Abstract
Context
Women with polycystic ovary syndrome (PCOS) have an increased risk of cardiovascular disease (CVD), and biomarkers can be used to detect early subclinical CVD. Midregional-pro-adrenomedullin (MR-proADM), midregional-pro-atrial natriuretic peptide (MR-proANP) and copeptin are all associated with CVD and part of the delicate system controlling fluid and hemodynamic homeostasis through vascular tonus and diuresis. The GLP-1 receptor agonist liraglutide, developed for treatment of type 2 diabetes (T2D), improves cardiovascular outcomes in patients with T2D including a decrease in particular MR-proANP.
Objective
To investigate if treatment with liraglutide in women with PCOS reduces levels of the cardiovascular biomarkers MR-proADM, MR-proANP and copeptin.
Methods
Seventy-two overweight women with PCOS were treated with 1.8 mg/day liraglutide or placebo for 26 weeks in a placebo-controlled RCT. Biomarkers, anthropometrics, insulin resistance, body composition (DXA) and visceral fat (MRI) were examined.
Results
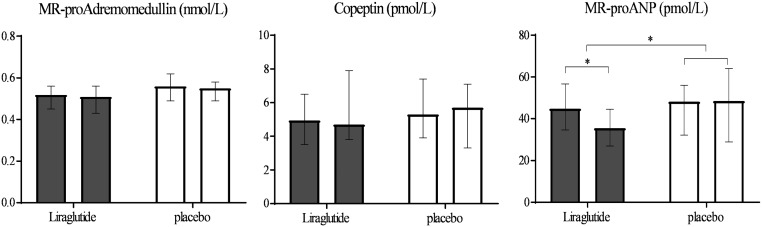
Baseline median (IQR) levels were as follows: MR-proADM 0.52 (0.45–0.56) nmol/L, MR-proANP 44.8 (34.6–56.7) pmol/L and copeptin 4.95 (3.50–6.50) pmol/L. Mean percentage differences (95% CI) between liraglutide and placebo group after treatment were as follows: MR-proADM −6% (−11 to 2, P = 0.058), MR-proANP −25% (−37 to −11, P = 0.001) and copeptin +4% (−13 to 25, P = 0.64). Reduction in MR-proANP concentration correlated with both increased heart rate and diastolic blood pressure in the liraglutide group. Multiple regression analyses with adjustment for BMI, free testosterone, insulin resistance, visceral fat, heart rate and eGFR showed reductions in MR-proANP to be independently correlated with an increase in the heart rate.
Conclusion
In an RCT, liraglutide treatment in women with PCOS reduced levels of the cardiovascular risk biomarkers MR-proANP with 25% and MR-proADM with 6% (borderline significance) compared with placebo. The decrease in MR-proANP was independently associated with an increase in the heart rate.
Keywords: PCOS, atrial natriuretic peptide, adrenomedullin, copeptin, GLP-1 receptor agonist
Introduction
Women with polycystic ovary syndrome (PCOS) are challenged with a metabolic aspect of the syndrome as they have increased prevalence of overweight with central obesity and insulin resistance. They present with an accumulation of cardiovascular risk factors, but their actual risk of developing cardiovascular disease (CVD) is difficult to assess since long-term, prospective studies with well-defined diagnostic criteria are sparse. Nevertheless, a meta-analysis recently reported that women with PCOS have a 30% increased risk of developing CVD and 44% increased risk of coronary heart disease compared with age and BMI-matched healthy women (1). Besides lifestyle intervention and treatment with metformin, a limited number of treatments that target the metabolic aspect of PCOS are available. As most PCOS patients are in the reproductive age and thus rather young, they rarely present with overt CVD; as a result, biomarkers of subclinical and early dysfunction of the heart and vascular endothelium could have a place in the work-up of patients. Adrenomedullin (ADM), atrial natriuretic peptide (ANP) and copeptin are all associated with CVD and part of the delicate system controlling fluid and hemodynamic homeostasis through vascular tonus and diuresis.
The vasodilating peptide ADM is ubiquitously expressed in the body, but secretion from vascular endothelium and smooth muscle cells is the main contributor to plasma levels (2). ADM is also considered an adipokine as it is expressed in adipocytes (3). Secretion of ADM is induced in response to inflammation, hypoxia and insulin resistance such that CVD and type 2 diabetes (T2D) are linked to increased levels of ADM (4, 5). The precursor prohormone midregional-pro-adrenomedullin (MR-proADM) is stable for measurement in the circulation and reflects ADM levels (6).
A- and B-type natriuretic peptides (NPs) (ANP and brain NP (BNP)) are synthesized in the cardiomyocytes from the pro-hormones midregional pro-A-type NP (MR-proANP) and N-terminal pro-B-type NP, and the NPs have a common receptor which is distributed widely (7). Both the intact peptides and the pro-hormones are released from the heart during hemodynamic stress as stretch of the cardiomyocytes during the development of heart failure, as well as during sympathetic stimulation (7). The NPs induce vasodilation and natriuresis, and reduce the renin–angiotensin–aldosterone system as well as the sympathetic nervous system, both activated in heart failure (7). Besides these main effects, the NPs can induce lipolysis, though this ability seems attenuated in obese individuals (8). Increased levels of the NPs are associated with CVD and early heart failure (9), but in contrast large cohort studies have shown that overweight and insulin resistance decrease levels of ANP (10). The preferred NPs to measure are the pro-hormones due to the fact that these assays are more stable and precise than assays measuring the intact peptides (7).
