (0.53 [0.37–0.79] vs. 0.50 [0.40–0.54] mitochondria/μm2; P = 0.52) (Figure 5D), and volume density (5.24 [3.25–7.44] vs. 6.29 [4.22–7.33]% mitochondrial area/total area; P = 0.36) (Figure 5E). Thus, no significant alterations in mitochondrial structure were observed in diaphragm fibers from critically ill patients.
Figure 5.
No major alterations of mitochondrial structure in diaphragm muscle fibers. (A) Electron micrograph of diaphragm biopsy from a control patient. Mitochondria are well aligned and have a normal general appearance. (B) Electron micrograph of a diaphragm biopsy from a critically ill patient. In A and B, the arrow indicates an example of a mitochondrion with a normal appearance. (C–E) Quantification of mitochondria in the micrographs revealed no differences in mitochondrial size, number, and density. Each data point represents the mean mitochondrial size/number/volume density as measured or counted in micrographs of a diaphragm biopsy of one patient. Data are presented as median and interquartile range. Crit. ill = critically ill; NS = not significant.
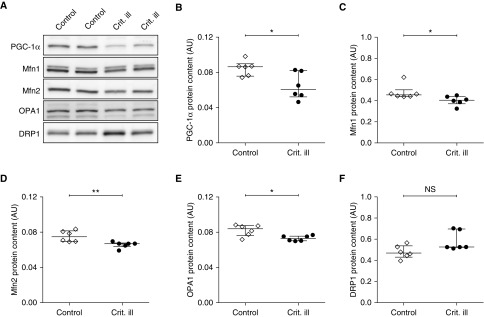
Mitochondrial structure is regulated by mitochondrial dynamics, which in turn are under the control of peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α), the master regulator of mitochondrial biogenesis. Overexpression of PGC-1α stimulates up-regulation of pro-fusion proteins mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optic atrophy 1 (OPA1) (35). We measured the contents of PGC1-α, the pro-fusion proteins, and the profission protein dynamin-related protein 1 (DRP1) (see Figure 6A and Figure E4 for typical examples of the Western blots). Compared with control subjects, PGC-1α content was significantly lower in critically ill patients (0.086 [0.076–0.089] vs. 0.060 [0.052–0.081]; P = 0.026) (Figure 6B). In line with this, the contents of pro-fusion proteins Mfn1 (0.45 [0.44–0.50] vs. 0.40 [0.37–0.44]; P = 0.026) (Figure 6C), Mfn2 (0.075 [0.070–0.082] vs. 0.067 [0.063–0.068]; P = 0.007) (Figure 6D), and OPA1 (0.084 [0.076–0.087] vs. 0.073 [0.070–0.076]; P = 0.015) (Figure 6E) were also significantly lower in critically ill patients than in control patients. However, the content of fission protein DRP1 was comparable in critically ill and control patients (0.47 [0.43–0.54] vs. 0.53 [0.52–0.67]; P = 0.13) (Figure 6F). These results indicate that mitochondria in diaphragm fibers have impaired fusion dynamics; however, this was not accompanied by major changes in mitochondrial function and structure (based on electron micrographs; Figure 5).
Figure 6.
Down-regulation of master regulator of biogenesis PGC-1α in diaphragm muscle fibers from critically ill patients. (A) Typical example of Western blot with antibodies for master regulator of biogenesis PGC-1α; fission proteins Mfn1, Mfn2, and OPA1; and fusion protein DRP1 from control and critically ill patients. (B) Compared with control subjects, PGC-1α protein content was lower in critically ill patients. (C–E) In line with lower PGC-1α, pro-fusion proteins Mfn1, Mfn2, and OPA1 were also lower in critically ill patients. This suggests that mitochondrial fusion dynamics are impaired in diaphragm fibers of critically ill patients, but without major changes in mitochondrial structure and function (see Figure 5). (F) Finally, content of pro-fusion protein DRP1 was comparable in critically ill patients and control patients. Each data point represents the mean protein content per group of diaphragm biopsies of three to four patients. Data are presented as median and interquartile range. AU = arbitrary units; Crit. ill = critically ill; DRP1 = dynamin-related protein 1; Mfn1 = mitofusin 1; Mfn2 = mitofusin 2; NS = not significant; OPA1 = optic atrophy 1; PGC-1α = peroxisome proliferator–activated receptor γ coactivator 1α. *P ≤ 0.05, **P ≤ 0.01.
