Abstract
Aims/Introduction
In previous studies, hydrogen gas (H2) administration has clearly shown effectiveness in inhibiting diabetes. Here, we evaluated whether subcutaneous injection of H2 shows enhanced efficacy against type 2 diabetes mellitus induced in mice by a high‐fat diet and low‐dose streptozotocin treatment.
Material and Methods
H2 was injected subcutaneously at a dose of 1 mL/mouse/week for 4 weeks. Type 2 diabetes mellitus‐associated parameters were then evaluated to determine the effectiveness of subcutaneous H2 administration.
Results
The bodyweight of H2‐treated mice did not change over the course of the experiment. Compared with the untreated control animals, glucose, insulin, low‐density lipoprotein and triglyceride levels in the serum were significantly lower in treated mice, whereas high‐density lipoprotein cholesterol in the serum was significantly higher. Glucose tolerance and insulin sensitivity were both improved in H2‐treated mice. Diabetic nephropathy analysis showed significant reductions in urine volume, urinary total protein and β2‐microglobulin, kidney/bodyweight ratio, and kidney fibrosis associated with subcutaneous injection of H2.
Conclusions
Subcutaneous injection of H2 significantly improves type 2 diabetes mellitus and diabetic nephropathy‐related outcomes in a mouse model, supporting further consideration of subcutaneous injection as a novel and effective route of clinical H2 administration.
Keywords: Diabetic nephropathy, Hydrogen, Subcutaneous administration
Introduction
Type 2 diabetes mellitus, the predominant form of diabetes, is characterized by high levels of blood sugar and insulin resistance. Approximately 170 million people in the world suffer from diabetes, a number expected to double by 20301. Although effective drugs are available for clinical use, including insulin, metformin and glucagon‐like peptide‐1, the World Health Organization reported 1.5 million deaths from diabetes in 2012, making it the eighth most prevalent cause of death2. Most diabetes deaths occur in developing countries3, and were associated with an estimated cost of $612 billion in 20144.
The anti‐oxidant properties of hydrogen gas (H2) have been recognized in recent years5, 6, and it has been used extensively to treat various conditions including ischemia/reperfusion7, sepsis8 and acute lung injury9. H2 has also shown to inhibit diabetes10, 11 and related diseases12. The low solubility of H2 renders therapeutic administration, and various routes have been used for specific treatments including high‐content (saturated) H2 water10, 12, inhalation7, 13, 14, electrically reduced water11 and H2‐producing intestinal bacteria15.
In the present study, we used a classical mouse model of type 2 diabetes mellitus to investigate the therapeutic effects of subcutaneously injected H2 by examining blood glucose, insulin and lipid levels, oxidative stress, and kidney function.
Materials and Methods
Drugs and chemicals
Streptozotocin (STZ) was purchased from Sigma (St. Louis, Missouri, USA). The 40% high‐fat diet was obtained from Slac Laboratory Animal Ltd. (Shanghai, China). The radioimmunoassay kits of β2‐microglobulin and insulin were purchased from Beijing North Institute of Biological Technology (Beijing, China). Urine total protein assay kit was purchased from Baoding Great Wall Clinical Reagents Co., Ltd. (Baoding, China). Detection kits for total superoxide dismutase (T‐SOD), catalase (CAT) and malondialdehyde (MDA) were purchased from Jiancheng Bioengineering Institute (Nanjing, China). Masson staining kit was obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
Animals
The 4‐week‐old male C57/BL6J mice were obtained from Slac Laboratory Animal Ltd. The animals were bred under standard conditions (12‐h light–dark cycle, 24°C), with free access to water and standard laboratory chow. All mice were carefully fed according to the standards of the Guide for the Care and Use of Laboratory Animals.