Copeptin is a splicing product that is released when vasopressin is activated. It is more stable for measurement than vasopressin itself and reflects vasopressin levels and thus responds to osmolality. Copeptin shows potential as a rule-out marker in combination with troponin in myocardial infarction, and increased copeptin levels in heart failure are associated with worsened outcome (11). Increased levels of copeptin have been demonstrated in obese individuals (12).
The three biomarkers have previously been evaluated in PCOS and healthy controls in cross-sectional studies, with contradicting results (13, 14, 15, 16, 17, 18). Weight loss and improved insulin sensitivity are cornerstones in the treatment of the metabolic syndrome, T2D and PCOS. Glucagon-like peptide 1 receptor agonists (GLP-1RA) were developed for treatment of hyperglycemia in T2D, but have additionally weight-reducing effect and have proven effective in smaller studies in women with PCOS (19). The LEADER study in high-risk patients with T2D reported that the GLP-1 RA liraglutide improved CVD outcomes (20). The mechanisms are not completely understood, though the available evidence suggests a beneficial effect on progression of atherosclerosis, endothelial dysfunction and inflammation. Based on this, we investigated the impact of 26 weeks of treatment with liraglutide in a PCOS cohort on the plasma levels of the cardiovascular biomarkers: MR-proADM, MR-proANP and copeptin in a double-blind placebo-controlled randomized clinical trial.
Subjects and methods
Design
The LIPT study was a double-blind placebo-controlled RCT. Women with PCOS were randomized in a 2:1 ratio to 1.8 mg liraglutide or placebo once daily for 26 weeks. Details of the study design and tolerability of the treatment have been described previously (21). In short, inclusion criteria were PCOS according to the Rotterdam criteria, BMI above 25 kg/m2 and/or insulin resistance defined as a fasting pro-insulin c-peptide above 600 pmol/L and use of non-hormonal contraception. Exclusion criteria were history of diabetes or CVD, treatment with hormonal contraceptives six weeks before or insulin-sensitizing drugs three months before randomization. The study protocol was approved by the Ethics Committee of Copenhagen (ID: H-2-2013-142), registered on www.clinicaltrials.gov (NCT02073929), and approved by the Danish Medicines Agency (EudraCT 2013-003862-15). The study was conducted in accordance with the Helsinki Declaration, monitored by the local GCP Unit, and oral and written informed consents were obtained from participants before study-related procedures were performed.
Data collection
Body weight was measured in light clothes on a single calibrated scale. Waist circumference was measured between the lowest rib and the iliac crest. Consultation blood pressure was measured three consecutive times after ten minutes of rest in the sitting position. Blood samples were collected in the morning after ten hours overnight fast. A 75 g-oral glucose tolerance test was performed; blood samples were collected at 0, 30, 60 and 120 min. The measurements of HOMA2-IR and Matsuda index were calculated accordingly (22, 23). Routine blood analyses were performed immediately and samples for biomarkers were stored in tubes with EDTA at −80°C until analyses. Plasma MR-proADM, MR-proANP and copeptin were analyzed before the randomization key was broken with an automated immunofluorescence assay on a KRYPTOR platform (Thermo Fischer). Specifications: MR-proANP: Detection limit 2.1 pmol/L, intra-assay CV <3.5% and inter-assay CV <6.5%. MR-proADM: Detection limit 0.05 nmol/L, intra-assay CV ≤10% and inter-assay CV ≤20%. Copeptin measured as C-terminal pro-Vasopressin: Detection limit 0.9 pmol/L, intra-assay CV <15% and inter-assay CV <17%. Total testosterone was analyzed with mass spectroscopy (Waters UPLC-TQS LC–MS/MS system, Milford, USA), inter-assay CV 10%. Free testosterone was calculated from total testosterone and sex hormone binding globulin (SHBG). SHBG was analyzed with a sandwich chemiluminescence immunometric assay, inter-assay CV 7%. Hemoglobin A1c (HbA1c) and fasting lipids were measured by routine methods. DXA whole beam fan scan (Hologic Discovery, Bedford, USA) was performed for whole body composition. MRI scans were performed on an Achieva 3.0 T MR-imaging system (Philips Medical Systems), and volumes of visceral and subcutaneous adipose tissue were measured in a 1-cm thick section at the L3 level.
The CONSORT statement for reporting clinical studies was used as a guidance for the study (24) and detailed information on design has previously been reported (21).
Statistical analyses
Change from baseline in the MR-proADM level was a secondary endpoint in the LIPT study. Power analysis performed on the primary endpoint, endogenous thrombin potential, dictated a sample size of 42:21 (liraglutide:placebo) (21). To allow for 10% dropout, at least 70 patients should be randomized. A power analysis of the secondary endpoint MR-proADM was performed before randomization as described (25). With a sample size of 42:21 (liraglutide:placebo), an in-house s.d. of 0.03 nmol/L and a two-sided significance level of 5%, we were able to detect a change in MR-proADM of 0.025 nmol/L (equal to approximately 5% of the baseline values) with a power of 90%.