Absence of Metabolic Oversupply in Diaphragm Muscle Fibers
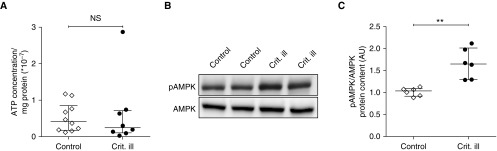
In mechanically ventilated brain-dead organ donors and rodents, a relative state of energetic oversupply has been observed in the diaphragm, which inhibits the mitochondrial electron flow and thereby reduces ATP synthesis rates (14). The preserved mitochondrial respiration in the diaphragm of critically ill patients suggests that ATP synthesis is unaffected, and we verified this by measuring the ATP concentration by bioluminescence. Compared with control patients, the ATP concentration in critically ill patients was not different (0.42 [0.18–0.85] vs. 0.25 [0.10–0.71] AU; P = 0.45) (Figure 7A). These findings suggest that metabolic oversupply does not occur in diaphragm fibers from critically ill patients.
Figure 7.
Absence of metabolic oversupply in diaphragm muscle fibers. (A) Compared with control patients, the ATP concentration was not changed in diaphragm muscle fibers of critically ill patients, suggesting the absence of metabolic oversupply in critically ill patients. Each data point represents the mean ATP concentration of a diaphragm biopsy of one patient. (B) Typical example of Western blot with antibodies for metabolic stress markers pAMPK and AMPK. (C) The ratio of pAMPK to AMPK was higher in diaphragm muscle fibers of critically ill patients than in those of control patients. Each data point represents the mean protein content per group of diaphragm biopsies from three to four patients. Data are presented as median and interquartile range. AMPK = AMP-activated protein kinase; AU = arbitrary units; Crit. ill = critically ill; NS = not significant; pAMPK = phosphorylated AMPK. **P ≤ 0.01.
To further investigate this, we determined AMP-activated protein kinase (AMPK) activity, which increases if the AMP-to-ATP ratio increases (36, 37). AMPK is activated by phosphorylation. Therefore, we measured the ratio of phosphorylated AMPK relative to total AMPK (pAMPK/AMPK) in the diaphragm biopsies (see Figure 7B and Figure E5 for typical examples of the Western blots). Compared with control patients, the pAMPK-to-AMPK ratio was significantly higher in critically ill patients (1.04 [0.92–1.09] vs. 1.65 [1.29–2.01]; P = 0.002) (Figure 7C). Thus, in diaphragm fibers from critically ill patients the ratio of pAMPK to AMPK is elevated without changes in ATP concentration.
Decreased Oxidative Stress Levels in Diaphragm Muscle Fibers
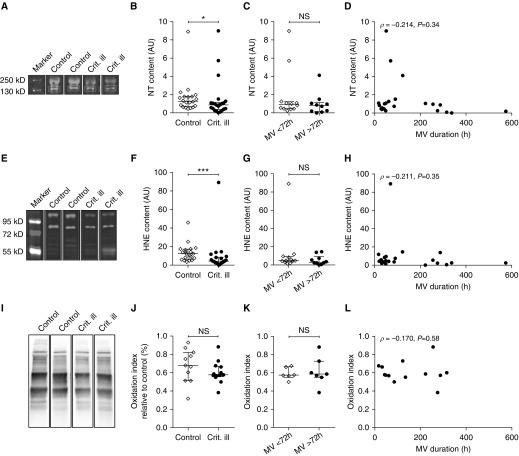
We hypothesized that oxidative stress levels are increased in the diaphragm of critically ill patients. However, no evidence supporting metabolic oversupply or mitochondrial dysfunction in diaphragm muscle fibers from critically ill patients was observed in the present study, suggesting that oxidative stress levels are comparable between control and critically ill patients. To verify this, we determined nitrosative stress marker nitrotyrosine, oxidative stress marker 4-hydroxy-2-nonenal (HNE), and the protein oxidation index in the diaphragm biopsies (see Figures 8A, 8E, and 8I, and Figures E6 and E7A for typical examples of Western blots and OxyBlots). Outcomes of nitrotyrosine and HNE assays were strongly correlated (ρ = 0.824; Figure E2A). Against our expectations, nitrotyrosine content (1.3 [0.7–1.8] vs. 0.9 [0.4–1.1]; P = 0.037) (Figure 8B) and HNE content (12.5 [6.5–17.2] vs. 4.5 [2.4–8.7]; P < 0.001) (Figure 8F) were lower in critically ill patients than in control patients, whereas the oxidation index was comparable (0.7 [0.5–0.8] vs. 0.6 [0.6–0.7]; P = 0.18) (Figure 8J). Because decrease in diaphragm thickness, as measured by ultrasound, is most pronounced during the first 72 hours of mechanical ventilation (38), we anticipated that oxidative stress would be increased during this period. Therefore, we compared nitrotyrosine, HNE, and oxidation indices of patients mechanically ventilated less than 72 hours with those of patients mechanically ventilated for more than 72 hours, but no differences were found (nitrotyrosine: 0.9 [0.5–1.2] vs. 0.8 [0.2–1.2] AU; P = 0.34; HNE: 4.7 [3.6–9.2] vs. 3.1 [1.9–9.0] AU; P = 0.37; oxidation index: 0.57 [0.55–0.67] vs. 0.59 [0.55–0.73]; P = 0.80) (Figures 8C, 8G, and 8K). Also, no correlation between nitrotyrosine content, HNE content, oxidation index, and the duration of mechanical ventilation was observed (Figures 8D, 8H, and 8L), further indicating that oxidative stress is not an early phenomenon in the diaphragm of mechanically ventilated critically ill patients. Finally, oxidative stress levels were not increased in critically ill patients ventilated with a controlled mode compared with those ventilated with an assist mode, and there was no difference between nonseptic and septic critically patients, patients with a low versus high disease severity (APACHE II score), or survivors versus nonsurvivors (see Figures E2B and E2C).
