Pulmonary fibrosis (PF) is a chronic, incurable interstitial lung disease characterized by irreversible destruction of lung architecture and abnormal wound healing, leading to disruption of gas exchange and death from respiratory failure. PF is either idiopathic (1) or arises from exposure to environmental toxins, such as fibers or asbestos. Idiopathic PF (IPF), which has a median mortality rate of 2–3 years after diagnosis, is characterized by radiographic or histologic findings of usual interstitial pneumonia in the absence of identifiable causes. IPF is pathologically characterized by the aberrant deposition of extracellular matrix as a result of repetitive injury to the alveolar epithelium, along with the accumulation of α-smooth muscle actin (αSMA)-expressing myofibroblasts. Soluble mediators released from epithelial cells, such as transforming growth factor (TGF)-β1, and mechanical influences, such as local tissue stiffness, are thought to activate fibroblast proliferation, leading to the presence of abundant foci, consisting of highly activated fibroblasts and myofibroblasts that are enriched for aerobic glycolysis and innate immune receptor activation (2, 3).
Although IPF is presumed to be an epithelial-driven disease, it also displays elements of a pathologic innate and adaptive immune response, including dysregulated macrophage, T, and B cell responses. The inflammatory pathways involved in the pathogenesis of IPF are not entirely understood, but the notion that endogenous damage-associated molecular patterns (DAMPs) released from epithelial cells, myofibroblasts, or innate immune cells, may amplify and exacerbate fibrosis is now being appreciated. Classically, it is thought that pathogen recognition receptors (PRRs) present on resident lung leukocytes recognize DAMPs and respond through the initiation of inflammatory cascades and the secretion of cytokines and other signaling molecules. During normal homeostasis, DAMP-associated inflammation allows the host to clear cellular debris, resolve inflammation, and repair tissue (4). However, in the context of IPF, the production and response to such DAMPs is dysregulated, and is thought to drive the unrelenting tissue remodeling response, in the absence of an overt infection (4). A number of DAMPs and danger receptors appear to contribute to the pathogenesis of lung fibrosis, including uric acid and ATP (4); however, until now, the concept that normal human lung fibroblasts (NHLFs) when damaged may act as the source, as well as the main recipient or responder to these DAMPs is particularly novel. In this issue of the Journal, Ryu and colleagues (pp. 1571–1581) propose that CpG-rich mitochondrial DNA (mtDNA), a DAMP previously associated with increased inflammation and mortality in other clinical settings (5, 6), may act as a driver of the proliferative myofibroblast phenotype in patients with IPF (7).
Specifically, the authors elegantly demonstrate that both TGF-β1 and direct contact with stiff surfaces (biomechanical model to mimic the IPF lung microenvironment) induce mtDNA release by NHLFs. In a similar manner, fibroblasts derived from patients with IPF spontaneously release mtDNA. The authors propose that such release of mtDNA associates with a metabolic shift of NHLFs to aerobic glycolysis. Using Seahorse technology, which provides information on mitochondrial metabolic function through real-time measurements of oxygen consumption rate (OCR; a marker of oxidative phosphorylation) and extracellular acidification rate (a surrogate for glycolysis), the authors demonstrate that NHLFs, as well as human IPF cells, have a higher extracellular acidification rate at baseline and relative to OCR, suggestive of more aerobic glycolysis. Such a decrease in mitochondrial-dependent OCR may occur as a result of lower mitochondrial numbers in the NHLFs; consistently, NHLFs stimulated with TGF-β1 (or by contact with stiff surfaces) displayed reduced mitochondrial mass. Interestingly, both NHLFs and their mitochondria appeared healthy, with normal mitochondrial membrane potentials and normal cell viability, suggesting that mtDNA release may occur via an active release mechanism, and not as a result of cell death. Although the link between mtDNA release and mechanotransduction is a particularly novel aspect of this study, what is most interesting about this study is that, when the authors stimulated NHLFs with isolated extracellular mtDNA from a parallel fibroblast culture, the mtDNA augmented αSMA expression in otherwise normal lung fibroblasts. This finding suggests that mtDNA may promote highly proliferative myofibroblasts in some sort of a paracrine autofeedback loop.