A mixed model with a repeated statement was used to test for differences between the treatment groups over time. Missing data at follow-up were handled by applying maximum likelihood in the mixed model procedure. The treatment groups were merged at baseline. Log2 transformation was applied when data were not Gaussian-distributed and consequently results from the mixed model are presented as ratios or percentage. Linear univariate and multiple regression analyses were performed in the liraglutide group with Δ-values of ΔMR-proADM, ΔMR-proANP and Δcopeptin as dependent variables. Δ-Values were calculated as baseline subtracted from follow-up values, and β coefficient and Pearson’s correlation coefficient are reported. For the multiple regression analyses, 6 clinical relevant variables (BMI, Matsuda index, heart rate, visceral adipose tissue (VAT), eGFR and free testosterone) were chosen for analyses. Test for collinearity was performed (the ‘VIF’ command was applied) in all regression analyses. A P-value of 0.05 was considered significant. Statistical analyses were performed with SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA).
Results
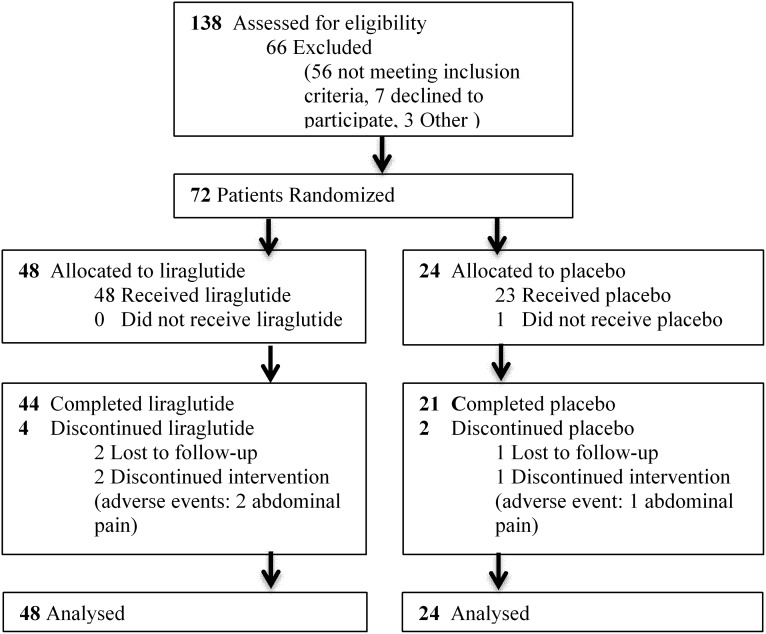
A total of 72 women were randomized in a 2:1 ratio allocating 42 women to liraglutide treatment and 21 to placebo. Seven participants dropped out during the study period (Fig. 1). The participants had a mean (s.d.) age of 29.9 (6.1) years and a BMI of 33.3 (4.9) kg/m2. The two groups were statistically comparable on all parameters at baseline.
Figure 1.
CONSORT flow.
Anthropometrics and body composition
On average (95% CI), the liraglutide group lost 5.2 kg (3.0–7.5, P < 0.001) equal to 5.6% body weight as mean difference compared with placebo. Systolic and diastolic blood pressure did not change during the study period, whereas heart rate increased with 6 bpm (2–10, P = 0.006) in the liraglutide group compared with placebo. We observed significant reductions in total fat and lean body mass as measured by DXA, as well as visceral and subcutaneous adipose tissues as measured by MRI (Table 1).
Table 1.
Effect of 26 weeks of treatment with liraglutide or place in a PCOS cohort.
| Liraglutide baseline | Change at follow-up | Placebo baseline | Change at follow-up | Difference between groups | P-Value | |
|---|---|---|---|---|---|---|
| Weight (kg) | 94.2 ± 15.4 | −5.2 ± 0.7 | 91.3 ± 13.6 | 0.2 ± 0.9 | −5.2 (−7.5 to −3.0) | <0.001 |
| BMI (kg/m2) | 33.3 ± 5.1 | −1.9 ± 0.3 | 33.3 ± 4.6 | 0.1 ± 0.3 | −1.8 (−2.7 to −1.0) | <0.001 |
| Waist (cm) | 102.6 ± 10.8 | −4.1 ± 1.1 | 102.6 ± 11.1 | 1.1 ± 1.5 | −5.7 (−9.3 to −1.9) | 0.01 |
| Systolic BP (mmHg) | 123 ± 9 | −3 ± 1 | 124 ± 9 | −2 ± 2 | −1.6 (−4.8 to 2.5) | 0.53 |
| Diastolic BP (mmHg) | 79 ± 8 | −1 ± 1 | 80 ± 7 | −1 ± 1 | −0.04 (−2.9 to 2.8) | 0.35 |
| Heart rate (bmp) | 76 ± 9 | 6 ± 1 | 79 ± 15 | −1 ± 2 | 6.4 (2.4–10.4) | 0.006 |
| Total cholesterol | 4.61 ± 0.80 | 0.03 ± 0.09 | 4.67 ± 0.57 | 0.08 ± 0.09 | −0.01 (−0.10 to 0.07) | 0.35 |
| LDL cholesterol | 2.83 ± 0.71 | 0.14 ± 0.09 | 2.99 ± 0.54 | 0.13 ± 0.09 | −0.01 (−0.29 to 0.26) | 0.92 |
| HDL cholesterol | 1.14 ± 0.25 | −0.01 ± 0.02 | 1.09 ± 0.28 | 0.01 ± 0.03 | −0.01 (−0.09 to 0.06) | 0.77 |
| Triglycerides | 1.23 (0.90–1.63) | −0.22 (−0.36 to −0.09) | 1.15 (0.90–1.47) | −0.11 (−0.37 to 0.14) | 0.94 (0.82–1.07) | 0.32 |
| HbA1C (mmol/mol) | 34.2 ± 2.8 | −1.3 ± 0.4 | 34.6 ± 3.4 | 0.1 ± 0.5 | −1.38 (−2.48 to −0.28) | 0.015 |
| HOMA2-IR | 2.29 (1.83–2.84) | −0.27 ± 0.15 | 2.42 (1.91–3.20) | −0.28 ± 0.20 | Ratio 0.93 (0.75–1.01) | 0.48 |
| Matsuda index | 2.13 (1.47–2.81) | 0.41 ± 0.19 | 2.04 (1.21–2.70) | 0.34 ± 0.24 | Ratio 1.08 (0.96–1.34) | 0.49 |
| eGFR (mL/min/1.73 m2) | 113 ± 13 | 1 ± 1 | 118 ± 10 | −2 ± 1 | 2 (−3 to 5) | 0.48 |