Figure 8.
Lower oxidative stress levels in diaphragm muscle fibers from critically ill patients. (A) Typical example of a Western blot stained for nitrosative stress marker nitrotyrosine (NT)-specific antibodies, indicating a footprint of nitrosative stress. (E) Typical example of a Western blot with 4-hydroxy-2-nonenal (HNE)-specific antibodies, a measure for lipid peroxidation. (I) Typical example of an OxyBlot, showing carbonylated proteins. (B, F, and J) NT and HNE contents were lower in diaphragm muscle fibers of critically ill patients than in those of control subjects, whereas the oxidation index was comparable. (C, G, and K) NT content, HNE content, and the oxidation index were not higher in critically ill patients mechanically ventilated for less than 72 hours than in critically ill patients mechanically ventilated longer than 72 hours. (D, H, and L) No correlation between the duration of ventilation and NT content, HNE content, and oxidation index was observed, suggesting that oxidative stress was not an early phenomenon in critically ill patients. Each data point represents the NT content, HNE content, or oxidation index in a diaphragm biopsy of one patient. Data in B, C, F, G, J, and K are presented as median and interquartile range. AU = arbitrary units; Crit. ill = critically ill; MV = mechanical ventilation; NS = not significant. *P ≤ 0.05, ***P ≤ 0.001.
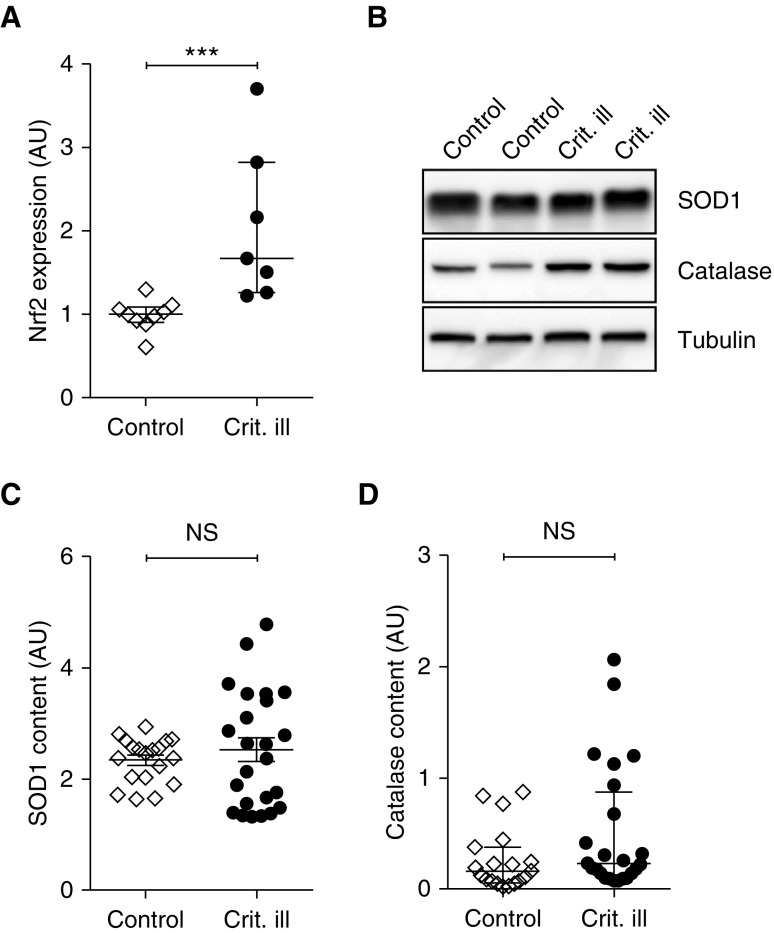
Finally, we determined the levels of superoxidase dismutase-1 (SOD1) and catalase, two antioxidant enzymes (see Figure 9B and Figure E7B for typical examples of the Western blots), as well as the expression level of nuclear erythroid 2–related factor 2 (Nrf2), a redox balance sensor and master regulator of antioxidant pathways. Nrf2 expression was increased in critically ill patients compared with control patients (1.0 [0.9–1.1] vs. 1.7 [1.3–2.8]; P < 0.001) (Figure 9A), whereas SOD1 and catalase protein contents were comparable in both groups (SOD1: 2.3 ± 0.1 vs. 2.5 ± 1.1; P = 0.47; catalase: 0.16 [0.07–0.38] vs. 0.23 [0.11–0.87]; P = 0.09) (Figures 9C and 9D). These data suggest that in diaphragm fibers of critically ill patients a redox imbalance might be developing, triggering Nrf2 expression.