Diabetic induction and animal grouping
Diabetes induction was carried out according to a previously reported protocol16. Briefly, mice were fed a high‐fat diet for 4 weeks and then intraperitoneally injected with 100 mg/kg STZ (Sigma) or an equal volume of vehicle as the control (n = 8). After 2 weeks of high‐fat diet feeding, glucose levels in the plasma were determined by a blood glucose meter (Andon Health Co., Ltd., Tianjin, China). Mice with glucose levels ≥10 mmol/L were considered diabetic, and were used for experiments if they continuously maintained hyperglycemia (≥10 mmol/L) over 10 days.
Mice were divided into three groups: mice without diabetes induction (normal control [NC], n = 8); diabetic mice receiving subcutaneous injection of air (DM; n = 15); diabetic mice receiving subcutaneous injections of H2 (SAH; n = 15). The SAH group was injected subcutaneously with H2 at a dose of 1 mL/mouse/week for 4 weeks. The same dose of air was given to the DM group by subcutaneous injection. In each group, the bodyweight was monitored daily, and blood glucose level was checked weekly.
Insulin tolerance test and glucose tolerance test
The insulin tolerance test (ITT) and glucose tolerance test (GTT) were carried out at the end of the experiment. For ITT, the mice were intraperitoneally injected with insulin at a dose of 0.5 U/kg bodyweight (Wanbang Biopharmaceuticals, Jiangsu, China) after a 15‐h fast. Blood samples (10 μL) were collected at 0, 30, 60 and 120 min after insulin administration. For GTT, the mice received oral glucose at 2 g/kg bodyweight of glucose after a 12‐h fast. Blood samples (10 μL) were taken at 0, 30, 60 and 120 min after glucose treatment. The ITT and GTT were carried out on mice without anesthetization. Glucose levels in the plasma were determined as described above.
Measurements of biochemical parameters
All measurements were carried out after 6 h of fasting. Blood samples from the common carotid artery were collected under anesthesia into chilled tubes treated with ethylenediamine tetraacetic acid disodium salt, immediately centrifuged and supernatants stored at −80. Plasma low‐density lipoprotein (LDL), triglyceride (TG), total cholesterol and high‐density lipoprotein levels were measured with the automatic biochemistry analyzer. Plasma insulin level was detected according to the instructions of the kit.
24‐h urine volume collection, and measurement of urinary total protein and β2‐microglobulin
Mice were fasted and supplied with water ad libitum for 24‐h urine collection. Urinary total protein and β2‐microglobulin were determined following the instructions supplied with the commercial kits.
Calculation of kidney weight/bodyweight ratio and examination of renal fibrosis
Under anesthesia, the kidneys were isolated and weighed to calculate the kidney weight/bodyweight ratio (Kw/Bw [mg/g]). The right kidney was fixed in 4% paraformaldehyde for paraffin embedding. Then, 4‐μm sections were stained with Masson and observed by microscopy.
Detection of oxidative stress indicator
The MDA content, T‐SOD, and CAT activities in the plasma and kidney tissue were determined by the thiobarbituric acid method17, xanthine oxidase18 and the ammonium molybdate colorimetric method19, respectively. The assays were carried out according to the instructions supplied with the commercial kits. The bicinchoninic acid assay was used to normalize the levels in kidney tissue.
Statistical analysis
All data are expressed as mean ± SD. Significant differences were determined by the Bonferroni test. A P‐value <0.05 was considered statistically significant.
Results
Subcutaneous administration of H2 improved hyperglycemia in diabetic mice induced by high‐fat diet and STZ
To initially investigate the effects of H2 on diabetic mice, bodyweight was tracked throughout the experimental period. Weight gains in all groups were similar without statistical significance (P > 0.05), suggesting that H2 administration does not affect bodyweight (Figure 1a).
Figure 1.
