Abstract
Atherosclerosis is one of the most common complications of rheumatoid arthritis (RA). The objective of this study is to evaluate differences in large artery compliance (C1) and small artery compliance (C2) between RA and controls and evaluating factors associated with reduced compliance in the RA population. The profiling of large and small arterial compliance was analyzed in 185 RA patients and 88 healthy controls using Cardiovascular Profiling Instrument. The correlations of arterial compliance and the relevant clinical data were determined in these subjects. Then correlation analysis and regression analysis were performed to find whether rheumatoid arthritis patients have more risk factors than healthy controls in artery compliance and to explore the possible element involved in RA patients including traditional cardiovascular risk factors, RA disease-related factors, and the therapy. Compared with healthy controls, levels of C1 and C2 were significantly decreased in RA patients. Having adjusted the traditional risk factors associated with atherosclerosis, C1 and C2 decline was still a significant indicator in RA patients [odds ratio = 7.411(95%CI 3.275, 16.771) and 10.184(95%CI 4.546, 22.817)]. Using multi-factor regression analysis to adjust traditional risk factors for arterial compliance, we found that the levels of ESR was correlated with the abnormal large artery compliance [odds ratio = 1.021(95%CI 1.007, 1.035)]. The HAQ values and the current usage of leflunomide were correlated with the abnormal small artery compliance in RA patients [odds ratio = 1.161(95%CI 1.046, 1.289) and 6.170(95%CI 1.510, 25.215)]. The values of C1 and C2 are indicators of artery compliance in RA patients. ESR, HAQ values, and the usage of leflunomide might be possible risk factors of artery compliance. The evaluation of artery compliance could be an easy and reliable test that could help us to screen and predict cardiovascular disorders in RA patients.
Electronic supplementary material
The online version of this article (10.1007/s10067-017-3899-8) contains supplementary material, which is available to authorized users.
Keywords: Arterial compliance, Atherosclerosis, Rheumatoid arthritis, Risk factors
Background
Rheumatoid arthritis (RA) is a common autoimmune disease involving multiple systems [1]. Atherosclerosis-induced cardiovascular disease (CVD) is one of the most common complications in patients with RA [2]. Compared with normal group, RA patients have significantly increased risk of atherosclerosis, earlier time of onset, higher mortality, and earlier time of death [3, 4].
According to previous studies, increases in traditional risk factors of atherosclerosis and inflammation are the two most important causes of increased atherosclerosis risk in RA patients [5, 6]. In addition, production of auto-antibodies and application of arthritis drugs can also increase the risk of atherosclerosis in RA patients [7, 8]. Therefore, early detection of subclinical atherosclerosis in RA patients by simple, fast, noninvasive, low-cost method is of positive significance to the improvement of their prognosis.
So far, there have been many means of assessing atherosclerosis in clinical settings, such as Doppler ultrasound measurement of patients’ carotid intima-media thickness and detection of presence/absence of arterial plaque formation [9], and coronary dual-source CT or coronary angiography detection of presence/absence of atherosclerosis and degree of coronary artery stenosis [10, 11]. Although the above approaches are highly sensitive, they have cumbersome procedures, which can only assess the already-formed atherosclerotic vessels as well, without having the predictive role in subclinical atherosclerosis.
Large artery compliance (C1), also known as the capacitive compliance, refers to the ratio of diastolic volume reduction to pressure decrease, while small artery compliance (C2), also known as the oscillatory compliance, is the ratio of oscillatory changes in diastolic volume to pressure [12]. The two reflect the stiffness and elasticity of large arteries and small arteries, respectively, which are reliable methods for early detection of vascular diseases [13]. The smaller the C1 and C2, the compliance of large and small arteries is poorer and their stiffness is higher. C1 and C2 detection results have good repeatability, with results of the same subject detected at 1–4 weeks intervals having very close means [14, 15]. Easily and noninvasively detectable, C1 and C2 are thus sensitive indices reflecting early changes of atherosclerosis [16].
This study assesses the large and small artery compliance of subjects by detecting the C1 and C2 levels of RA patients and healthy controls underwent routine physical examination at the physical examination center; meanwhile, the relationship of clinical and treatment conditions with arterial compliance in RA patients is analyzed, with the aims of evaluating differences in arterial compliance between RA and controls and evaluating factors associated with reduced compliance in the RA population.
Methods
Clinical data collection
Totally, 273 subjects were enrolled in this study, including 185 RA outpatients treated at the Department of Rheumatology, the First Affiliated Hospital of Nanjing Medical University, and 88 volunteers received routine physical examination at the same hospital’s physical examination center. Exclusion criteria were as follows: patients with severe infection, perioperative patients, patients with severe liver and kidney dysfunctions, cancer patients, menstrual patients, or patients who underwent coronary stent implantation or bypass graft. This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. Furthermore, all subjects signed an informed consent.