Figure 9.
Antioxidant response in diaphragm muscle fibers from critically ill patients. (A) Expression of Nrf2, a master regulator of antioxidant pathways, was increased in critically ill patients, indicating activation of antioxidant pathways. (B) Typical example of a Western blot stained for antioxidant enzymes SOD1 and catalase, and loading control tubulin. (C and D) Antioxidant enzymes SOD1 and catalase were comparable in critically ill patients and control patients. Each data point represents the Nrf2 expression or SOD1 or catalase content in a diaphragm biopsy of one patient. Data in A and D are presented as median and interquartile range, and data in C are presented as mean ± SEM. AU = arbitrary units; Crit. ill = critically ill; Nrf2 = nuclear erythroid 2–related factor 2; NS = not significant; SOD1 = superoxidase dismutase-1. ***P ≤ 0.001.
Discussion
The aim of this study was to establish that contractile weakness of the diaphragm in mechanically ventilated critically ill patients is associated with mitochondrial dysfunction. Our findings reveal that diaphragm muscle fibers from these patients display significant atrophy and contractile weakness in the absence of major mitochondrial alterations and oxidative stress. Thus, mitochondrial alterations and oxidative stress do not play a causative role in the development of atrophy and contractile weakness of diaphragm muscle fibers in critically ill patients.
Preserved Mitochondrial Function in Diaphragm Muscle Fibers of Critically Ill Patients
Contractile weakness of diaphragm fibers in critically ill patients can be largely explained by fiber atrophy and activation of proteolytic pathways (10, 11). The mechanisms underlying the activation of proteolysis are unknown. Studies on brain-dead organ donors and mechanically ventilated animals have suggested a major role for mitochondrial dysfunction: diaphragm unloading during mechanical ventilation causes a state of energetic oversupply (14, 24), promoting fission (25), leading to mitochondrial dysfunction (22, 25) and oxidative stress (16), and hence activation of proteolytic pathways (13–15, 17, 18).
Surprisingly, in the present study in mechanically ventilated critically ill patients we observed atrophy and contractile weakness of diaphragm muscle fibers, in the absence of mitochondrial dysfunction, oxidative stress, and metabolic oversupply. Impaired fusion dynamics were present in critically ill patients (Figure 6), similar to findings in ventilated animals (14, 24). Typically, impaired mitochondrial fusion leads to impaired mitochondrial function (22), as occurs during a state of metabolic oversupply. However, the normal ATP concentration does not support such a state in the diaphragm of critically ill patients (Figure 7), and electron microscopy images do not show major morphological changes in mitochondria. In line with these findings, mitochondrial respiration was not impaired in critically ill patients, and also not in those who were septic (Figure E1). The latter findings corroborate data from Fredriksson and colleagues, which indicated normal ATP levels, and mitochondrial respiration and morphology, in the intercostal muscles of septic intensive care patients (39). Note that we cannot rule out that, eventually, the imbalance in mitochondrial dynamics impairs mitochondrial respiration and increases levels of oxidative stress in the diaphragm of critically ill patients. However, as the patients already displayed manifest diaphragm fiber atrophy and weakness at the time of biopsy (Figure 1), changes in mitochondrial dynamics cannot be the cause of diaphragm weakness.
Furthermore, the present findings do not support a concept in which mitochondrial dysfunction and oxidative stress peak during the first days of ICU admission to initiate proteolytic pathways, after which they rapidly normalize: markers of oxidative stress did not correlate with duration of mechanical ventilation (Figures 8D, 8H, and 8L). Patients who received less than 72 hours of mechanical ventilation before biopsy (a duration that has been associated with the most prominent diaphragm thinning on ultrasound [38]) had similar levels of oxidative stress markers as patients who were ventilated for longer durations. It should be noted that biopsies were obtained after at least 17 hours of mechanical ventilation, and thus we cannot rule out that oxidative stress levels were elevated during the first 17 hours.
