Abstract
Mechanical ventilation (MV) is critical in the management of many patients with acute respiratory distress syndrome (ARDS). However, MV can also cause ventilator-induced lung injury (VILI). The selection of an appropriate Vt is an essential part of a lung-protective MV strategy. Since the publication of a large randomized clinical trial demonstrating the benefit of lower Vts, the use of Vts of 6 ml/kg predicted body weight (based on sex and height) has been recommended in clinical practice guidelines. However, the predicted body weight approach is imperfect in patients with ARDS because the amount of aerated lung varies considerably due to differences in inflammation, consolidation, flooding, and atelectasis. Better approaches to setting Vt may include limits on end-inspiratory transpulmonary pressure, lung strain, and driving pressure. The limits of lowering Vt have not yet been established, and some patients may benefit from Vts that are lower than those in current use. However, lowering Vts may result in respiratory acidosis. Tactics to reduce respiratory acidosis include reductions in ventilation circuit dead space, increases in respiratory rate, higher positive end-expiratory pressures in patients who recruit lung in response to positive end-expiratory pressure, recruitment maneuvers, and prone positioning. Mechanical adjuncts such as extracorporeal carbon dioxide removal may be useful to normalize pH and carbon dioxide levels, but further studies will be necessary to demonstrate benefit with this technology.
Keywords: acute respiratory distress syndrome, tidal volume, mechanical ventilation
Mechanical ventilation (MV) is critical for survival of many patients with acute respiratory distress syndrome (ARDS). However, MV can also cause ventilation-induced lung injury (VILI), which can exacerbate or perpetuate injury from the original cause of ARDS, such as pneumonia, sepsis, or trauma (1–5). One mechanism of VILI is overdistention of aerated lung tissue (6). Patients with ARDS are especially vulnerable to this, because the volume of aerated lung available for ventilation is reduced greatly in some patients. Therefore, a Vt that might be gentle and harmless in a normal subject may cause serious injury from overdistention in patients with ARDS. To prevent or reduce VILI from overdistention, we usually use Vts that are substantially smaller than those that were used in the past, before the potential for VILI from overdistention was recognized (7, 8).
Setting Vt is an important part of MV management of patients with ARDS. Several approaches for adjusting Vt to prevent VILI have been suggested. In this review, we explain the rationale behind different methods, review data that support them, and comment on certain practical aspects of setting Vt for patients with ARDS. This discussion is written with the assumption that the ventilator mode allows direct control over Vt, as with the volume-assist/control mode, or indirect control, as with pressure-assist/control mode in patients who are not making respiratory efforts. Moreover, additional considerations are needed when patients are making spontaneous inspiratory efforts even when on controlled modes of ventilation. The risks and management of spontaneous breathing during mechanical ventilation have been expertly reviewed recently (9).
Volume-Control versus Pressure-Control Modes
In volume-assist/control modes, depending on the patient’s respiratory system compliance, the resulting inspiratory alveolar pressure may be low, intermediate, or high. If it is too high, the clinician may reduce the set Vt. With a mode that allows direct control of the pressure during inspiration, as with the pressure control mode, the Vt that results from the inspiratory pressure increment depends on the magnitude and duration of the increment and a patient’s respiratory system compliance (lung and chest wall combined) and airway resistance. The pressure control increment and duration can be adjusted if the initial Vt is not at the desired volume. Some modes, such as pressure-regulated volume control and autoflow, allow control over the Vt if the patient has no respiratory efforts. However, control over Vt is lost in these modes when patients begin to make spontaneous respiratory efforts (9).