Clinical data of all subjects were collected in detail, including name, contact information, and traditional atherosclerosis risk factors (such as age, gender, body mass index, history of smoking, systolic blood pressure, diastolic blood pressure, fasting blood glucose, triglycerides, total cholesterol, low density lipoprotein, and high density lipoprotein), as well as RA-related factors (including course of disease, tender joint count, swollen joint count, morning stiffness, erythrocyte sedimentation rate, C-reactive protein, visual analogue scale scoring, patient global assessment scoring, evaluator global assessment scoring, simplified disease activity index scoring, clinical disease activity index scoring, disease activity score 28 joint count, health assessment questionnaire scoring, rheumatoid factor and anti-CCP antibody positive/ negative, drug in use or not, including non-steroidal anti-inflammatory drugs, glucocorticoids, disease modifying anti-rheumatic drugs, methotrexate, leflunomide and hydroxychloroquine).
Determination of arterial compliance and measurement of C1 and C2
Radial arterial pulse waveforms were recorded with radial artery diastolic pulse waveform analyzer (CVProfilor DO-2020, HDI, USA). According to the operation instruction, patients were connected to the instrument properly. The instrument’s software system automatically identified and collected waveform data and calculated, displayed the pressure waveform and arterial elastic function data, including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), pulse pressure (PP), pulse rate (PR), larger artery compliance index C1 (ml/mmHg*10), and small artery compliance index C2 (ml/mmHg*100).
According to the operating instructions of the instrument and relevant previous literature, a subject was considered to have abnormal large arterial compliance when his/her mean C1 of three test results was less than 10 ml/mmHg*10 and considered to have abnormal small arterial compliance when the mean C2 was less than 4 ml/mmHg*100. The test was performed three times for each subject.
Statistics
Data were entered and self-tested using Microsoft Office Excel 2007 and statistically analyzed using IBM SPSS Statistics 19.0. Before the analysis, hypothesis testing was performed using normal probability plots to observe whether the detected values followed normal distribution. Normal distribution measurement data were expressed as mean ± standard deviation, whereas the skewed distribution measurement data were expressed as median. The two sets of measurement data were compared by Student’s t test, while comparison of categorical data was done by Pearson’s chi-square test. p < 0.05 was considered statistically significant, and all statistical tests were two-tailed probability tests. We performed correlation analysis between the value of C1, C2, and RA patients or healthy controls, items of traditional atherosclerosis risk factors and RA-related factors, respectively. Also, we put RA patients or not and traditional atherosclerosis risk factors into the regression analysis of model 1, and all items of traditional atherosclerosis risk factors and RA-related factors were putted into the regression analysis of model 2. Correlation analysis and regression analysis were performed using SPSS 19.0.
Results
Analysis of basic information, clinical data, and arterial compliance