In fact, increased Nrf2 expression and a trend toward increased catalase levels in the diaphragm of critically ill patients (Figures 9A and 9D) may reflect a (developing) redox imbalance. This is not unlikely, given the infiltration of neutrophils and macrophages into the diaphragm of critically ill patients (11). Clearly, the absence of increased oxidative stress markers indicates an adequate antioxidant response in the diaphragm, a response that is known to be well developed in respiratory muscles (39, 40). Indeed, catalase and levels of oxidative stress markers showed a significant negative correlation (HNE: r = −0.558, P = 0.01; nitrotyrosine: r = −0.456, P = 0.04; data not shown)
Catalase decomposes hydrogen peroxide, and its induction in diaphragm fibers of critically patients is apparently sufficient to prevent the development of oxidative stress. A redox imbalance due to hydrogen peroxide generation, independent of changes in ATP levels, can explain the activation of AMPK (Figure 7C) (41). AMPK activation induces FOXO (Forkhead box, class O) via the AMPK–FOXO pathway (42), which activates the ubiquitin–proteasome pathway. The latter pathway was shown to be activated in diaphragm fibers of critically ill patients from a similar cohort as studied in the present study (11). Thus, we propose that a redox imbalance triggers atrophy of diaphragm muscle fibers in critically ill patients, in the absence of mitochondrial dysfunction and oxidative stress.
The discrepancy between our findings and those from mechanically ventilated animal models and brain-dead organ donors can be explained by differences in their clinical features. For instance, critically ill patients can have sepsis, and do not have the excessive catecholamine release and hormonal disturbances that characterize brain-dead organ donors (43, 44). Another clinical feature that differs between critically ill patients and brain-dead organ donors relates to arterial blood gases. Hypercapnia can attenuate diaphragm contractile dysfunction (45), whereas hyperoxia contributes to oxidative stress formation (46) and impairs contractile function in skeletal muscle (47). The PaCO2 levels in our study (42 ± 5.6 mm Hg) were significantly higher than the levels in two other studies on brain-dead organ donors (Levine and colleagues [12]: 34 ± 6 mm Hg [P = 0.0004], and Hussain and colleagues [13]: 37 ± 1.8 mm Hg [P = 0.04]). In addition, those brain-dead organ donors had significantly higher PaO2 levels (Levine and colleagues [12]: 147 ± 88 mm Hg [P = 0.02]; Hussain and colleagues [13]: 260 ± 53 mm Hg [P < 0.0001]; Picard and colleagues [14]: 273 ± 132 mm Hg [P < 0.0001]) than did the critically ill patients in the present study (average pressure and pressure at biopsy, respectively; 100 ± 14 and 96 ± 26 mm Hg). Thus, it is conceivable that lower PaCO2 and extremely high PaO2 might have contributed to the elevated levels of oxidative stress markers in the diaphragm of brain-dead organ donors.
Furthermore, brain-dead organ donors and controlled mechanically ventilated animals completely lack diaphragm activity (48, 49), whereas in critically ill patients the ventilation mode—and thus diaphragm activity—depends on respiratory drive, level of sedation, the use neuromuscular blocking agents, and clinician preference. Studies have shown that even low levels of diaphragm activity are sufficient to improve mitochondrial function (50, 51) and decrease oxidative stress levels (50, 51). In these studies, the diaphragm of patients undergoing cardiothoracic surgery was activated by applying a magnetic stimulus to one phrenic nerve. The stimulus was applied every 30 minutes for 1 minute, which is a low frequency compared with the normal breathing frequency. Thus, even low levels of diaphragm activity in critically patients receiving controlled ventilation might be sufficient to protect against mitochondrial dysfunction and oxidative stress (see also Figures E2B and E2C).
Study Limitations
First, we did not determine whether all critically ill patients had in vivo diaphragm weakness, although it seems likely that the majority had, given the markedly reduced force-generating capacity of slow-twitch diaphragm fibers (fibers that are predominantly recruited during inspiration). Second, the patients in this study represent a subgroup of critically ill patients: they required abdominal or thoracic surgery, and had an average APACHE II score of 26 ± 1.4, which is at the high end of the ICU population. Surprisingly, even in this subgroup of severely ill patients, mitochondrial function in the diaphragm was preserved and oxidative stress levels were not increased. Thus, it is unlikely that in patients with less severe illness mitochondrial dysfunction develops and triggers diaphragm weakening. Third, our control group consisted of patients with lung cancer undergoing surgery for tumor removal. Lung cancer, a history of smoking, and surgery have been associated with systemic oxidative stress, and we cannot rule out that these systemic factors have negatively affected mitochondrial function and oxidative stress levels in diaphragm muscle fibers of our control patients (52–54). However, the control patients in the present study underwent surgery at an early stage of lung cancer, and they were not cachectic (Table 1). Furthermore, several control patients were diagnosed with COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD]) stage I or II, which has been associated with impaired diaphragm fiber contractility (55). However, in the present study we observed no difference in diaphragm fiber cross-sectional area and contractility between control patients with or without COPD (data not shown), indicating that COPD did not confound our results. Note that studies on brain-dead organ donors (12–14) with comparable control groups did show significant differences in mitochondrial function and oxidative stress in the diaphragm. This suggests that absence of mitochondrial dysfunction and oxidative stress in the present study was not due to our selection of control patients. Finally, we acknowledge that, because of size limitations of these human biopsies, not all experiments could be performed on all patients (for an overview of the number of biopsies studied per parameter, see Table E1).