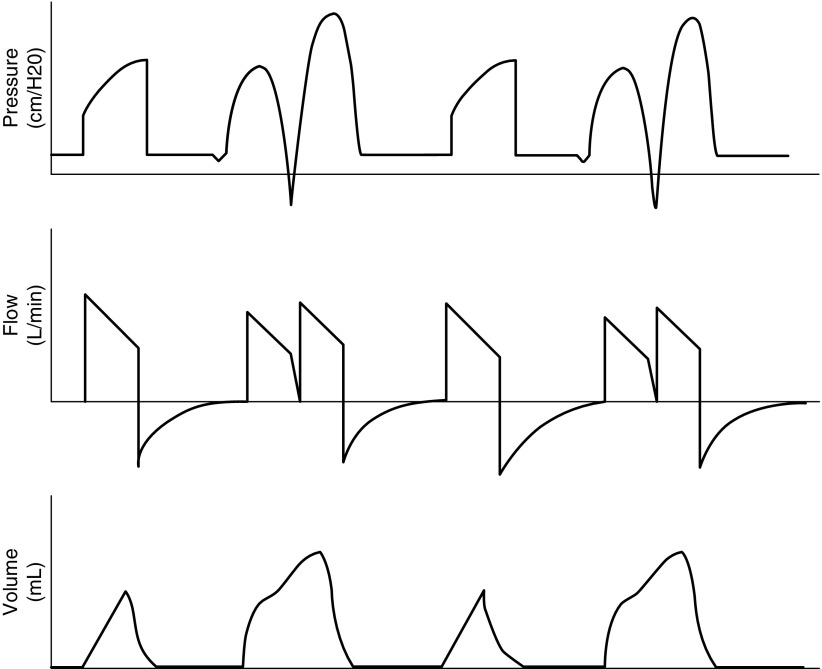
Pressure and volume are inextricably entwined according to each patient’s respiratory system pressure–volume relationship. Therefore, theoretically we can achieve the same Vt and airway pressure pattern of ventilation with either mode. The choice of modes is usually dictated by clinicians’ experiences and preferences. Some prefer the pressure-control mode because they can set the pressure during inspiration to a level that they think is safe. This may have some merit when patients are passive, not making any efforts to breath. However, if patients make inspiratory efforts in the pressure-control mode, the resulting Vts can be large even though the airway pressure is not high, causing substantial overdistention (10, 11). Thus, one advantage of the volume-control mode is that we have better control over Vts. A disadvantage of the volume-control mode is that the inspiratory flow is preset. In patients who have a high inspiratory drive, the flow setting may result in frequent double breaths, which can cause VILI from overdistention. (Figure 1). Nevertheless, although the volume- and pressure-control modes are very different, current data do not show any difference in clinical outcomes between the modes (11).
Figure 1.
Patient–ventilator dyssynchrony with triggering of double breaths from the ventilator in volume-assist control mode. The first channel demonstrates airway pressure with a normal breath followed by a double breath triggered by the patient. The second channel shows flow during a normal breath followed by a double breath. Patient inspiratory effort continues beyond the set ventilator inspiratory time, resulting in airway pressure decreasing below the positive end-expiratory pressure and triggering a second breath during the same patient effort. This may result in high lung pressures (during the double breath: top panel) and high volumes. The third channel demonstrates increased volumes during double-triggered breaths. If patients consistently trigger double breaths, they will not be receiving low Vts for lung protection during the double breaths and are at increased risk for overdistention.
Methods for Setting Vt
Setting Vt according to Body Weight
A historical approach set Vts according to actual body weight. Larger patients generally have larger lungs, so patients with larger lungs would usually receive larger Vts. In a comprehensive review of acute respiratory failure in adults from 1972, the authors recommended generous Vts of 10 to 15 ml/kg (7). There were two problems with this recommendation. First, although lung size correlates with body weight, body weight varies substantially between patients because of differences in adipose tissue, muscle mass, and extravascular fluid, independent of lung size. Second, these large Vts would tend to cause VILI from overdistention in patients at risk for or with established ARDS.
Four randomized clinical trials of traditional, generous Vts versus lower Vts in patients with or at risk for ARDS used estimations of lean body weight to set Vts (12–15). One trial used an equation for “ideal body weight” that did not account for variations in lung size according to sex (13). Two of the trials used equations for “predicted body weight” (PBW) that accounted for both sex and height (14, 15):
Several subsequent clinical studies and randomized trials used PBW to set Vts (16–20), and some clinical guidelines recommended this approach (21, 22).
According to the National Institutes of Health ARDS Network protocol, the goal for Vts is 6 ml/kg PBW, with an inspiratory plateau pressure (Pplat) limit of 30 cm H2O. If the Pplat exceeds 30 cm H2O on a Vt of 6 ml/kg PBW, the protocol recommends a reduction in Vt to 5 or 4 ml/kg PBW if arterial pH is greater than 7.15. The goal of 6 ml/kg PBW was chosen to be lung protective without causing severe respiratory acidosis in most patients. The ARDS Network trial demonstrated improved clinical outcomes with a Vt goal of 6 ml/kg PBW as opposed to a Vt goal of 12 ml/kg PBW. Some investigators suggested that it is not necessary to reduce Vts if the Pplat is less than 30 or 32 cm H2O (23, 24). However, an analysis of the ARDS Network Vt trial suggested that there were beneficial effects of Vt reduction even among patients whose Pplats would have been ≤26 cm H2O if they received Vts of 12 ml/kg PBW (25). Two other studies suggested that Pplats in the mid-20s were safer than Pplats in the high 20s (20, 26).
A shortcoming of the PBW approach is that the volume of aerated lung varies substantially among patients with ARDS because, at a given sex and height, there is a substantial variability in normal lung volumes (27). Moreover, among patients with ARDS, there are large differences in the extent of the lung inflammation, edema, atelectasis, and consolidation. These are the main reasons that, at a given Vt, Pplats vary greatly among patients with ARDS of the same sex and height. The Pplat limit of 30 cm H2O leads to additional reductions in Vt in patients with more extensive disease. However, in some patients, high inspiratory pressures are caused by high chest wall elastance and weight rather than extensive lung disease (28, 29). A better approach could be to set Vt according to some objective measure of aerated lung volume, such as the functional residual capacity (30, 31).