RA group and healthy control group did not show significant differences (p > 0.05) in gender ratio, age, BMI, waist circumference, SBP, DBP, MAP, PP, fasting blood glucose (FBG), triglycerides, high density lipoprotein (HDL), history of smoking, history of hypertension, history of diabetes or history of hyperlipemia. Compared with the healthy control group, bodyheight, weight, total cholesterol (TC), and low density lipoprotein (LDL) were significantly lower in the RA group (p < 0.05), while PR was significantly higher (p < 0.05). In addition, compared with the healthy control group, C1 and C2 significantly reduced in the RA group, while proportions of abnormal large and small artery compliance increased significantly (p < 0.001) (Table 1).
Table 1.
Basic information and laboratory index of subjects
| RA | HC | p value | |
|---|---|---|---|
| Case number | 185 | 88 | |
| Gender | 153; 82.7% | 66; 75.0% | 0.146 |
| Age (years) | 51.66 ± 11.77 | 49.67 ± 7.18 | 0.086 |
| Height (centimeter) | 161.09 ± 6.69 | 164.18 ± 6.96 | 0.001 |
| Weight (kilogram) | 61.28 ± 9.726 | 65.19 ± 11.70 | 0.004 |
| BMI | 23.59 ± 3.33 | 24.09 ± 3.30 | 0.244 |
| Waist (centimeter) | 85.82 ± 10.10 | 85.08 ± 9.96 | 0.569 |
| SBP (mmHg) | 128.70 ± 17.24 | 125.20 ± 16.91 | 0.117 |
| DBP (mmHg) | 74.84 ± 10.29 | 73.53 ± 10.63 | 0.334 |
| MAP (mmHg) | 92.22 ± 17.37 | 92.17 ± 13.53 | 0.981 |
| PP (mmHg) | 53.28 ± 12.76 | 51.82 ± 8.95 | 0.275 |
| PR (per minute) | 77.84 ± 15.57 | 73.58 ± 10.68 | 0.021 |
| FBG (mmol/L) | 5.37 ± 0.82 | 5.20 ± 0.51 | 0.056 |
| TG (mmol/L) | 1.20 ± 0.69 | 1.38 ± 0.96 | 0.093 |
| TC (mmol/L) | 4.94 ± 1.06 | 5.37 ± 1.01 | 0.003 |
| LDL (mmol/L) | 3.12 ± 0.80 | 3.37 ± 0.74 | 0.017 |
| HDL (mmol/L) | 1.40 ± 0.34 | 1.43 ± 0.33 | 0.422 |
| History of smoking | 21 (11.4%) | 5 (5.7%) | 0.186 |
| History of hypertension | 40 (21.6%) | 23 (26.1%) | 0.444 |
| History of diabetes | 5 (2.7%) | 2 (2.3%) | 1.000 |
| History of hyperlipemia | 5 (2.7%) | 1 (1.1%) | 0.668 |
| C1 (ml/mmHg*10) | 10.23 ± 4.29 | 12.38 ± 3.24 | 0.000 |
| C2 (ml/mmHg*100) | 3.24 ± 1.88 | 5.23 ± 2.31 | 0.000 |
| Large artery compliance abnormalities | 99 (53.5%) | 12 (13.6%) | 0.000 |
| Small artery compliance abnormalities | 141 (76.2%) | 28 (31.8%) | 0.000 |
Student’s t test and Pearson’s chi-square test were used to analysis. Data are mean + SD or N(%). Gender are N(%) of female
*RA rheumatoid arthritis, HC healthy controls, BMI Body Mass Index, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean blood pressure, PP pulse pressure, PR pulse rate, FBG fasting blood glucose, TG triglycerides, TC total cholesterol, LDL low density lipoprotein, HDL high density lipoprotein
Characteristics of RA cohort were listed on Table 2. Duration of RA varies from initial attack to over 40 years, with a median of 4 years, which covers a wide range. Disease activity in RA patients was assessed using DAS28 scoring system, which revealed that 63 patients were experiencing severe disease activity, 70 patients were experiencing moderate disease activity, and 39 patients were experiencing mild disease activity or remission. So in this cohort, there are relatively many patients with active disease. Among them, 116 used disease-modifying anti-rheumatic drugs, while 69 were baseline patients who did not receive regular treatment (Table 2).