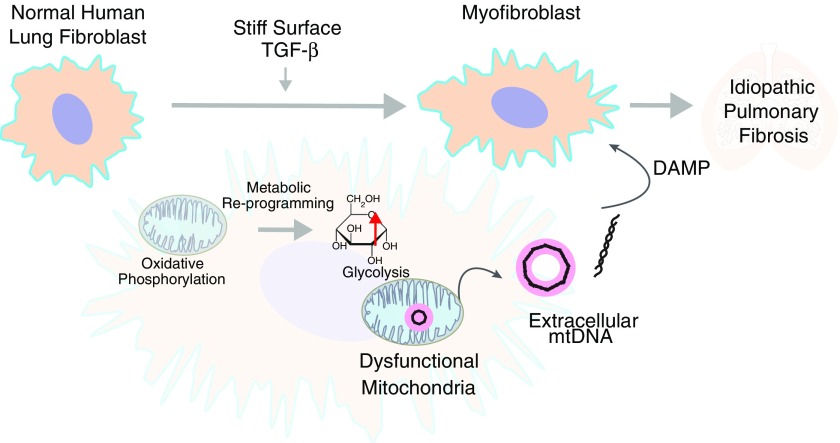
The authors therefore hypothesize that the αSMA-expressing, glycolytically reprogrammed phenotype of IPF fibroblasts drives a microenvironment that is enriched for mtDNA (Figure 1). To support this hypothesis, the authors proceeded to measure mtDNA in the bronchoalveolar lavage fluid of patients with IPF. Importantly they are able to show that mtDNA is increased in the bronchoalveolar lavage fluid of subjects with IPF when compared with control subjects. Subsequently, they demonstrate that extracellular mtDNA levels increase in the plasma of patients with IPF, where mtDNA levels strikingly associate with disease progression and reduced event-free survival in two independent cohorts. Finally, the authors show that circulating plasma mtDNA concentrations are reduced in patients who respond to pirfenidone, suggesting that mtDNA measurements may have prognostic use for responses to new therapies.
Figure 1.
Glycolytically reprogrammed idiopathic pulmonary fibrosis (IPF) fibroblasts drive a microenvironment that is enriched for mitochondrial DNA (mtDNA). Transforming growth factor (TGF)-β1 and direct contact with stiff surfaces (biomechanical model to mimic the IPF lung microenvironment) induce mtDNA release by normal human lung fibroblasts (NHLFs). Such a release of mtDNA is associated with a shift in metabolism of an NHLF from oxidative phosphorylation (mitochondrial dependent) to glycolysis. DAMP = damage-associated molecular pattern.
Although this is an elegant, well-designed study with high translational relevance, demonstrating clinical prognostic applicability for human IPF, these findings bring up a number of thought-provoking questions related to mitochondrial biology and disease pathogenesis in the lung. While it is well known that TGF-β1 critically regulates the alterations in mitochondrial function and bioenergetics that are required to fuel these activated fibroblasts, little is known about how metabolic rearrangements trigger mtDNA release. Are defects in the somatic sequence of mtDNA, an observation noted by Gazdhar and colleagues (8) in a scleroderma model of lung fibrosis, responsible for this altered metabolism and, in turn, release of mtDNA? Other questions include: how does mtDNA escape from the cell in this apparently active process? Is mtDNA being secreted in vesicular form, or are mitochondria themselves being released from damaged cells and acting in some sort of adaptive transfer mechanism with a recipient cell? Are other mitochondrial DAMPS also released in a similar manner in IPF? Putative mechanisms regulating mtDNA release may involve processes such as mitophagy, the selective removal of damaged or dysfunctional mitochondria by autophagy, of which a loss of this process (which would, in turn, lead to more extracellular mtDNA) has been associated with enhanced myofibroblast differentiation and profibrotic signaling pathways in IPF (9, 10); mitochondrial sirtuins, class III histone deacetylases that control the transformation of fibroblasts into myofibroblasts via suppression of TGF-β1 signaling (11), and regulators of mitochondrial dynamics and biogenesis (11, 12). Finally the exact cellular source of mtDNA in human IPF remains to be firmly solidified—do other cells that have been documented to have abnormal mitochondria, such as the epithelial cell (1, 9, 11, 13), also release this danger signal? The authors also speculate on the downstream effects of such mtDNA release, including the direct activation of macrophages or the role of mtDNA in the formation of neutrophil extracellular traps (14). Despite these unanswered questions, this is a solid study that provides supportive evidence to explore the use of circulating mtDNA as a mechanism-based, prognostic biomarker of IPF. This study also significantly contributes to our understanding of the role of mitochondrial dynamics and signaling in lung biology and disease.