| DXA fat mass (kg) | 35.9 ± 8.5 | −2.6 ± 0.5 | 35.7 ± 7.2 | 0.3 ± 0.7 | −2.8 (−4.6 to 1.1) | 0.002 |
| DXA lean mass (kg) | 58.8 ± 8.0 | −2.4 ± 0.4 | 56.0 ± 7.0 | 0.1 ± 0.4 | −2.3 (−3.5 to −1.2) | <0.001 |
| MRI VAT (cm3) | 115.7 ± 51.4 (n = 44) | −17.2 ± 4.3 (n = 37) | 120.9 ± 42.3 (n = 23) | 4.50 ± 7.0 (n = 20) | −21.9 (−37.3 to −6.5) | 0.006 |
| MRI SAT (cm3) | 407.4 ± 121.4 (n = 44) | −40.1 ± 10.5 (n = 37) | 409.6 ± 125.3 (n = 23) | −0.35 ± 9.94 (n = 20) | −40.7 (−72.3 to −9.1) | 0.013 |
| MR-proADM (nmol/L) | 0.52 (0.45–0.56) | −0.02 (−0.04 to 0.002) | 0.55 (0.49–0.62) | 0.003 (−0.018 to 0.024) | Ratio 0.94 (0.89–1.002) | 0.057 |
| Copeptin (pmol/L) | 4.95 (3.50–6.50) | 0.48 (−0.39 to 1.34) | 5.30 (3.90–7.40) | 0.28 (−0.51 to 1.08) | Ratio 1.04 (0.87–1.25) | 0.64 |
| MR-proANP (pmol/L) | 44.8 (34.6–56.7) | −11.5 (−17.0 to −6.1) | 48.1 (32.1–56.0) | 1.4 (−7.5 to 10.2) | Ratio 0.75 (0.63–0.89) | 0.001 |
Baseline values, changes at 26 weeks follow, and between-group difference after treatment. Mean ± s.d., median (interquartile range), (95% CI).
MR-proADM, midregional proadrenomedullin; MR-proANP, midregional proatrial natriuretic peptide; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Biomarkers
At baseline, the median (IQR) levels of biomarkers in the whole group were MR-proADM 0.52 (0.45–0.56) nmol/L, MR-proANP 44.8 (34.6–56.7) pmol/L and copeptin 4.95 (3.50–6.50) pmol/L. Mean differences between treatment groups over time showed a change in MR-proANP of −25% (95% CI −37 to −11, P = 0.001), a trend in MR-proADM of −6% (95% CI −11 to 2, P = 0.057) and no change in levels of copeptin in the liraglutide group compared with placebo (Fig. 2 and Table 1).
Figure 2.
Effect of liraglutide or placebo treatment on levels of MR-pro-adrenomedullin, copeptin, and MR-proANP. Median and interquartile range before and after 26 weeks of treatment.
Biochemistry
We observed a reduction in mean difference of HbA1c in the liraglutide group compared with placebo, whereas there was no difference between treatment groups neither regarding markers of insulin resistance, HOMA2-IR and Matsuda index, nor eGFR. Fasting total, HDL and LDL cholesterol and triglycerides did not change during liraglutide/placebo treatment. Plasma SHBG increased and a trend toward a reduction in free testosterone in the liraglutide group compared with placebo was found (Table 1).
Regression analyses
Regression analyses between within-group changes (Δ) in ΔMR-proANP and all covariates presented in Table 1 were assessed for the liraglutide group. Tests for collinearity in the multiple regression analyses were negative. As ΔMR-proADM and copeptin did not change during the treatment period, regressions analyses were not performed. ΔMR-proADM did not correlate significantly in univariate analysis with any of the tested parameters. ΔMR-proANP correlated inversely with Δheart rate (β −0.69, r 0.33, P = 0.03) and Δdiastolic blood pressure (β −0.89, r 0.34, P = 0.03). Multiple regression analyses with adjustment for BMI, free testosterone, Matsuda index, VAT, heart rate and eGFR showed ΔMR-proANP and heart rate to be independently correlated (Table 2).
Table 2.
Multiple regressions between changes in metabolic parameters after 26 weeks of treatment in the liraglutide group in women with PCOS.
| Liraglutide group | ΔMR-proANP β (s.e.) | P |
|---|---|---|
| ΔBMI | −1.40 (2.05) | 0.50 |
| ΔFree testosterone | −462 (240) | 0.06 |
| ΔMatsuda index | −0.75 (2.46) | 0.76 |
| ΔVAT | −0.02 (0.13) | 0.88 |
| ΔHeart rate | −0.77 (0.36) | 0.045 |
| ΔeGFR | 0.29 (0.39) | 0.46 |
Δ, changes during treatment period; MR-proANP, midregional proatrial natriuretic peptide; r, Pearson’s correlation coefficient; s.e., standard error; VAT, visceral adipose tissue.