Table 2.
Characteristics of RA cohort
| Disease duration (month), median (IQR) | 48 (16.5–120) |
| RF or anti-CCP positive, n(%) | 119 (64.32%) |
| ESR (mm/H), mean + SE | 33.20 ± 2.30 |
| CRP (mg/L), mean + SE | 19.48 ± 2.44 |
| Tender Joint Count, mean + SE | 6.55 ± 0.46 |
| Swollen joint count, mean + SE | 3.66 ± 0.33 |
| DAS28, mean + SE | 4.06 ± 0.11 |
| SDAI, mean + SE | 21.04 ± 1.21 |
| CDAI, mean + SE | 19.48 ± 1.04 |
| HAQ, median (IQR) | 5 (1–13) |
| Treatment—NSAIDs in use, n(%) | 77 (41.62%) |
| Glucocorticoids in use, n(%) | 56 (30.27%) |
| DMARDs in use, n(%) | 116 (62.70%) |
| -- MTX in use, n(%) | 61 (52.59%) |
| LEF in use, n(%) | 53 (45.69%) |
| HCQ in use, n(%) | 56 (48.28%) |
Data are median, mean ± SE or N(%)
RA rheumatoid arthritis, RF rheumatoid factor, anti-CCP anti-cyclic citrullinated peptide antibody, ESR erythrocyte sedimentation rate, CRP C-reactive protein, DAS28 disease activity score 28 joint count, SDAI simplified disease activity index scoring, CDAI clinical disease activity index scoring, HAQ health assessment questionnaire scoring, NSAIDs non-steroidal anti-inflammatory drugs, DMARDs disease modifying anti-rheumatic drugs, MTX methotrexate, LEF leflunomide, HCQ hydroxychloroquine
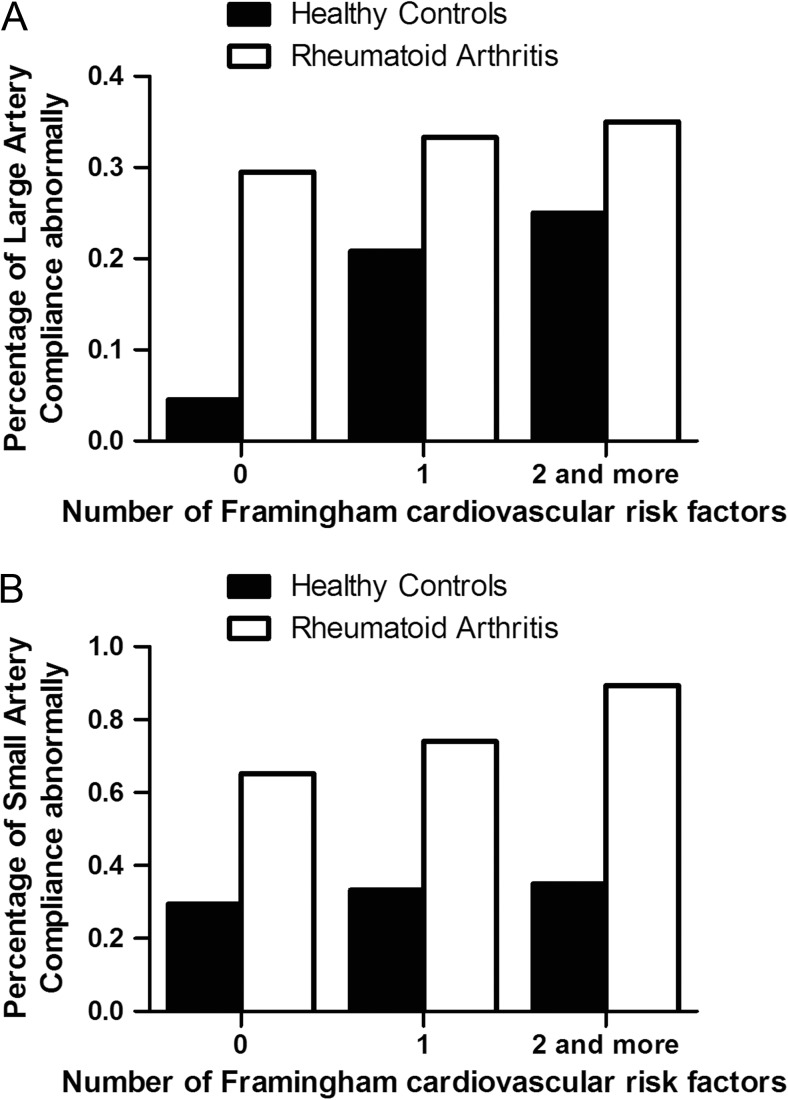
Framingham cardiovascular risk score is a common-used and credible way to assess the possibility of cardiovascular incidence occurred in the future. Age, total cholesterol, high density lipoprotein, systolic blood pressure, and smoking are contained in Framingham cardiovascular risk score. We calculated the number of risk factors in each subjects, and we found that with the increasing traditional cardiovascular risk factors, subjects’ proportions of large and small arterial compliance abnormalities increased. Moreover, in the same state of traditional risk factors, the proportions of large and small arterial compliance abnormalities were both higher for RA patients than the healthy controls (Fig. 1).
Fig. 1.
Ratio of arterial compliance abnormalities in different numbers of Framingham cardiovascular risk score. a–b The percentage of large and small artery compliance abnormally are determined by Cardiovascular Profiling Instrument between the subgroups with different number of Framingham cardiovascular risk factors
Univariate analysis of factors influencing C1 and C2
With respect to univariate analysis among RA patients and healthy controls, age, SBP, DBP, and FBG were negatively correlated with C1 (p < 0.05); besides, C1 was significantly lower for RA patients than healthy controls, and for women than men (p < 0.05). Age, SBP, and DBP were negatively correlated with C2 (p < 0.05); besides, C2 was significantly lower for RA patients than healthy controls, for women than men, for men with a history of smoking than those without (p < 0.05).
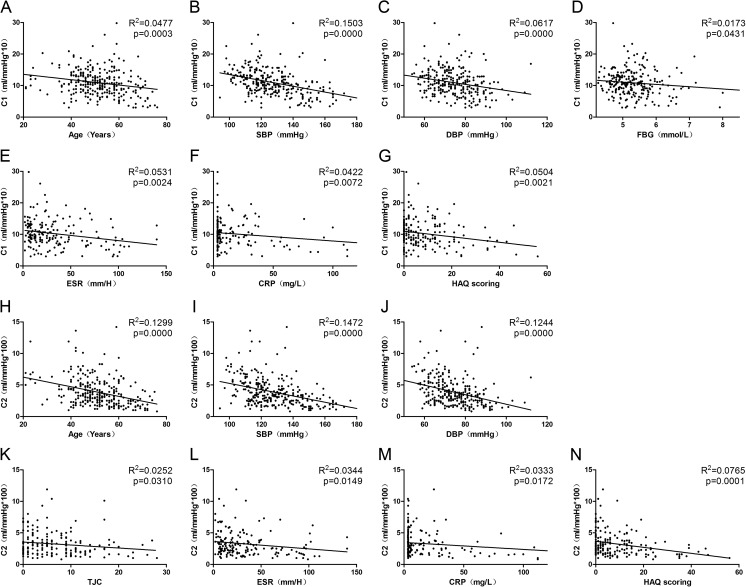
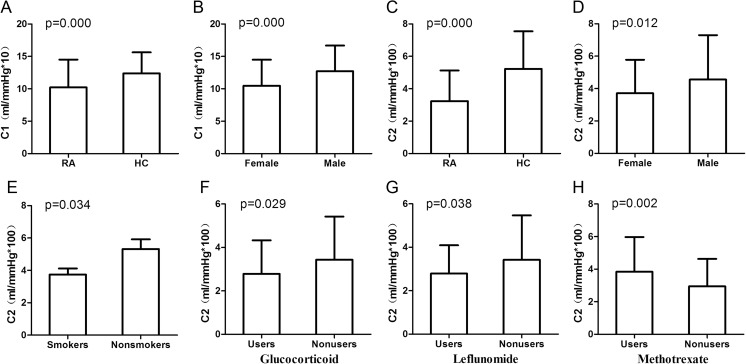
And in the univariate analysis of RA patients, we found ESR, CRP, and HAQ score were negatively correlated with C1 and C2 (p < 0.05); besides, TJC was also negatively correlated with C2 (p < 0.05). C2 was significantly lower for glucocorticoid users than nonusers, and for leflunomide users than nonusers (p < 0.05). Meanwhile, methotrexate users had significantly higher C2 than nonusers (p < 0.05) (Figs. 2 and 3).