Clinical Implications
Diaphragm weakness is an important factor in the development of weaning failure in critically ill patients. The results of the present study suggest that treatment aimed at targeting mitochondrial function may not be beneficial for critically ill patients with clinical features comparable to those of the patients studied here.
Conclusions
Diaphragm weakness in critically ill patients is not caused by mitochondrial dysfunction and oxidative stress. Future exploration of alternative pathways is necessary to understand the development of diaphragm weakness and to combat weaning failure.
Acknowledgments
Acknowledgment
The authors thank the research nurses, intensivists, and surgeons of the VU University Medical Center, Netherlands Cancer Institute–Antoni van Leeuwenhoek, and Medisch Spectrum Twente who were involved with patient inclusion and biopsy obtaining, and Sylvia Bogaards, Stefan Conijn, and Ruud Zaremba for help in conducting experiments.
Footnotes
Supported by a VIDI grant from the Netherlands Foundation for Scientific Research and by NHLBI grant HL-121500 (C.A.C.O.).
Author Contributions: Study design: A.B., M. A. Paul, and C.A.C.O. Study coordination: M.v.d.B., P.E.H., and C.A.C.O. Patient inclusion: A.B., M.C.d.W., M. A. Paul, and K.J.H. Biopsy obtainment: M. A. Paul and K.J.H. Data collection and analysis: M.v.d.B. (biopsy handling, contractility experiments, and bioluminescence); P.E.H. (biopsy handling, contractility and histology experiments, and mitochondrial complexes); H.W.H.v.H. (oxidative stress markers), M.W.L. (electron microscopy); L.B., R.B., and M. A. Pellegrino (Nrf2, OxyBlots, mitochondrial biogenesis, dynamics, and antioxidant enzymes); and R.C.I.W. (mitochondrial respiration). Data interpretation: M.v.d.B., P.E.H., H.W.H.v.H., M.W.L., L.B., R.B., M. A. Pellegrino, G.J.M.S., L.M.A.H., R.C.I.W., and C.A.C.O. Manuscript drafting: M.v.d.B., P.E.H., and C.A.C.O. Figure creation: M.v.d.B. and P.E.H. Critical revision and final approval of the draft to be published: M.v.d.B., P.E.H., A.B., M.C.d.W., M. A. Paul, K.J.H., H.W.H.v.H., M.W.L., L.B., R.B., M. A. Pellegrino, G.J.M.S., L.M.A.H., R.C.I.W., and C.A.C.O.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201703-0501OC on August 8, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med. 2001;29:1325–1331. doi: 10.1097/00003246-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hermans G, Agten A, Testelmans D, Decramer M, Gayan-Ramirez G. Increased duration of mechanical ventilation is associated with decreased diaphragmatic force: a prospective observational study. Crit Care. 2010;14:R127. doi: 10.1186/cc9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaber S, Petrof BJ, Jung B, Chanques G, Berthet JP, Rabuel C, Bouyabrine H, Courouble P, Koechlin-Ramonatxo C, Sebbane M, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–371. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman E, McCool FD. Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med. 1997;155:1570–1574. doi: 10.1164/ajrccm.155.5.9154859. [DOI] [PubMed] [Google Scholar]
- 5.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, Rittayamai N, Lanys A, Tomlinson G, Singh JM, et al. Evolution of diaphragm thickness during mechanical ventilation: impact of inspiratory effort. Am J Respir Crit Care Med. 2015;192:1080–1088. doi: 10.1164/rccm.201503-0620OC. [DOI] [PubMed] [Google Scholar]
- 6.Dres M, Dubé BP, Mayaux J, Delemazure J, Reuter D, Brochard L, Similowski T, Demoule A. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195:57–66. doi: 10.1164/rccm.201602-0367OC. [DOI] [PubMed] [Google Scholar]
- 7.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, et al. Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 8.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013;17:R120. doi: 10.1186/cc12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, Matecki S, Duguet A, Similowski T, Jaber S. Diaphragm dysfunction on admission to the intensive care unit: prevalence, risk factors, and prognostic impact—a prospective study. Am J Respir Crit Care Med. 2013;188:213–219. doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- 10.Hooijman PE, Beishuizen A, de Waard MC, de Man FS, Vermeijden JW, Steenvoorde P, Bouwman RA, Lommen W, van Hees HW, Heunks LM, et al. Diaphragm fiber strength is reduced in critically ill patients and restored by a troponin activator. Am J Respir Crit Care Med. 2014;189:863–865. doi: 10.1164/rccm.201312-2260LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra-de Man AM, Niessen HW, Manders E, van Hees HW, van den Brom CE, et al. Diaphragm muscle fiber weakness and ubiquitin–proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191:1126–1138. doi: 10.1164/rccm.201412-2214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 13.Hussain SN, Mofarrahi M, Sigala I, Kim HC, Vassilakopoulos T, Maltais F, Bellenis I, Chaturvedi R, Gottfried SB, Metrakos P, et al. Mechanical ventilation–induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182:1377–1386. doi: 10.1164/rccm.201002-0234OC. [DOI] [PubMed] [Google Scholar]