Discussion
In this placebo-controlled RCT, evaluating the effect of liraglutide treatment on the cardiovascular biomarkers MR-proANP, MR-proADM and copeptin in women with PCOS, we observed a 25% reduction in MR-proANP, a trend toward a 6% reduction in MR-proADM and no change in copeptin levels. The reduction in MR-proANP was independently correlated with increased heart rate as seen during liraglutide treatment.
The borderline reduction in MR-proADM levels is in accordance with previous clinical studies demonstrating reduced MR-proADM levels after diet-induced weight loss (26) and gastric bypass surgery (27), although changes in MR-proADM and BMI did not correlated despite excessive weight loss (27). This is in accordance with our results of a 6% borderline significant reduction in MR-proADM levels (P = 0.057) with no correlation to changes in BMI. ADM is also considered to be an adipokine as its expression has been demonstrated in human pre-adipocytes, stromal cells and activated macrophages in adipose tissue (28). Furthermore, ADM secretion from omental adipose tissue was increased in obese compared with lean women, and the secretion was correlated with features of the metabolic syndrome (28). Despite these reports and a substantial reduction in VAT in the present study, we were unable to find any correlations between MR-proADM levels and BMI or VAT, both at baseline and after weight reduction.
With respect to insulin resistance, previous studies are conflicting. During a euglycemic hyperinsulinemic clamp in obese individuals, plasma ADM increased in response to hyperinsulinemia (29). In contrast, considerable reduction in insulin resistance after gastric bypass was not associated with changes in ADM levels in obese individuals (27). We did not find any association between changes in MR-proADM levels and changes in insulin resistance as measured by HOMA2-IR and Matsuda index. To our knowledge, no clinical studies have previously studied the effect of liraglutide on MR-proADM levels. The study on gastric bypass has the closest resemblance as this procedure increased endogenous GLP-1 secretion considerably (27). Thus, the present and previous studies seem to acknowledge that weight loss does reduce ADM; however, whether this is driven by weight loss, change in insulin resistance or another factor remains unclear.
Low ANP levels and NPs in general have been linked to the development of hypertension, maybe by a direct action on the vessel wall (7, 29). Similarly, but with a potential different mechanism, ANP and other NP levels are low in obesity and seem restored to some extent after weight loss (7, 10, 26), although conflicting data exist, since unchanged ANP levels during excessive weight loss have also been reported (30). In obese individuals, the ratios of the expression of the activating to clearance receptors of ANP in adipose tissue have been found reduced with some degree of reversibility after weight loss (31).
GLP-1RA treatment in mice led to increased ANP levels, reduced blood pressure and increased natriuresis (32). In contrast, in human studies on T2D patients, liraglutide treatment led to reduction in both MR-proANP and blood pressure, but also increased natriuresis (33), which suggests that the antihypertensive effect of liraglutide might not be driven by MR-proANP levels, but rather to increased natriuresis. In line with this, Skov and coworkers (34) found a reduced tubular reabsorption in sodium during short-term studies on liraglutide on kidney function in T2D patients. In another clinical trial, studies on both short- and long-term effects of liraglutide in T2D patients found an increase in natriuresis, however no change in MR-proANP levels (35). In our study on women with PCOS, we found a similar reduction in MR-proANP as in T2D patients, whereas blood pressure remained unaltered. Unfortunately, we did not measure natriuresis. Both in the T2D study and in our PCOS study, the patients lost a considerable amount of weight, including fat mass and VAT. This could potentially lead to an increase in MR-proANP levels due to the above-mentioned shift in activating and clearance receptors of NPs, but did not seem to occur in this cohort.
Liraglutide is known to increase heart rate with approximately 3 bpm (36), by yet unknown mechanisms, and accordingly we observed an increase of 6 bpm during liraglutide treatment in our PCOS cohort. GLP-1 receptors have been identified in the human heart both in the sinus node (37) and the atria (32). In our study, the reduction in MR-proANP levels correlated strongly with the increase in the heart rate, and remained independently correlated after adjustment for changes in BMI, VAT, Matsuda index, free testosterone and eGFR. From a previous cross-sectional study on 98 PCOS women (38), we were able to extract data on MR-proANP and heart rate, and could reproduce the significant negative correlation, between log2 MR-proANP and heart rate (β = −0.02 (s.e. 0.004), r 0.43, P < 0.001) (unpublished data). A mechanism which could affect circulating MR-proANP levels during liraglutide treatment is an increased natriuresis and decreased extracellular volume. These changes in natriuresis and extracellular volume have been demonstrated together with 20% decrease in MR-proANP levels and a 6.5-bpm increase in the heart rate in T2D patients after liraglutide treatment (39), and liraglutide infusion increased heart rate and natriuresis in healthy individuals; however, neither ANP nor BNP levels changed (40). We did not address these issues in our PCOS population. The LEADER study demonstrated improvement in CVD outcomes despite an increase in the heart rate of 3 bpm (20).
Regarding testosterone, studies in other populations and a study administering testosterone therapy in women with hypoandrogenemia point to an inverse correlation between NPs and testosterone (41, 42). We have previously reported a significant inverse correlation in a case-control study (38), but the association vanished after adjustment for BMI, age, pro-insulin c-peptide, LDL cholesterol, eGFR and systolic blood pressure. Based on these findings, the relatively low endogenous testosterone levels in women with PCOS compared with men do not seem to have any major influence on MR-proANP levels.