Fig. 2.
Correlation between clinical data and C1 and C2, respectively. a–n The levels of C1 and C2 are negatively correlated with clinical data
Fig. 3.
The value of C1 and C2 between different subgroups. a–h. The levels of C1 and C2 are presented between different subgroups
Multivariate analysis of influences of traditional risk factors and RA on arterial compliance
In the multivariate regression analysis after adjusting traditional risk factors in the Framingham cardiovascular risk score, C1 and C2 decline was still a significant indicator in RA patients, where OR values were 7.411 and 10.184, respectively (p < 0.05) (Table 3).
Table 3.
Multivariate analysis of artery compliance influenced by traditional risk factors, rheumatoid arthritis and RA-related factors
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| Large arterial compliance | ||||
| Model 1 | ||||
| Female | 2.272 (1.169–4.415) | 0.016 | 4.185 (1.506–11.627) | 0.006 |
| BMI | 0.982 (0.912–1.057) | 0.623 | 0.875 (0.784–0.977) | 0.018 |
| SBP | 1.060 (1.041–1.079) | 0.000 | 1.124 (1.078–1.172) | 0.000 |
| HDL | 0.567 (0.251–1.283) | 0.173 | 0.309 (0.109–0.877) | 0.027 |
| RA | 7.291 (3.716–14.303) | 0.000 | 7.411 (3.275–16.771) | 0.000 |
| Model 2 | ||||
| SBP | 1.060 (1.041–1.079) | 0.000 | 1.058 (1.030–1.088) | 0.000 |
| ESR | 1.014 (1.004–1.026) | 0.008 | 1.021 (1.007–1.035) | 0.003 |
| Small arterial compliance | ||||
| Model 1 | ||||
| Age | 1.078 (1.048–1.109) | 0.000 | 1.085 (1.042–1.129) | 0.000 |
| Female | 2.026 (1.110–3.699) | 0.021 | 7.966 (2.777–22.850) | 0.000 |
| BMI | 0.999 (0.928–1.075) | 0.976 | 0.821 (0.720–0.937) | 0.004 |
| SBP | 1.062 (1.041–1.083) | 0.000 | 1.059 (1.009–1.112) | 0.021 |
| DBP | 1.094 (1.061–1.127) | 0.000 | 1.097 (1.021–1.178) | 0.012 |
| RA | 6.867 (3.915–12.045) | 0.000 | 10.184 (4.546–22.817) | 0.000 |
| Model 2 | ||||
| Age | 1.078 (1.048–1.109) | 0.000 | 1.127 (1.056–1.202) | 0.000 |
| BMI | 0.999 (0.928–1.075) | 0.976 | 0.760 (0.620–0.931) | 0.008 |
| DBP | 1.094 (1.061–1.127) | 0.000 | 1.204 (1.106–1.312) | 0.000 |
| HAQ scoring | 1.068 (1.017–1.122) | 0.008 | 1.161 (1.046–1.289) | 0.005 |
| Leflunomide | 2.559 (1.060–6.178) | 0.037 | 6.170 (1.510–25.215) | 0.011 |
Binary logistical regression analysis was used in regression equation of both models. Rheumatoid arthritis patients or not and all items of traditional cardiovascular risk factors (including age, female, body mass index, history of smoking, systolic blood pressure, diastolic blood pressure, fasting blood glucose, triglycerides, total cholesterol, low-density lipoprotein, and high-density lipoprotein) were putted into the regression analysis of model1. All items of traditional cardiovascular risk factors and disease-related factors (including course of disease, tender joint count, swollen joint count, morning stiffness, erythrocyte sedimentation rate, C-reactive protein, visual analogue scale scoring, patient global assessment scoring, evaluator global assessment scoring, simplified disease activity index scoring, clinical disease activity index scoring, disease activity score 28 joint count, health assessment questionnaire scoring, rheumatoid factor and anti-CCP antibody positive/ negative, drug in use or not, including non-steroidal anti-inflammatory drugs, glucocorticoids, disease modifying anti-rheumatic drugs, methotrexate, leflunomide and hydroxychloroquine) were putted into the regression analysis of model2
Multivariate analysis of influences of traditional risk factors and RA-related factors on arterial compliance
In the multivariate regression analysis after adjusting traditional risk factors in the Framingham cardiovascular risk score, ESR was the risk factor for abnormal large arterial compliance (OR value = 1.021), whereas HAQ score (OR value = 1.161) and use of leflunomide (OR value = 6.170) were the risk factors of abnormal small arterial compliance (p < 0.05) (Table 3).
Discussion
By measuring the large artery compliance C1 and small artery compliance C2 of subjects using radial artery diastolic pulse waveform analyzer, this study confirms that the C1 and C2 indices are significantly lower in RA patients compared with the healthy control group. Furthermore, after removing traditional risk factors, OR values of RA against large and small artery compliance abnormalities were 7.411 and 10.184, respectively, indicating that RA patients have evident abnormal arterial compliance and significantly increased risk of atherosclerosis.