Setting Vt to Reduce Driving Pressure
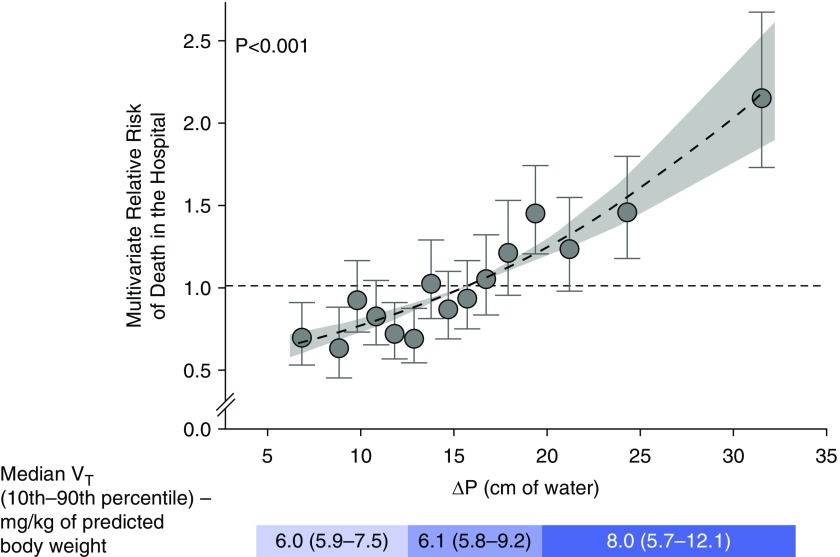
Driving pressure (DP) is easily calculated at the bedside as the difference between the airway inspiratory plateau pressure and the positive end-expiratory pressure (PEEP) (DP = Pplat − PEEP). It is determined by the ratio of the Vt to the compliance of the respiratory system (CRS) (DP = Vt/CRS). Because CRS is directly related to the size of the lung participating in ventilation, driving pressure reflects the size of the Vt in relation to the aerated lung volume. Assuming no auto-PEEP, DP estimates the average increase in alveolar pressure during inspiration and decrease during exhalation. Unlike Pplat and PEEP, which are static estimates of stress in the respiratory system, DP is a dynamic indicator, the change in stress with each breath. An individual patient data meta-analysis of predictors of mortality included more than 3,000 patients with ARDS enrolled in clinical trials of lung-protective ventilation strategies (32). All patients in the meta-analysis were ventilated with a PEEP of at least 5 cm H2O. DP was the strongest predictor of mortality among the mechanical ventilation variables that clinicians can manipulate (Vt, Pplat, PEEP, and respiratory rate). At a constant DP, variations in Vt, PEEP, and Pplat did not predict mortality. The median DP was approximately 14 cm H2O, and the slope of the relationship of mortality to DP appeared to increase above 14 cm H2O (Figure 2). This might suggest that we should reduce Vt until DP is below 14 cm H2O. When DP is less than 14 cm H2O with Vt greater than 6 ml/kg PBW, the value of further Vt reduction is likely less, as other factors may take on more importance. The magnitude of the effect of DP on mortality in this meta-analysis may be partly attributable to the higher range of DPs included from the studies examining higher versus lower Vt (33). In the Vt trials, DP ranged from approximately 10 to 15 cm H2O in the low Vt groups and approximately 20 to 25 cm H2O in the control groups. Subsequent mechanical ventilation trials on higher versus lower PEEP levels limited Vt to 6 ml/kg PBW, and DP ranged from 12 to 17 cm H2O in both control and intervention groups. However, in the patient data meta-analysis, the mortality rate among patients with DP below 14 cm H2O was still approximately 20%. Furthermore, the slope of the relationship between DP and mortality appears to be positive even at DP below 14 cm H2O, suggesting that there is no safe upper limit for DP in patients with ARDS on positive pressure ventilation.
Figure 2.
Relative risk of death in the hospital versus driving pressure in the combined cohort after multivariate adjustment. Even below the median driving pressure of 14 cm H2O, there is still a significant risk of death in the hospital. Reprinted by permission from Reference 32.
Another problem with using DP to set Vt is that Pplat, one of the components of DP, is influenced by the chest wall. Two patients with the same DP might have very different risks for VILI. At a given DP, a patient with a stiff or heavy chest wall likely has less overdistention of the lung than a patient with a normal chest wall. Therefore, a better indicator of dynamic stress in the lung would be the transpulmonary driving pressure (DP-Pl), which is the difference in transpulmonary pressure (Pl = airway pressure minus pleural pressure) between end-expiration and end-inspiration (34). However, there are several assumptions and potential limitations of measuring Pl, and we do not know the safe upper limit for DP-Pl (35). This probably varies depending on the severity of the lung injury from pneumonia, sepsis, or whatever the inciting cause of ARDS.