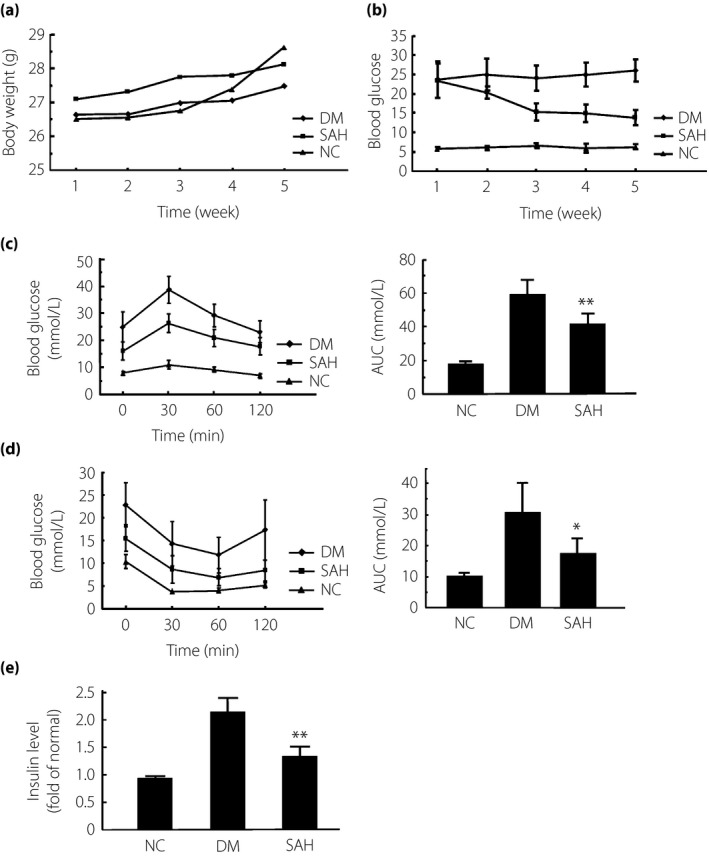
Subcutaneous administration of hydrogen gas (H2) suppressed hyperglycemia in mice with diabetes mellitus (DM) induced by a high‐fat diet and a low dose of streptozotocin. Diabetic mice were subcutaneously injected H2 at 1 mL/mouse/week for 4 weeks. The same dose of air was given as a control. Biochemical analysis was carried out to obtain plasma parameters. (a) Bodyweight after H2 treatment for each group was recorded weekly. There was no significant difference in bodyweight between the SAH group and DM group. (b) The level of blood glucose after H2 treatment for each group was recorded weekly. At the 4‐week time‐point, the level of blood glucose was significantly reduced in the subcutaneous administration of H2 group (SAH) compared with the DM group. (c,d) At day 28, the glucose tolerance test and insulin tolerance test were carried out to check the levels of blood glucose for each group at the time‐points of 0, 30, 60 and 120 min. The area under the curve (AUC) for each group is also shown. (e) The level of plasma insulin was measured after 4‐week H2 treatment. The data are expressed as mean ± SD (n = 8–16). *P < 0.05, **P < 0.01. NC, normal control group.
We carried out biochemical analysis on blood samples to further investigate the antidiabetic effects of H2. Glucose plasma levels were significantly reduced by H2 injection (P < 0.01 or P < 0.05; Figure 1b). ITT and GTT were carried out at day 28. As shown in Figure 1c,d, blood glucose levels in the diabetic group reached the maximum at 30 min, and then gradually decreased. In contrast, blood glucose in the H2‐treated mice declined faster. Serum glucose levels in the diabetic mice were significantly increased compared with the control animals, and this was clearly inhibited by subcutaneous administration of H2 (Figure 1d). Thus, glucose tolerance and insulin sensitivity in diabetic mice were significantly improved by subcutaneous H2 treatment. Additionally, the area under the corresponding curve of GTT and ITT in the H2 group was significantly decreased compared with the DM group (P < 0.01 or P < 0.05; Figure 1c,d). Plasma insulin levels also showed a dramatic reduction in the H2‐treated group compared with the DM group (Figure 1e). These data suggest that subcutaneous injection of H2 improves hyperglycemia in diabetic mice.