Conventional view holds that atherosclerosis is a lipid metabolism disorder involving arteries [17]. However, with the deepening of research on vascular injury mechanisms, it has been discovered that inflammation plays an important role in the occurrence and development of atherosclerosis [18, 19]. Risk of atherosclerotic cardiovascular diseases increased significantly in patients with chronic inflammation and autoimmune diseases [20, 21]. In a chronic inflammatory state, some inflammatory mediators such as tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6 can cause endothelial cell dysfunction and increased protein levels of adhesion molecules and chemokines on endothelial cell surface [22–25], thus contributing to the recruitment of monocytes into the arterial wall composed of endothelial cells; meanwhile, macrophages express scavenger receptors to promote the formation of foam cells, ultimately leading to the occurrence and development of atherosclerosis [26]. In addition, increased traditional risk factors of atherosclerosis are considered to be another important cause of increased atherosclerosis risk in RA patients [27]. RA patients have significantly increased traditional risk factors of atherosclerosis such as smoking, hypertension, diabetes, dyslipidemia, obesity, and decreased activity than the general population. Therefore, EULAR recommended in 2016 that Framingham scores should be multiplied by 1.5 in the calculation of atherosclerosis risk factors for patients with RA [28].
In this study, arterial compliance of 185 RA patients and 88 healthy controls are measured with radial artery diastolic pulse waveform analyzer and analyzed by combining relevant clinical data. On the basis of matching gender and age, we found no significant difference in the degree of obesity, blood pressure, FBG level, smoking history, or chronic disease history between the two groups. However, RA patients had reduced height, weight, TC level, LDL level, and elevated PR compared with healthy controls. These differences have been published in the literatures, which, on the one hand, are considered to be associated with the poor living standard and nutritional status of RA patients and, on the other hand, are possibly associated with the occurrence of rheumatoid “cachexia” state.
In the analysis of arterial compliance between the two groups, we found that the C1 and C2 absolute values significantly reduced while the proportions of large and small arterial compliance abnormalities increased significantly in RA patients. Meanwhile, we recommended all RA patients with abnormal large and small artery compliance receive ECG and carotid B-ultrasound examinations. Some patients already presented with myocardial ischemia, carotid intima-media thickening, plaque formation, and other atherosclerotic manifestations. Besides, we will follow up other RA patients with abnormal large and small artery compliance but without presenting abnormalities on ECG or carotid B-ultrasound in the long term, in order to clarify the value of this method for predicting atherosclerosis in RA patients.
This study suggests that ESR, CRP, and HAQ score are important risk factors of abnormal arterial compliance in RA patients [29]. Therefore, patients’ disease activity should be reduced as early as possible, in order to reduce inflammatory markers of patients with active RA and improve their activity function, which help reduce their risk of atherosclerosis. In the treatment of arthritis, methotrexate is a protective factor against abnormal arterial compliance and atherosclerosis, while glucocorticoids and leflunomide are the risk factors. Thus, minimization of the dose and duration of glucocorticoids is conducive to reducing the atherosclerosis risk in RA patients. Meanwhile, the relationship between leflunomide and atherosclerosis needs further investigation.
It has been recognized that arthritis disease control and resumption of patients’ normal activity can both significantly improve the atherosclerosis risk of RA patients [30]. Powerful anti-inflammatory action of glucocorticoids can rapidly relieve joint symptoms and systemic inflammation and help reduce the risk of atherosclerosis in RA patients. But on the other hand, the hypertensive, hyperglycemic, and fat redistribution-promoting side effects of glucocorticoids may increase the atherosclerosis risk in RA patients. Some scholars have confirmed that although glucocorticoids can significantly relieve patient conditions, they can also significantly increase RA patients’ risk of atherosclerosis [31, 32]. In addition, disease-modifying anti-rheumatic drugs represented by methotrexate, leflunomide, and hydroxychloroquine significantly improved and delayed the disease progression in RA patients [33]. At present, some small-sample studies have confirmed the positive effects of methotrexate, hydroxychloroquine, and related drugs on atherosclerosis risk reduction in RA patients [30, 34]. But extensive large-sample cohort studies, randomized controlled trials, and long-term follow-up studies are still needed for confirmation of such effects.
The effect of leflunomide on atherosclerotic risk in RA patients is still unclear in part due to sample size. The association between leflunomide and artery compliance is an interesting point that we found in our research. Im CH et.al performed carotid B-ultrasound examination in 406 RA patients [35]. Their results showed that among 148 patients with carotid plaque formation, 101 used leflunomide, while among 258 patients without carotid plaque formation, 161 used leflunomide, exhibiting a significant difference (p = 0.047) between two groups. In our cohort, when analyzing the effect of leflunomide to the artery compliance, we divided 185 RA patients into two groups according to leflunomide treatment or not. We analyze the effect of leflunomide to the artery compliance only based on medical treatment. However, the impact of the interactions of drugs, doses, dosing time, and etc. were ignored. Our data only suggest a potential link between leflunomide and artery compliance rather than a causal link between them. In the future, a randomized, double-blind, placebo-controlled studies and follow-up study are needed to confirm the link between leflunomide and artery compliance.