Setting Vt to Reduce Lung Stress
The stress of an object is the force applied to it divided by its surface area. In the lungs, a static Pl represents the stress in the lungs at a given lung volume (34). At the end of inspiration, Pl represents the highest level of stress during the respiratory cycle, which is determined by the PEEP and the size of the Vt relative to the amount of aerated lung. Thus, Pl may be used as a marker of potential overdistention injury, and Vt could be limited to maintain end-inspiratory Pl below a critical limit. A previous investigation suggested a critical limit of 27 cm H2O for end-inspiratory Pl in humans (36). Other models, however, have proposed much lower limits for acceptable lung stress during inspiration. Using a theoretical model, Gattinoni and colleagues suggested the limit to avoid overdistention was an end-inspiratory Pl of 15 cm H2O, which corresponds to approximately 70 to 75% of total lung capacity (TLC) in normal humans (37). In healthy pigs, ventilator-induced pulmonary edema occurred at an average inspiratory Pl of 13 cm H2O (38).
At this time, the only method for estimating pleural pressure in patients is esophageal manometry, and several questionable assumptions are required when we use this technique (35). Furthermore, there are different methods of calculating Pl, which yield results that sometimes differ substantially depending on which calculation is used (39). At this time, there do not appear to be clear boundaries for Pl in humans. In the future, clinicians will need reliable measurements for Pl and reliable targets for safe limits of stress before this measure can be used to optimize Vts.
Setting Vt to Reduce Strain
Strain is the ratio of the stretch of an object to its resting length. In the lung, strain at end-inspiration can be represented by the Vt normalized to the FRC measured at an airway opening pressure of 0 cm H2O. Lung strain is directly related to stress through the specific lung elastance and, like stress, may be used as a marker of overdistention (36). Similar to lung stress, the strain resulting from a given Vt varies substantially among patients with ARDS because of variations in aerated lung volume at FRC (36). In a study by Chiumello and colleagues (36), the same measured strain and stress could be generated with both low Vts of 6 ml/kg PBW and high Vts of 12 ml/kg within subgroups of patients that included some without lung disease and some with ARDS, further demonstrating the inadequacy of Pplat and Vt per PBW for predicting overdistention injury.
VILI related to excessive strain may be related to both its dynamic and static components. Static strain results from the application of PEEP, which causes an isotonic deformation of the aerated lung above FRC at end-expiration. Dynamic strain results from the cyclic inflation of aerated lung with each tidal breath. Protti and colleagues demonstrated in healthy pigs that ventilator-induced pulmonary edema developed only when Vts resulted in dynamic lung strains greater than 1.5 to 2.0 (38). In further investigations, lung injury was primarily related to dynamic strain rather than static strain. At the same peak inspiratory strain, ventilation with smaller dynamic and larger static strains was associated with less lung injury than ventilation with smaller static and larger dynamic strains (40).
An individualized setting of Vt based on reducing dynamic strain would be a more direct method to reduce stretch-induced lung injury than setting Vt according to PBW. This method, however, requires a valid measurement of FRC, which can be difficult in critically ill patients. Values of FRC can be measured by gas dilution, nitrogen wash-in/wash-out, and quantitative CT analysis. Unfortunately, these methods are relatively complex, cumbersome, or risky, which has limited the clinical implementation of these techniques.
Another limitation to using strain to set Vt is that in many patients with ARDS, recruitment of lung tissue occurs during inspiration. When this occurs, the ratio of Vt/FRC overestimates the strain in the aerated lung parenchyma because some of the Vt is distributed to some alveoli that open during inspiration rather than stretching of previously aerated alveoli. To reduce this error, strain could be assessed from the ratio of Vt to TLC (41). A challenge to this approach is to define TLC in a patient with ARDS. Another challenge is that we do not know a safe upper limit of strain measured with this approach.
There is increasing interest in electrical impedance tomography (EIT) for monitoring regional changes in lung volume at the bedside. EIT may be useful for monitoring regional compliance and to assess changes in end-expiratory lung volume during PEEP titration. It is unclear if the regional assessment of volume change is an adequate surrogate for global volume change. However, it is noninvasive, is relatively easy to implement, and may be helpful in adjusting Vt. As research using EIT continues to grow, its use in the clinical setting may also expand (42).
Preventing or Reducing Hypercapnia and Acidosis When Using Small Vt
Smaller Vt may lead to respiratory acidosis, especially in patients with high physiologic dead space, as in ARDS. Some ventilator circuits contain unnecessary dead space. Endotracheal tube extenders and heat and moisture exchangers are attached to the tip of the endotracheal tube and fill with alveolar gas at end-expiration. This CO2-laden gas is then delivered back into the lungs with the next inspiration. Some heat and moisture exchangers contain as much as 80 ml of volume, which converts otherwise effective alveolar ventilation into dead space. Heated humidifiers are more effective for conditioning inspired air and, because of their location in the ventilator circuit, do not impose additional dead space. In addition, endotracheal tube extenders can be removed from the circuit to reduce instrumental dead space.