To the best of our knowledge, this is the first report on the effect of liraglutide treatment on circulating levels of copeptin in humans. As levels of copeptin are increased in individuals with obesity and IR (12, 43), a reduction in weight would favor a reduction in copeptin levels in our PCOS cohort, whereas a possible liraglutide-induced diuresis would favor an increase through osmotic stimuli. In rats, increased and decreased vasopressin levels induced by injections of vasopressin or increased fluid intake in lean and obese rats (44) demonstrated increased vasopressin levels to induce increased glycemia in lean rats and increased IR in obese rats. Large population studies report that high copeptin levels might predict the development of T2D (11). We could not demonstrate an otherwise hypothesized reduction in copeptin during liraglutide treatment. Whether copeptin levels are influenced by a counter regulation to a hypothesized liraglutide-induced natriuresis is a question for further investigation, or perhaps our participants were too young and therefore without subclinical CVD.
Limitations: 24 h ambulatory blood pressure and heart rate recordings would have given more precise estimates, but were not performed. A pre-study power analysis based on MR-proADM was performed; however, the expected change in MR-proADM was not met, and the study might thus be underpowered to some degree. We used the CONSORT statement as a guideline (24) for reporting a clinical study and one issue needs attention: Clinical biomarkers have an inherent and large biological and analytical variation. The analytical variation is reflected in usually a rather large CV of the assay, especially the inter-assay CV, whereas the biological variation is reflected in day-to-day changes in the individual. With regard to MR-proANP measurements (and probably also measurements of MR-proADM and copeptin), such rather large biological variations have been reported, and estimates for clinically relevant changes in terms of percent changes over time have been suggested (45). The small changes that we observed are less than these estimates, which weaken our results, and might be the reason for the diversity of findings on MR-proANP measurements discussed above. In order to minimize this potential bias, we analyzed samples from one individual in the same assay (avoiding inter-assay CV), and used the placebo-controlled study design.
One could argue that we should have measured BNP and not ANP, since BNP is the established biomarker for evaluating heart failure. ANP and BNP seem to be secreted and metabolized in a quite similar fashion, and they have the same receptor (7). We studied ANP since the MR-proANP has the longest plasma half-lifetime which is translated into the most robust assay performance within the NP technology. This is also reflected in the fact that most studies focusing on metabolic disturbances and the effect of liraglutide have used MR-proANP measurements. We did not measure other established biomarkers as galactin-3 or troponins since these biomarkers mainly reflect cardiac fibrosis and necrosis, respectively, and we did not expect to find changes in such parameters in our cohort of relatively young PCOS women.
Conclusion
During 26 weeks of treatment with 1.8 mg/day liraglutide in a PCOS cohort, we saw a 25% reduction in MR-proANP levels and a trend toward a 6% reduction in MR-proADM, whereas copeptin levels did not change compared with placebo. The reduction in MR-proANP was associated with an increase in the heart rate, and the mechanism behind we can only speculate on, but taking current literature together, we believe that these changes represent a signal of beneficial metabolic adaptations. Further research works regarding the direct effect of liraglutide on MR-proANP levels and natriuresis are warranted.
Declaration of statement
S F, M N and S O S have nothing to disclose. C K and J F have given lectures at Novo Nordisk A/S sponsored symposia and are members of a Novo Nordisk A/S advisory board with regard to the use of liraglutide in diabetes.
Funding
The work was supported with grants from Herlev Gentofte Hospital Research Foundation, Department of Internal Medicine, Herlev Gentofte Hospital and an unrestricted grant as well as study medication from Novo Nordisk A/S. The study was investigator-initiated and the authors own the intellectual property of the study. Novo Nordisk A/S had access to the manuscript prior to publication, but no influence on the content.