Subcutaneous administration of H2 improved hyperlipemia in diabetic mice induced by a high‐fat diet and a low dose of STZ
Hyperlipemia is an important feature of type 2 diabetes mellitus. We examined plasma lipids in diabetic mice after subcutaneous H2 administration. Levels of LDL and TG, but not total cholesterol, were significantly attenuated, whereas the level of high‐density lipoprotein was increased after H2 treatment in the SAH group (**P < 0.01; Figure 2). These observations show significant improvement of hyperlipemia by subcutaneous H2 administration in diabetic mice induced by a high‐fat diet and a low dose of STZ.
Figure 2.
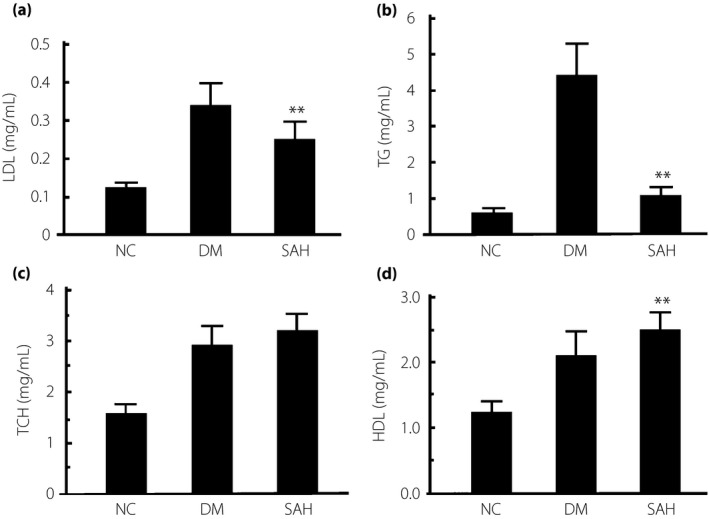
Subcutaneous administration of hydrogen gas (H2) suppressed hyperlipemia in the mice with diabetes mellitus (DM) induced by a high‐fat diet and a low dose of streptozotocin. After 4 weeks of H2 treatment, plasma samples were collected from each group to measure the levels of plasma lipids including (a) low‐density lipoprotein (LDL), (b) triglyceride (TG), (c) total cholesterol (TCH) and (d) high‐density lipoprotein (HDL). The levels of plasma LDL and TG in the subcutaneous administration of H2 group (SAH) group were significantly lower than those in the DM group. The data are expressed as mean ± SD (n = 8–16). **P < 0.01. NC, normal control group.
Subcutaneous administration of H2 reduced the oxidative stress of diabetic mice induced by a high‐fat diet and a low dose of STZ
The effect of H2 on oxidative stress was examined by measuring the levels of T‐SOD, CAT and MDA in plasma (Figure 3a–c). The MDA level in plasma was significantly reduced in the H2‐administered mice (P < 0.01). The activity of T‐SOD and CAT in plasma was prominently reduced in the diabetic mice compared with the NC group, whereas these changes were reversed by subcutaneous administration of H2 (P < 0.01 or P < 0.05). Thus, H2 suppressed oxidative stress in the diabetic mice. We also measured the levels of T‐SOD, CAT and MDA to evaluate the oxidative stress in the kidney after H2 administration (Figure 3d–f). H2 administration significantly reduced MDA compared with the DM group. The activity of T‐SOD and CAT in the kidney tissue of diabetic mice was prominently attenuated, which was enhanced by subcutaneous administration of H2. These data show that H2 inhibited renal oxidative stress in the diabetic mice.
Figure 3.