- 14.Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, Godin R, Danialou G, Chaturvedi R, Rygiel K, et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med. 2012;186:1140–1149. doi: 10.1164/rccm.201206-0982OC. [DOI] [PubMed] [Google Scholar]
- 15.McClung JM, Whidden MA, Kavazis AN, Falk DJ, Deruisseau KC, Powers SK. Redox regulation of diaphragm proteolysis during mechanical ventilation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1608–R1617. doi: 10.1152/ajpregu.00044.2008. [DOI] [PubMed] [Google Scholar]
- 16.Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med. 2009;46:842–850. doi: 10.1016/j.freeradbiomed.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation–induced protease activation in the diaphragm. J Appl Physiol. 1985;2010:1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation–induced diaphragm weakness. Crit Care Med. 2011;39:1749–1759. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Weaver D, Shirihai O, Hajnóczky G. Mitochondrial “kiss-and-run”: interplay between mitochondrial motility and fusion–fission dynamics. EMBO J. 2009;28:3074–3089. doi: 10.1038/emboj.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 23.Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picard M, Azuelos I, Jung B, Giordano C, Matecki S, Hussain S, White K, Li T, Liang F, Benedetti A, et al. Mechanical ventilation triggers abnormal mitochondrial dynamics and morphology in the diaphragm. J Appl Physiol. 1985;2015:1161–1171. doi: 10.1152/japplphysiol.00873.2014. [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Jin P, Yu L, Wang Y, Han L, Shi T, Li X. Impaired mitochondrial dynamics and bioenergetics in diabetic skeletal muscle. PLoS One. 2014;9:e92810. doi: 10.1371/journal.pone.0092810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Berg M, Hooijman PE, Beishuizen A, de Waard MC, Girbes ARJ, Spoelstra-de Man A, Zaremba R, Lawlor MW, Stienen GJM, Hartemink KJ, et al. Late-breaking abstract: disturbed mitochondrial dynamics and network in diaphragm muscle fibers of mechanically ventilated critically ill patients [abstract] Eur Respir J. 2015;46(Suppl 59):PA850. [Google Scholar]
- 27.Wüst RC, Helmes M, Stienen GJ. Rapid changes in NADH and flavin autofluorescence in rat cardiac trabeculae reveal large mitochondrial complex II reserve capacity. J Physiol. 2015;593:1829–1840. doi: 10.1113/jphysiol.2014.286153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wüst RC, Myers DS, Stones R, Benoist D, Robinson PA, Boyle JP, Peers C, White E, Rossiter HB. Regional skeletal muscle remodeling and mitochondrial dysfunction in right ventricular heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H402–H411. doi: 10.1152/ajpheart.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wüst RC, de Vries HJ, Wintjes LT, Rodenburg RJ, Niessen HW, Stienen GJ. Mitochondrial complex I dysfunction and altered NAD(P)H kinetics in rat myocardium in cardiac right ventricular hypertrophy and failure. Cardiovasc Res. 2016;111:362–372. doi: 10.1093/cvr/cvw176. [DOI] [PubMed] [Google Scholar]
- 30.Cannavino J, Brocca L, Sandri M, Bottinelli R, Pellegrino MA. PGC1-α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol. 2014;592:4575–4589. doi: 10.1113/jphysiol.2014.275545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schellekens WJ, van Hees HW, Linkels M, Dekhuijzen PN, Scheffer GJ, van der Hoeven JG, Heunks LM. Levosimendan affects oxidative and inflammatory pathways in the diaphragm of ventilated endotoxemic mice. Crit Care. 2015;19:69. doi: 10.1186/s13054-015-0798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ottenheijm CA, Heunks LM, Geraedts MC, Dekhuijzen PN. Hypoxia-induced skeletal muscle fiber dysfunction: role for reactive nitrogen species. Am J Physiol Lung Cell Mol Physiol. 2006;290:L127–L135. doi: 10.1152/ajplung.00073.2005. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Heunks LM, Versteeg EM, van der Heijden HF, Ennen L, van Kuppevelt TH, Vina J, Dekhuijzen PN. Hypoxia-induced dysfunction of rat diaphragm: role of peroxynitrite. Am J Physiol Lung Cell Mol Physiol. 2005;288:L16–L26. doi: 10.1152/ajplung.00412.2003. [DOI] [PubMed] [Google Scholar]
- 34.Brocca L, Pellegrino MA, Desaphy JF, Pierno S, Camerino DC, Bottinelli R. Is oxidative stress a cause or consequence of disuse muscle atrophy in mice? A proteomic approach in hindlimb-unloaded mice. Exp Physiol. 2010;95:331–350. doi: 10.1113/expphysiol.2009.050245. [DOI] [PubMed] [Google Scholar]