Footnotes
Supported by National Institute of Health/NHLBI grant K99-HL125899 and by American Lung Association Biomedical Research grant RG-348928 (S.M.C.).
Originally Published in Press as DOI: 10.1164/rccm.201708-1593ED on August 16, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, et al. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS One. 2015;10:e0121246. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Dwyer DN, Ashley SL, Moore BB. Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2016;311:L590–L601. doi: 10.1152/ajplung.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V, Egan JJ, Lambrecht BN, Lories R, Parfrey H, et al. The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J. 2013;41:1207–1218. doi: 10.1183/09031936.00073012. [DOI] [PubMed] [Google Scholar]
- 4.Ellson CD, Dunmore R, Hogaboam CM, Sleeman MA, Murray LA. Danger-associated molecular patterns and danger signals in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;51:163–168. doi: 10.1165/rcmb.2013-0366TR. [DOI] [PubMed] [Google Scholar]
- 5.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10:e1001577. doi: 10.1371/journal.pmed.1001577. [Discussion, e1001577.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krychtiuk KA, Ruhittel S, Hohensinner PJ, Koller L, Kaun C, Lenz M, Bauer B, Wutzlhofer L, Draxler DF, Maurer G, et al. Mitochondrial DNA and Toll-like receptor-9 are associated with mortality in critically ill patients. Crit Care Med. 2015;43:2633–2641. doi: 10.1097/CCM.0000000000001311. [DOI] [PubMed] [Google Scholar]
- 7.Ryu C, Sun H, Gulati M, Herazo-Maya JD, Chen Y, Osafo-Addo A, Brandsdorfer C, Winkler J, Blaul C, Faunce J, et al. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:1571–1581. doi: 10.1164/rccm.201612-2480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazdhar A, Lebrecht D, Roth M, Tamm M, Venhoff N, Foocharoen C, Geiser T, Walker UA. Time-dependent and somatically acquired mitochondrial DNA mutagenesis and respiratory chain dysfunction in a scleroderma model of lung fibrosis. Sci Rep. 2014;4:5336. doi: 10.1038/srep05336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, Sanchez CG. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1. Aging Cell. 2015;14:774–783. doi: 10.1111/acel.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi K, Araya J, Minagawa S, Hara H, Saito N, Kadota T, Sato N, Yoshida M, Tsubouchi K, Kurita Y, et al. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J Immunol. 2016;197:504–516. doi: 10.4049/jimmunol.1600265. [DOI] [PubMed] [Google Scholar]
- 11.Sundaresan NR, Bindu S, Pillai VB, Samant S, Pan Y, Huang JY, Gupta M, Nagalingam RS, Wolfgeher D, Verdin E, et al. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol Cell Biol. 2015;36:678–692. doi: 10.1128/MCB.00586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mora AL, Bueno M, Rojas M. Mitochondria in the spotlight of aging and idiopathic pulmonary fibrosis. J Clin Invest. 2017;127:405–414. doi: 10.1172/JCI87440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueno M, Lai Y-C, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]