References
- 1.Zhao L, Zhu Z, Lou H, Zhu G, Huang W, Zhang S, Liu F. Polycystic ovary syndrome (PCOS) and the risk of coronary heart disease (CHD): a meta-analysis. Oncotarget 2016. 7 33715–33721. ( 10.18632/oncotarget.9553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocrine Reviews 2000. 21 138–167. ( 10.1210/edrv.21.2.0396) [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Jiang C, Wang X, Zhang Y, Shibahara S, Takahashi K. Adrenomedullin is a novel adipokine: adrenomedullin in adipocytes and adipose tissues. Peptides 2007. 28 1129–1143. ( 10.1016/j.peptides.2007.03.005) [DOI] [PubMed] [Google Scholar]
- 4.Wong HK, Tang F, Cheung TT, Cheung BMY. Adrenomedullin and diabetes. World Journal of Diabetes 2014. 5 364–371. ( 10.4239/wjd.v5.i3.364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong HK, Cheung TT, Cheung BMY. Adrenomedullin and cardiovascular diseases. JRSM Cardiovascular Disease 2012. 1 ( 10.1258/cvd.2012.012003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clinical Chemistry 2005. 51 1823–1829. ( 10.1373/clinchem.2005.051110) [DOI] [PubMed] [Google Scholar]
- 7.Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP. Natriuretic peptides in cardiometabolic regulation and disease. Nature Reviews Cardiology 2014. 11 403–412. ( 10.1038/nrcardio.2014.64) [DOI] [PubMed] [Google Scholar]
- 8.Rydén M, Bäckdahl J, Petrus P, Thorell A, Gao H, Coue M, Langin D, Moro C, Arner P. Impaired atrial natriuretic peptide-mediated lipolysis in obesity. International Journal of Obesity 2016. 40 714–720. ( 10.1038/ijo.2015.222) [DOI] [PubMed] [Google Scholar]
- 9.Tzikas S, Keller T, Wild PS, Schulz A, Zwiener I, Zeller T, Schnabel RB, Sinning C, Lubos E, Kunde J, et al. Midregional pro-atrial natriuretic peptide in the general population/insights from the Gutenberg Health Study. Clinical Chemistry and Laboratory Medicine 2013. 51 1125–1133. ( 10.1515/cclm-2012-0541) [DOI] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PWF, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004. 109 594–600. ( 10.1161/01.CIR.0000112582.16683.EA) [DOI] [PubMed] [Google Scholar]
- 11.Enhörning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, Struck J, Morgenthaler NG, Bergmann A, Lindholm E, et al. Plasma copeptin and the risk of diabetes mellitus. Circulation 2010. 121 2102–2108. ( 10.1161/CIRCULATIONAHA.109.909663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asferg CL, Andersen UB, Linneberg A, Goetze JP, Jeppesen JL. Copeptin, a surrogate marker for arginine vasopressin secretion, is associated with higher glucose and insulin concentrations but not higher blood pressure in obese men. Diabetic Medicine 2014. 31 728–732. ( 10.1111/dme.12411) [DOI] [PubMed] [Google Scholar]
- 13.Ucar B, Noyan V, Caglayan O, Yucel A, Sagsoz N. Plasma adrenomedullin levels in patients with polycystic ovary syndrome. Fertility and Sterility 2006. 86 942–948. ( 10.1016/j.fertnstert.2006.02.119) [DOI] [PubMed] [Google Scholar]
- 14.Sahin I, Celik O, Celik N, Keskin L, Dogru A, Dogru I, Yürekli M, Yologlu S. Adrenomedullin: possible predictor of insulin resistance in women with polycystic ovary syndrome. Journal of Endocrinological Investigation 2012. 35 553–556. ( 10.3275/7872) [DOI] [PubMed] [Google Scholar]
- 15.Lauria PBM, Del Puerto HL, Reis AM, Candido AL, Reis FM. Low plasma atrial natriuretic peptide: a new piece in the puzzle of polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 2013. 98 4882–4889. ( 10.1210/jc.2013-2141) [DOI] [PubMed] [Google Scholar]
- 16.Karbek B, Ozbek M, Karakose M, Topaloglu O, Bozkurt NC, Cakır E, Aslan MS, Delibasi T. Copeptin, a surrogate marker for arginine vasopressin, is associated with cardiovascular risk in patients with polycystic ovary syndrome. Journal of Ovarian Research 2014. 7 31 ( 10.1186/1757-2215-7-31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taskin MI, Bulbul E, Adali E, Hismiogulları AA, Inceboz U. Circulating levels of obestatin and copeptin in obese and nonobese women with polycystic ovary syndrome. European Journal of Obstetrics and Gynecology and Reproductive Biology 2015. 189 19–23. ( 10.1016/j.ejogrb.2015.03.006) [DOI] [PubMed] [Google Scholar]
- 18.Deveer M, Deveer R, Basaran O, Turkcu UO, Akbaba E, Cullu N, Turhan N, Kucuk M, Kasap B. Serum copeptin, pentraxin 3, anti-mullerian hormone levels with echocardiography and carotid artery intima-media thickness in adolescents with polycystic ovary syndrome. Journal of Clinical Medicine Research 2015. 7 989–994. ( 10.14740/jocmr2375w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niafar M, Pourafkari L, Porhomayon J, Nader N. A systematic review of GLP-1 agonists on the metabolic syndrome in women with polycystic ovaries. Archives of Gynecology and Obstetrics 2016. 293 509–515. ( 10.1007/s00404-015-3976-7) [DOI] [PubMed] [Google Scholar]
- 20.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2016. 375 311–322. ( 10.1056/NEJMoa1603827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nylander M, Frøssing S, Kistorp C, Faber J, Skouby SO. Liraglutide in polycystic ovary syndrome: a randomized trial, investigating effects on thrombogenic potential. Endocrine Connections 2017. 6 89–99. ( 10.1530/EC-16-0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004. 27 1487–1495. ( 10.2337/diacare.27.6.1487) [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999. 22 1462–1470. ( 10.2337/diacare.22.9.1462) [DOI] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D. & CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010. 340 c332 ( 10.1136/bmj.c332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frøssing S, Nylander M, Kistorp C, Skouby SO, Faber J. The LIPT-Study: on risk markers of vascular thrombosis in polycystic ovary syndrome a randomized, double-blind, placebo-controlled study of the effect of liraglutide. Journal of Obesity and Weight Loss Therapy 2015. 5 254 ( 10.4172/2165-7904.1000254) [DOI] [Google Scholar]