- 35.Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R, Pellegrino MA. The role of alterations in mitochondrial dynamics and PGC-1α over-expression in fast muscle atrophy following hindlimb unloading. J Physiol. 2015;593:1981–1995. doi: 10.1113/jphysiol.2014.286740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats’ skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 37.Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2006;290:E471–E479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- 38.Schepens T, Verbrugghe W, Dams K, Corthouts B, Parizel PM, Jorens PG. The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Crit Care. 2015;19:422. doi: 10.1186/s13054-015-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fredriksson K, Hammarqvist F, Strigård K, Hultenby K, Ljungqvist O, Wernerman J, Rooyackers O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291:E1044–E1050. doi: 10.1152/ajpendo.00218.2006. [DOI] [PubMed] [Google Scholar]
- 40.Pascual-Guardia S, Árbol F, Sánchez E, Casadevall C, Merlo V, Gea J, Barreiro E. [Inflammation and oxidative stress in respiratory and limb muscles of patients with severe sepsis] [article in Spanish] Med Clin (Barc) 2013;141:194–200. doi: 10.1016/j.medcli.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong JF, Yan X, Zhu MJ, Du M. AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes. J Cell Biochem. 2009;108:458–468. doi: 10.1002/jcb.22272. [DOI] [PubMed] [Google Scholar]
- 43.McKeown DW, Bonser RS, Kellum JA. Management of the heartbeating brain-dead organ donor. Br J Anaesth. 2012;108:i96–i107. doi: 10.1093/bja/aer351. [DOI] [PubMed] [Google Scholar]
- 44.Smith M. Physiologic changes during brain stem death: lessons for management of the organ donor. J Heart Lung Transplant. 2004;23(9) Suppl:S217–S222. doi: 10.1016/j.healun.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 45.Schellekens WJ, van Hees HW, Kox M, Linkels M, Acuña GL, Dekhuijzen PN, Scheffer GJ, van der Hoeven JG, Heunks LM. Hypercapnia attenuates ventilator-induced diaphragm atrophy and modulates dysfunction. Crit Care. 2014;18:R28. doi: 10.1186/cc13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flandin P, Donati Y, Barazzone-Argiroffo C, Muzzin P. Hyperoxia-mediated oxidative stress increases expression of UCP3 mRNA and protein in skeletal muscle. FEBS Lett. 2005;579:3411–3415. doi: 10.1016/j.febslet.2005.04.084. [DOI] [PubMed] [Google Scholar]
- 47.Eu JP, Hare JM, Hess DT, Skaf M, Sun J, Cardenas-Navina I, Sun QA, Dewhirst M, Meissner G, Stamler JS. Concerted regulation of skeletal muscle contractility by oxygen tension and endogenous nitric oxide. Proc Natl Acad Sci USA. 2003;100:15229–15234. doi: 10.1073/pnas.2433468100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quality Standards Subcommittee of the American Academy of Neurology. Practice parameters for determining brain death in adults (summary statement) Neurology. 1995;45:1012–1014. doi: 10.1212/wnl.45.5.1012. [DOI] [PubMed] [Google Scholar]
- 49.Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol. 1985;2002:2585–2595. doi: 10.1152/japplphysiol.01213.2001. [DOI] [PubMed] [Google Scholar]
- 50.Martin AD, Joseph AM, Beaver TM, Smith BK, Martin TD, Berg K, Hess PJ, Deoghare HV, Leeuwenburgh C. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit Care Med. 2014;42:e152–e156. doi: 10.1097/CCM.0b013e3182a63fdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mankowski RT, Ahmed S, Beaver T, Dirain M, Han C, Hess P, Martin T, Smith BK, Someya S, Leeuwenburgh C, et al. Intraoperative hemidiaphragm electrical stimulation reduces oxidative stress and upregulates autophagy in surgery patients undergoing mechanical ventilation: exploratory study. J Transl Med. 2016;14:305. doi: 10.1186/s12967-016-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esme H, Cemek M, Sezer M, Saglam H, Demir A, Melek H, Unlu M. High levels of oxidative stress in patients with advanced lung cancer. Respirology. 2008;13:112–116. doi: 10.1111/j.1440-1843.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 53.Barreiro E, del Puerto-Nevado L, Puig-Vilanova E, Pérez-Rial S, Sánchez F, Martínez-Galán L, Rivera S, Gea J, González-Mangado N, Peces-Barba G. Cigarette smoke–induced oxidative stress in skeletal muscles of mice. Respir Physiol Neurobiol. 2012;182:9–17. doi: 10.1016/j.resp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeldt F, Wilson M, Lee G, Kure C, Ou R, Braun L, de Haan J. Oxidative stress in surgery in an ageing population: pathophysiology and therapy. Exp Gerontol. 2013;48:45–54. doi: 10.1016/j.exger.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:200–205. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]