In addition, there might be association between artery compliance and treatment of leflunomide in RA patients; more clinical studies are needed to improve it.
Study limitation
It is not matched perfectly in some items related with atherosclerosis between RA patients and healthy controls, such as age, gender, BMI, blood pressure, level of serum lipid and blood glucose, and life style including smoking and alcohol consumption. And we just receive the cross-sectional date of the patients and healthy controls at present. So, further large-sample cohort studies and randomized clinical trials should be done to demonstrate the aforementioned findings and acquire the change of artery compliance after the therapy.
Conclusion
The values of C1 and C2 are indicators of artery compliance in RA patients. ESR, HAQ values, and the usage of leflunomide might be possible risk factors of artery compliance. The evaluation of artery compliance could be an easy and reliable test that could help us to screen and predict cardiovascular disorders in RA patients.
Electronic supplementary material
(DOCX 16 kb)
Author contributions
Study concept and design: Miaojia Zhang, Wenfeng Tan, FangWang, and Lei Wang; Recruit patients: Miaojia Zhang, Wenfeng Tan, Youxuan Shen, Huanping Mei, Yanyan Wang, Yao Ke, Lei Gu, and Qiang Wang; Acquisition of data: Lei Wang; Analysis and interpretation of the data: Miaojia Zhang, Wenfeng Tan, and Lei Wang; Write the paper: Miaojia Zhang, Wenfeng Tan and Lei Wang. All authors reviewed the manuscript before submission.
Funding
This study was supported by the Special Project of Clinical Medicine from Jiangsu Province (BL2013034), National Natural Science Foundation of China (NSFC) (81671615, 81273294, 81471610, 81172845 and 81471611), National Science and Technology Major Project of China (2014ZX10003003, AD14) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Compliance with ethical standards
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. Furthermore, all subjects signed an informed consent.
Disclosure
None.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10067-017-3899-8) contains supplementary material, which is available to authorized users.
References
- 1.Jalil SF, Arshad M, Bhatti A, et al. Rheumatoid arthritis: what have we learned about the causing factors? Pak J Pharm Sci. 2016;29(2):629–645. [PubMed] [Google Scholar]
- 2.Svensson AL, Christensen R, Persson F, et al. Multifactorial intervention to prevent cardiovascular disease in patients with early rheumatoid arthritis: protocol for a multicentre randomised controlled trial. BMJ Open. 2016;6(4):e009134. doi: 10.1136/bmjopen-2015-009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougados M. Comorbidities in rheumatoid arthritis. Curr Opin Rheumatol. 2016;28(3):282–288. doi: 10.1097/BOR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 4.Pujades-Rodriguez M, Duyx B, Thomas SL, et al. Rheumatoid arthritis and incidence of twelve initial presentations of cardiovascular disease: a population record-linkage cohort study in England. PLoS One. 2016;11(3):e0151245. doi: 10.1371/journal.pone.0151245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agca R, Heslinga SC, van Halm VP, et al. Atherosclerotic cardiovascular disease in patients with chronic inflammatory joint disorders. Heart. 2016;102(10):790–795. doi: 10.1136/heartjnl-2015-307838. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Chang Y, Wei W. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediat Inflamm. 2016;2016:6813016. doi: 10.1155/2016/6813016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbarroja N, Perez-Sanchez C, Ruiz-Limon P, et al. Anticyclic citrullinated protein antibodies are implicated in the development of cardiovascular disease in rheumatoid arthritis. Arterioscler Thromb Vasc Biol. 2014;34(12):2706–2716. doi: 10.1161/ATVBAHA.114.304475. [DOI] [PubMed] [Google Scholar]
- 8.Naerr GW, Rein P, Saely CH, et al. Effects of synthetic and biological disease modifying antirheumatic drugs on lipid and lipoprotein parameters in patients with rheumatoid arthritis. Vasc Pharmacol. 2016;81:22–30. doi: 10.1016/j.vph.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Ishizu T, Kawakami Y. Utility of ultrasonography in point of care for cardiovascular disease. Rinsho byori The Japanese. J Clin Pathol. 2015;63(6):709–716. [PubMed] [Google Scholar]
- 10.Booij R, Dijkshoorn ML, van Straten M, et al. Cardiovascular imaging in pediatric patients using dual source CT. J Cardiovasc Comput Tomogr. 2016;10(1):13–21. doi: 10.1016/j.jcct.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Li S, Guo YL, et al. Is monocyte to HDL ratio superior to monocyte count in predicting the cardiovascular outcomes: evidence from a large cohort of Chinese patients undergoing coronary angiography. Ann Med. 2016;48(5):305–312. doi: 10.3109/07853890.2016.1168935. [DOI] [PubMed] [Google Scholar]
- 12.Mocnik M, Nikolic S, Varda NM. Arterial compliance measurement in overweight and hypertensive children. Indian J Pediatr. 2016;83(6):510–516. doi: 10.1007/s12098-015-1965-2. [DOI] [PubMed] [Google Scholar]