A simple approach to reducing hypercapnia and respiratory acidosis is to increase the respiratory rate. This technique was used in the ARDS Network lower Vt protocol, in which levels of respiratory acidosis on average were quite mild. Recruiting lung can also improve CO2 clearance (43). The use of higher levels of PEEP and recruitment maneuvers may help to reduce hypercapnia and acidosis in patients who respond with recruitment, by reducing physiologic dead space and shunt (43). In those who do not recruit, higher PEEP can worsen dead space (44). Prone positioning may lead to substantial recruitment in many patients with ARDS, which may also reduce dead space and respiratory acidosis. In addition, increasing the duration of inspiration, as with an end-inspiratory pause, can decrease PaCO2 in small amounts to decrease acidosis from smaller Vts (45, 46).
Mechanical adjuncts, such as extracorporeal carbon dioxide removal (ECCO2R), may facilitate the use of very low Vts by mitigating hypercapnia. Recent trials have demonstrated the feasibility of an ultraprotective ventilation strategy with concomitant use of low-flow venovenous ECCO2R to reduce respiratory acidosis (47, 48). In 15 patients, Vt was reduced to approximately 4 ml/kg PBW, and ECCO2R was effective for preventing severe respiratory acidosis. Six of the patients, however, experienced persistent hypoxemia and required either prone positioning or extracorporeal membrane oxygenation. This unanticipated hypoxemia may be a consequence of lower airway pressures causing increased atelectasis (49). These effects may be counteracted by periodic recruitment maneuvers or higher levels of PEEP. In the second trial, 79 patients were randomized to Vt of 3 ml/kg PBW combined with ECCO2R or to a control group with Vt of 6 ml/kg PBW. Patients with a PaO2/FiO2 ≤150 demonstrated significantly improved ventilator-free days after 60 days in the ECCO2R study group, although overall mortality rate did not differ between the two groups (48). More investigation is warranted before fully embracing this technologic adjunct. A multicenter randomized clinical trial is currently enrolling to test the feasibility, safety, and efficacy of using ECCO2R to enable ultraprotective ventilation (SUPERNOVA [Strategy of Ultraprotective Lung Ventilation with Extracorporeal CO2 Removal for New-Onset Moderate to Severe ARDS]; Clinicaltrials.gov NCT02282657).
High-frequency oscillatory ventilation (HFOV) delivers small Vts of approximately 1 to 3 ml/kg PBW at very high respiratory rates (50). These very small Vts limit overdistention, while relatively high mean airway pressures prevent cyclic alveolar collapse and reopening (51). An early randomized clinical trial of HFOV versus conventional MV in ARDS demonstrated a trend toward lower mortality with HFOV. However, the conventional MV group used generous Vts of greater than 10 ml/kg PBW, which are now known to be injurious (52). More recent, larger randomized trials demonstrated either no difference in mortality or possible harm with HFOV compared with conventional MV with lower Vts (18, 19). Each of the HFOV trials were challenged by limitations in their design, which may have led to the HFOV groups receiving more sedation, fluids, vasopressors, and neuromuscular blocking agents. In the absence of convincing evidence of efficacy, the use of HFOV is now largely limited to patients who have failed other therapies or who are not candidates for mechanical support such as extracorporeal gas exchange.
Lower Vt Reduces VILI Even at Lower Expiratory Pressures and Volumes
VILI may also occur from ventilation with low end-expiratory volume and pressure, perhaps from repeated opening and reclosing of small bronchioles and alveoli and from excessive stress at the margins between aerated and atelectatic or consolidated lung tissue (5, 53). This form of VILI may be reduced with higher levels of PEEP than were used in the past, before VILI was recognized (5, 53). However, recruitment and derecruitment occur at almost all lung volumes in patients with ARDS, even when relatively high levels of PEEP are used (54, 55). Regardless of the volume at end-expiration, the recruiting and derecruiting pressure swings are smaller when low Vts are used. Therefore, in addition to reducing VILI from overdistention, lower Vts can also reduce VILI from opening and reclosing or from excessive stress at the margins between aerated and atelectatic air spaces (56, 57). Ultimately, the mechanical power that is applied to the lung decreases exponentially with reductions in Vt, which is a powerful mechanism for reducing VILI (58).