- 26.Kistorp C, Bliddal H, Goetze JP, Christensen R, Faber J. Cardiac natriuretic peptides in plasma increase after dietary induced weight loss in obesity. BMC Obesity 2014. 1 24 ( 10.1186/s40608-014-0024-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vila G, Riedl M, Maier C, Struck J, Morgenthaler NG, Handisurya A, Prager G, Ludvik B, Clodi M, Luger A. Plasma MR-proADM correlates to BMI and decreases in relation to leptin after gastric bypass surgery. Obesity 2009. 17 1184–1188. ( 10.1038/oby.2009.22) [DOI] [PubMed] [Google Scholar]
- 28.Paulmyer-Lacroix O, Desbriere R, Poggi M, Achard V, Alessi M-C, Boudouresque F, Ouafik L, Vuaroqueaux V, Labuhn M, Dutourand A, et al. Expression of adrenomedullin in adipose tissue of lean and obese women. European Journal of Endocrinology 2006. 155 177–185. ( 10.1530/eje.1.02170) [DOI] [PubMed] [Google Scholar]
- 29.Letizia C, Iacobellis G, Caliumi C, Leonetti F, Cotesta D, Ribaudo MC, Petramala L, Cianci R, Celi M, D’Erasmo E, et al. Acute hyperinsulinemia is associated with increased plasma adrenomedullin concentrations in uncomplicated obesity. Experimental and Clinical Endocrinology and Diabetes 2005. 113 171–175. ( 10.1055/s-2005-837519) [DOI] [PubMed] [Google Scholar]
- 30.Haufe S, Kaminski J, Utz W, Haas V, Mähler A, Daniels MA, Birkenfeld AL, Lichtinghagen R, Luft FC, Schulz-Menger J, et al. Differential response of the natriuretic peptide system to weight loss and exercise in overweight or obese patients. Journal of Hypertension 2015. 33 1458–1464. ( 10.1097/HJH.0000000000000573) [DOI] [PubMed] [Google Scholar]
- 31.Kovacova Z, Tharp WG, Liu D, Wei W, Xie H, Collins S, Pratley RE. Adipose tissue natriuretic peptide receptor expression is related to insulin sensitivity in obesity and diabetes. Obesity 2016. 24 820–828. ( 10.1002/oby.21418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nature Medicine 2013. 19 567–575. ( 10.1038/nm.3128) [DOI] [PubMed] [Google Scholar]
- 33.von Scholten BJ, Persson F, Rosenlund S, Eugen-Olsen J, Pielak T, Faber J, Hansen TW, Rossing P. Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: a sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes, Obesity and Metabolism 2017. 19 901–905. ( 10.1111/dom.12884) [DOI] [PubMed] [Google Scholar]
- 34.Skov J, Pedersen M, Holst JJ, Madsen B, Goetze JP, Rittig S, Jonassen T, Frøkiaer J, Dejgaard A, Christiansen JS. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes, Obesity and Metabolism 2016. 18 581–589. ( 10.1111/dom.12651) [DOI] [PubMed] [Google Scholar]
- 35.Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 2015. 38 132–139. ( 10.2337/dc14-1958) [DOI] [PubMed] [Google Scholar]
- 36.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open 2013. 3 e001986 ( 10.1136/bmjopen-2012-001986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014. 155 1280–1290. ( 10.1210/en.2013-1934) [DOI] [PubMed] [Google Scholar]
- 38.Frøssing S, Nylander M, Aziz M, Skouby SO, Kistorp C, Faber J. Atrial natriuretic peptide, copeptin and adrenomedullin levels in polycystic ovary syndrome: a case-control study. Gynecological Endocrinology 2016. 33 30–33. ( 10.1080/09513590.2016.1202915) [DOI] [PubMed] [Google Scholar]
- 39.von Scholten BJ, Lajer M, Goetze JP, Persson F, Rossing P. Time course and mechanisms of the anti-hypertensive and renal effects of liraglutide treatment. Diabetic Medicine 2015. 32 343–352. ( 10.1111/dme.12594) [DOI] [PubMed] [Google Scholar]
- 40.Skov J, Holst JJ, Gøtze JP, Frøkiær J, Christiansen JS. Glucagon-like peptide-1: effect on pro-atrial natriuretic peptide in healthy males. Endocrine Connections 2014. 3 11–16. ( 10.1530/EC-13-0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam CSP, Cheng S, Choong K, Larson MG, Murabito JM, Newton-Cheh C, Bhasin S, McCabe EL, Miller KK, Redfield MM, et al. Influence of sex and hormone status on circulating natriuretic peptides. Journal of the American College of Cardiology 2011. 58 618–626. ( 10.1016/j.jacc.2011.03.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin E, McCabe E, Newton-Cheh C, Bloch K, Buys E, Wang T, Miller KK. Effects of transdermal testosterone on natriuretic peptide levels in women: a randomized placebo-controlled pilot study. Fertility and Sterility 2012. 97 489–493. ( 10.1016/j.fertnstert.2011.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enhörning S, Struck J, Wirfält E, Hedblad B, Morgenthaler NG, Melander O. Plasma copeptin a unifying factor behind the metabolic syndrome. Journal of Clinical Endocrinology and Metabolism 2011. 96 E1065–E1072. ( 10.1210/jc.2010-2981) [DOI] [PubMed] [Google Scholar]
- 44.Taveau C, Chollet C, Waeckel L, Desposito D, Bichet DG, Arthus M-F, Magnan C, Philippe E, Paradis V, Foufelle F, et al. Vasopressin and hydration play a major role in the development of glucose intolerance and hepatic steatosis in obese rats. Diabetologia 2015. 58 1081–1090. ( 10.1007/s00125-015-3496-9) [DOI] [PubMed] [Google Scholar]
- 45.Wu AHB. Biological and analytical variation of clinical biomarker testing: implications for biomarker-guided therapy. Current Heart Failure Reports 2013. 10 434–440. ( 10.1007/s11897-013-0156-6) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a