- 13.Setia S, Fung SS, Waters DD. Doctors' knowledge, attitudes, and compliance with 2013 ACC/AHA guidelines for prevention of atherosclerotic cardiovascular disease in Singapore. Vasc Health Risk Manag. 2015;11:303–310. doi: 10.2147/VHRM.S82710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duprez DA, Jacobs DR, Jr, et al. Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011;174(5):528–536. doi: 10.1093/aje/kwr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, Adeney KL, Shlipak MG, et al. Structural and functional vascular alterations and incident hypertension in normotensive adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2010;171(1):63–71. doi: 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkins JT, McDermott MM, Liu K, et al. Associations of noninvasive measures of arterial compliance and ankle-brachial index: the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2012;25(5):535–541. doi: 10.1038/ajh.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahebkar A, Watts GF. Developing role of microRNA-33 in lipid metabolism and atherosclerosis. Curr Opin Lipidol. 2016;27(2):197–199. doi: 10.1097/MOL.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 18.Allen S, Liu YG, Scott E. Engineering nanomaterials to address cell-mediated inflammation in atherosclerosis. Regen Eng Transl Med. 2016;2(1):37–50. doi: 10.1007/s40883-016-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennuyer N, Duplan I, Paquet C, et al. The novel selective PPARalpha modulator (SPPARMalpha) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis. 2016;249:200–208. doi: 10.1016/j.atherosclerosis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Signorelli SS, Candido S, Salemi R, et al. Low levels of inflammation and the absence of subclinical atherosclerosis in rheumatoid arthritis. Mol Med Rep. 2016;13(4):3521–3524. doi: 10.3892/mmr.2016.4962. [DOI] [PubMed] [Google Scholar]
- 21.Jia R, Hashizume-Takizawa T, Du Y et al (2015) Aggregatibacter actinomycetemcomitans induces Th17 cells in atherosclerotic lesions. Pathog Dis 73(3):1–8. 10.1093/femspd/ftu027 [DOI] [PubMed]
- 22.Edsfeldt A, Grufman H, Asciutto G, et al. Circulating cytokines reflect the expression of pro-inflammatory cytokines in atherosclerotic plaques. Atherosclerosis. 2015;241(2):443–449. doi: 10.1016/j.atherosclerosis.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35(27):1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caselli C, De Graaf MA, Lorenzoni V, et al. HDL cholesterol, leptin and interleukin-6 predict high risk coronary anatomy assessed by CT angiography in patients with stable chest pain. Atherosclerosis. 2015;241(1):55–61. doi: 10.1016/j.atherosclerosis.2015.04.811. [DOI] [PubMed] [Google Scholar]
- 25.Ellulu MS, Patimah I, Khaza'ai H, et al. Atherosclerotic cardiovascular disease: a review of initiators and protective factors. Inflammopharmacology. 2016;24(1):1–10. doi: 10.1007/s10787-015-0255-y. [DOI] [PubMed] [Google Scholar]
- 26.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547–563. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 27.Skeoch S, Bruce IN. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nature reviews. Rheumatology. 2015;11(7):390–400. doi: 10.1038/nrrheum.2015.40. [DOI] [PubMed] [Google Scholar]
- 28.Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 29.Hjeltnes G, Hollan I, Forre O, et al. Anti-CCP and RF IgM: predictors of impaired endothelial function in rheumatoid arthritis patients. Scand J Rheumatol. 2011;40(6):422–427. doi: 10.3109/03009742.2011.585350. [DOI] [PubMed] [Google Scholar]
- 30.Kisiel B, Kruszewski R, Juszkiewicz A, et al. Methotrexate, cyclosporine a, and biologics protect against atherosclerosis in rheumatoid arthritis. J Immunol Res. 2015;2015:759610. doi: 10.1155/2015/759610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szwebel TA, Le Jeunne C. Cardiovascular risks of corticosteroids. Presse Med. 2012;41(4):384–392. doi: 10.1016/j.lpm.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Johannsson G, Ragnarsson O. Cardiovascular and metabolic impact of glucocorticoid replacement therapy. Front Horm Res. 2014;43:33–44. doi: 10.1159/000360556. [DOI] [PubMed] [Google Scholar]
- 33.Negrei C, Bojinca V, Balanescu A, et al. Management of rheumatoid arthritis: impact and risks of various therapeutic approaches. Exp Ther Med. 2016;11(4):1177–1183. doi: 10.3892/etm.2016.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araiza-Casillas R, Diaz-Molina R, Gonzalez-Ortiz M, et al. Effects of hydroxychloroquine on insulin sensitivity and lipid profile in patients with rheumatoid arthritis. Rev Med Chil. 2013;141(8):1019–1025. doi: 10.4067/S0034-98872013000800008. [DOI] [PubMed] [Google Scholar]
- 35.Im CH, Kim NR, Kang JW, et al. Inflammatory burden interacts with conventional cardiovascular risk factors for carotid plaque formation in rheumatoid arthritis. Rheumatology. 2015;54(5):808–815. doi: 10.1093/rheumatology/keu376. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)