Rethinking Goals for Setting Vt
Several studies strongly suggest that in patients with ARDS, VILI from overdistention can occur at Vts and Pplats that are lower than those that are in current use in most intensive care units (20, 25, 26). Unfortunately, there is no reliable test for ongoing VILI from overdistention, like troponin for ongoing cardiac injury. Moreover, despite current guidelines and recommendations for Vt, Pplat, stress, strain, and DP, we do not know safe levels of any of these parameters. Therefore, we propose that any of the methods for setting Vt reviewed here can be used to set the initial Vt. We advocate that the initial Vt should be within the range of 4 to 8 ml/kg PBW, with Pplat less than 30 cm H2O. The upper part of this range, 7 to 8 ml/kg, should be reserved for patients who have frequent double breaths or severe dyspnea with airway pressure less than PEEP for much of the inspiratory cycle. In patients with moderate and severe ARDS, and especially in patients whose DPs and plateau pressures are not comfortably low (DP > 14 cm H2O and Pplat > 25 cm H2O, respectively), we suggest that clinicians push the limits of reducing Vt beyond their current practice, setting Vts in the lower part of our range of 4 to 6 ml/kg. Vt can then be reduced in small decrements (e.g., 0.5 ml/kg PBW) over several hours while monitoring individual patients’ responses for signs of intolerance to the adverse effects of lower Vts, including hemodynamic changes, gas exchange, and respiratory mechanics (Table 1). In some patients, the ultimate Vt may be considerably lower than those that are in common use today (59). Furthermore, regardless of the method to set the initial Vt, if the same signs of intolerance are used, the same ultimate Vt will be attained. A shortcoming of this approach is that the signs of intolerance are not very specific to intolerance to hypercapnia and acidosis. However, if we monitor these signs closely over a period of a few hours while lowering Vt in steps, changes in these signs should more specifically represent effects of the lower Vt implementation. If signs of intolerance develop, adjunctive therapies, such as neuromuscular blockade or ECCO2R, should be considered to facilitate tolerance of low Vt, especially for patients who would likely benefit the most from reduced Vt
Table 1.
Signs of Intolerance to Low Vt and Hypercapnia
| Signs of Low Vt Intolerance |
|---|
| Tachycardia |
| Hypertension |
| Hypotension |
| Tachypnea |
| Patient–ventilator dyssynchrony (e.g., double triggering) |
| Inspiratory airway pressure below PEEP, indicating high work of breathing |
| Respiratory acidosis |
| Hypoxemia despite high FiO2 |
| Agitation |
Definition of abbreviation: PEEP = positive end-expiratory pressure.
Physiologic signs that can be assessed for evidence of intolerance to mechanical ventilation with low Vts and hypercapnia. Patient–ventilator dyssynchrony often manifests as a double breath (Figure 1), which means the end-inspiratory pressure and end-inspiratory lung volume are higher than at the end of synchronous breath, which is a major risk for alveolar overdistention.
Conclusions
The optimal method for setting Vt for patients with ARDS remains unknown. The use of smaller Vts has been accepted by many since the publication of the National Institutes of Health ARDS Network study (15, 60). However, some patients remain at risk for overdistention injury even when small Vts are used, because their aerated lung volumes are greatly reduced (20, 25). As PBW does not correlate well with aerated lung volume, better approaches to setting Vt may involve limiting lung stress, strain, or driving pressure as potential surrogates for VILI from parenchymal overdistention injury. Whatever method is initially used, for patients with moderate to severe ARDS or more severely impaired respiratory mechanics, we suggest the careful, continued further reduction of Vt in a stepwise manner until a patient begins to exhibit physiologic signs of intolerance or the DP or Pplat is in a safer range (Table 1). As is frequently the case, this intervention will have competing effects that must be balanced on a patient-by-patient basis. Higher levels of PEEP may be necessary to prevent atelectasis that may result from the smaller Vts. However, higher PEEP may cause overdistention or hemodynamic instability (61). The need for strict control of PaCO2, as in intracranial hypertension, may outweigh the need to lower Vt to protect the lung. Nevertheless, we recommend keeping Vt at the lowest possible value as dictated by patient tolerance and appropriateness of respiratory mechanics, gas exchange, and hemodynamic parameters. As the technology of ECCO2R continues to evolve, the limits of MV with low Vts as a lung-protective strategy should continue to be tested. Further investigations are warranted to determine if pushing the limits of lowering Vt will improve clinical outcomes.
Footnotes
Supported by the NHLBI of the National Institutes of Health under award number T32HL007534.
CME will be available for this article at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201708-1629CI on September 20, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures: protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 2.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 3.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenfield LJ, Ebert PA, Benson DW. Effect of positive pressure ventilation on surface tension properties of lung extracts. Anesthesiology. 1964;25:312–316. doi: 10.1097/00000542-196405000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 6.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema: respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 7.Pontoppidan H, Geffin B, Lowenstein E. Acute respiratory failure in the adult. 3. N Engl J Med. 1972;287:799–806. doi: 10.1056/NEJM197210192871605. [DOI] [PubMed] [Google Scholar]
- 8.Brochard L, Lemaire F. Tidal volume, positive end-expiratory pressure, and mortality in acute respiratory distress syndrome. Crit Care Med. 1999;27:1661–1663. doi: 10.1097/00003246-199908000-00055. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Fujino Y, Amato MB, Kavanagh BP. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation: risks, mechanisms, and management. Am J Respir Crit Care Med. 2017;195:985–992. doi: 10.1164/rccm.201604-0748CP. [DOI] [PubMed] [Google Scholar]
- 10.de Asua I, McKechnie S. Caveats of pressure control: lung non-protective ventilation. Br J Anaesth. 2014;113:1058. doi: 10.1093/bja/aeu394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittayamai N, Katsios CM, Beloncle F, Friedrich JO, Mancebo J, Brochard L. Pressure-controlled vs volume-controlled ventilation in acute respiratory failure: a physiology-based narrative and systematic review. Chest. 2015;148:340–355. doi: 10.1378/chest.14-3169. [DOI] [PubMed] [Google Scholar]
- 12.Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondéjar E, Clémenti E, Mancebo J, Factor P, Matamis D, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome: the Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158:1831–1838. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 13.Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, Mazer CD, McLean RF, Rogovein TS, Schouten BD, et al. Pressure- and Volume-Limited Ventilation Strategy Group. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. N Engl J Med. 1998;338:355–361. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 14.Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P, Jr, Wiener CM, Teeter JG, Dodd-o JM, Almog Y, Piantadosi S. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999;27:1492–1498. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, et al. Expiratory Pressure (Express) Study Group. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 17.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, et al. Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, et al. OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 19.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 20.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, Gandini G, Herrmann P, Mascia L, Quintel M, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 21.Fan E, Needham DM, Stewart TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA. 2005;294:2889–2896. doi: 10.1001/jama.294.22.2889. [DOI] [PubMed] [Google Scholar]
- 22.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 23.Tobin MJ. Culmination of an era in research on the acute respiratory distress syndrome. N Engl J Med. 2000;342:1360–1361. doi: 10.1056/NEJM200005043421808. [DOI] [PubMed] [Google Scholar]
- 24.Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med. 2002;166:1510–1514. doi: 10.1164/rccm.200208-956OC. [DOI] [PubMed] [Google Scholar]
- 25.Hager DN, Krishnan JA, Hayden DL, Brower RG ARDS Clinical Trials Network. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–1245. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasso S, Stripoli T, De Michele M, Bruno F, Moschetta M, Angelelli G, Munno I, Ruggiero V, Anaclerio R, Cafarelli A, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end-expiratory pressure. Am J Respir Crit Care Med. 2007;176:761–767. doi: 10.1164/rccm.200702-193OC. [DOI] [PubMed] [Google Scholar]
- 27.Stocks J, Quanjer PH Official Statement of The European Respiratory Society. Reference values for residual volume, functional residual capacity and total lung capacity: ATS workshop on lung volume measurements. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 28.Ranieri VM, Brienza N, Santostasi S, Puntillo F, Mascia L, Vitale N, Giuliani R, Memeo V, Bruno F, Fiore T, et al. Impairment of lung and chest wall mechanics in patients with acute respiratory distress syndrome: role of abdominal distension. Am J Respir Crit Care Med. 1997;156:1082–1091. doi: 10.1164/ajrccm.156.4.97-01052. [DOI] [PubMed] [Google Scholar]
- 29.Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol (1985) 2010;108:212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31:776–784. doi: 10.1007/s00134-005-2627-z. [DOI] [PubMed] [Google Scholar]
- 31.Gattinoni L, Marini JJ, Pesenti A, Quintel M, Mancebo J, Brochard L. The “baby lung” became an adult. Intensive Care Med. 2016;42:663–673. doi: 10.1007/s00134-015-4200-8. [DOI] [PubMed] [Google Scholar]
- 32.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 33.Bugedo G, Retamal J, Bruhn A. Driving pressure: a marker of severity, a safety limit, or a goal for mechanical ventilation? Crit Care. 2017;21:199. doi: 10.1186/s13054-017-1779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loring SH, Topulos GP, Hubmayr RD. Transpulmonary pressure: the importance of precise definitions and limiting assumptions. Am J Respir Crit Care Med. 2016;194:1452–1457. doi: 10.1164/rccm.201512-2448CP. [DOI] [PubMed] [Google Scholar]
- 35.Sahetya SK, Brower RG. The promises and problems of transpulmonary pressure measurements in acute respiratory distress syndrome. Curr Opin Crit Care. 2016;22:7–13. doi: 10.1097/MCC.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178:346–355. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 37.Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D. Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J Suppl. 2003;47:15s–25s. doi: 10.1183/09031936.03.00021303. [DOI] [PubMed] [Google Scholar]
- 38.Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, Chierichetti M, Coppola S, Conte G, Gatti S, et al. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med. 2011;183:1354–1362. doi: 10.1164/rccm.201010-1757OC. [DOI] [PubMed] [Google Scholar]
- 39.Gulati G, Novero A, Loring SH, Talmor D. Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results. Crit Care Med. 2013;41:1951–1957. doi: 10.1097/CCM.0b013e31828a3de5. [DOI] [PubMed] [Google Scholar]
- 40.Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, Votta E, Gatti S, Lombardi L, Leopardi O, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med. 2013;41:1046–1055. doi: 10.1097/CCM.0b013e31827417a6. [DOI] [PubMed] [Google Scholar]
- 41.Brower RG, Hubmayr RD, Slutsky AS. Lung stress and strain in acute respiratory distress syndrome: good ideas for clinical management? Am J Respir Crit Care Med. 2008;178:323–324. doi: 10.1164/rccm.200805-733ED. [DOI] [PubMed] [Google Scholar]
- 42.Kobylianskii J, Murray A, Brace D, Goligher E, Fan E. Electrical impedance tomography in adult patients undergoing mechanical ventilation: a systematic review. J Crit Care. 2016;35:33–50. doi: 10.1016/j.jcrc.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 44.Grasso S, Fanelli V, Cafarelli A, Anaclerio R, Amabile M, Ancona G, Fiore T. Effects of high versus low positive end-expiratory pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:1002–1008. doi: 10.1164/rccm.200407-940OC. [DOI] [PubMed] [Google Scholar]
- 45.Aboab J, Niklason L, Uttman L, Brochard L, Jonson B. Dead space and CO2 elimination related to pattern of inspiratory gas delivery in ARDS patients. Crit Care. 2012;16:R39. doi: 10.1186/cc11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguirre-Bermeo H, Morán I, Bottiroli M, Italiano S, Parrilla FJ, Plazolles E, Roche-Campo F, Mancebo J. End-inspiratory pause prolongation in acute respiratory distress syndrome patients: effects on gas exchange and mechanics. Ann Intensive Care. 2016;6:81. doi: 10.1186/s13613-016-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fanelli V, Ranieri MV, Mancebo J, Moerer O, Quintel M, Morley S, Moran I, Parrilla F, Costamagna A, Gaudiosi M, et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care. 2016;20:36. doi: 10.1186/s13054-016-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bein T, Weber-Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J, Muellenbach R, Dembinski R, Graf BM, Wewalka M, et al. Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39:847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gattinoni L. Ultra-protective ventilation and hypoxemia. Crit Care. 2016;20:130. doi: 10.1186/s13054-016-1310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hager DN, Fessler HE, Kaczka DW, Shanholtz CB, Fuld MK, Simon BA, Brower RG. Tidal volume delivery during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2007;35:1522–1529. doi: 10.1097/01.CCM.0000266586.04676.55. [DOI] [PubMed] [Google Scholar]
- 51.Krishnan JA, Brower RG. High-frequency ventilation for acute lung injury and ARDS. Chest. 2000;118:795–807. doi: 10.1378/chest.118.3.795. [DOI] [PubMed] [Google Scholar]
- 52.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Carlin B, Lowson S, Granton J Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial (MOAT) Study Investigators. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–808. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 53.Corbridge TC, Wood LDH, Crawford GP, Chudoba MJ, Yanos J, Sznajder JI. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. Am Rev Respir Dis. 1990;142:311–315. doi: 10.1164/ajrccm/142.2.311. [DOI] [PubMed] [Google Scholar]
- 54.Richard JC, Brochard L, Vandelet P, Breton L, Maggiore SM, Jonson B, Clabault K, Leroy J, Bonmarchand G. Respective effects of end-expiratory and end-inspiratory pressures on alveolar recruitment in acute lung injury. Crit Care Med. 2003;31:89–92. doi: 10.1097/00003246-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Jonson B, Richard JC, Straus C, Mancebo J, Lemaire F, Brochard L. Pressure-volume curves and compliance in acute lung injury: evidence of recruitment above the lower inflection point. Am J Respir Crit Care Med. 1999;159:1172–1178. doi: 10.1164/ajrccm.159.4.9801088. [DOI] [PubMed] [Google Scholar]
- 56.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 57.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:149–158. doi: 10.1164/rccm.201308-1567OC. [DOI] [PubMed] [Google Scholar]
- 58.Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, Brochard L, Clarkson K, Esteban A, Gattinoni L. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42:1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 59.Retamal J, Libuy J, Jiménez M, Delgado M, Besa C, Bugedo G, Bruhn A. Preliminary study of ventilation with 4 ml/kg tidal volume in acute respiratory distress syndrome: feasibility and effects on cyclic recruitment - derecruitment and hyperinflation. Crit Care. 2013;17:R16. doi: 10.1186/cc12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 61.Sahetya SK, Goligher EC, Brower RG. Fifty years of research in ARDS: setting positive end-expiratory pressure in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1429–1438. doi: 10.1164/rccm.201610-2035CI. [DOI] [PMC free article] [PubMed] [Google Scholar]