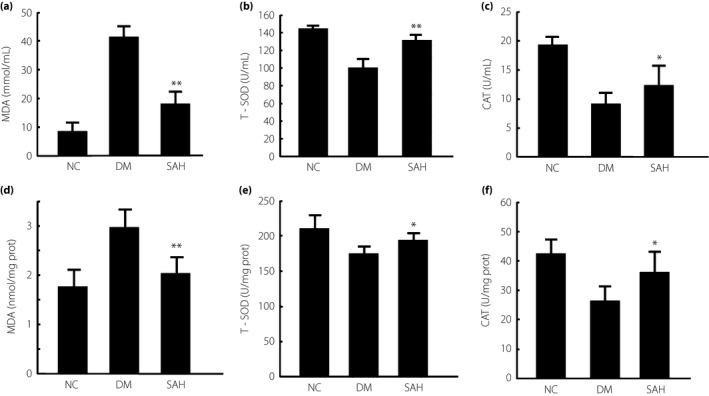
Levels of malondialdehyde (MDA), total superoxide dismutase (T‐SOD) and catalase (CAT) activity in the plasma and kidney of mice with diabetes mellitus (DM) induced by a high‐fat diet and a low dose of streptozotocin. Subcutaneous administration of hydrogen gas (H2) to the mice (a) decreased the levels of MDA, and promoted the activities of (b) T‐SOD and (c) CAT in plasma. After 4 weeks of H2 treatment, the plasma samples from each group were collected to detect the levels of indicators for plasma oxidative stress. The oxidative stress was significantly reduced in the subcutaneous administration of H2 group (SAH) group compared with the DM group. Subcutaneous administration of H2 (d) reduced the content of MDA and promoted the activities of (e) T‐SOD and (f) CAT in the kidney. Renal tissues were homogenized to examine T‐SOD and CAT activity, and MDA content. bicinchoninic acid assay was used to determine protein levels in renal samples to normalize oxidative parameters. The data are expressed as mean ± SD (n = 8–16). *P < 0.05; **P < 0.01. NC, normal control group.
Subcutaneous administration of H2 reduced diabetic renal injury
Because the kidney was sensitive to injury induced by diabetes, we evaluated 24‐h urine volume, total urine protein and β2‐microglobulin, Kw/Bw, and renal fibrosis in the experimental and control groups. As shown in Figure 4a, the 24‐h urine volume of mice in the DM group was significantly increased, and this was ameliorated by subcutaneous injection of H2 (P < 0.01). The levels of total urinary protein and β2‐microglobulin, and the ratio of Kw/Bw were significantly higher in the DM vs NC mice, and subcutaneous administration of H2 attenuated all DM‐associated parameters (P < 0.01; Figure 4b–d). The fibrosis observed in the kidneys of diabetic mice (blue) was alleviated by H2 treatment (Figure 4e). These data strongly show that subcutaneous administration of H2 is able to reduce renal injury in diet‐ and STZ‐induced diabetic mice.
Figure 4.
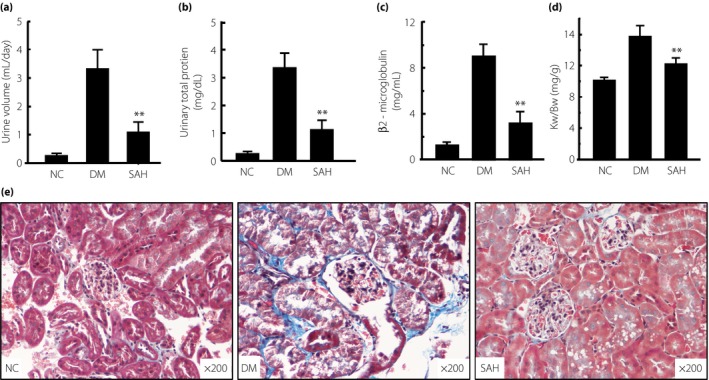
Subcutaneous administration of hydrogen gas (H2) reduced 24‐h urine volume, urinary total protein, β2‐microglobulin, kidney weight/bodyweight ratio (Kw/Bw) and renal fibrosis resulting from diabetes. After 4 weeks of H2 treatment, (a) 24‐h urine volume, (b) urinary total protein, (c) β2‐microglobulin and (d) Kw/Bw were detected, and (e) kidney tissues were fixed for Masson staining. The 24‐h urine volume, urinary total protein, β2‐microglobulin, Kw/Bw and renal fibrosis in the group with H2 treatment were significantly reduced, compared with the diabetes mellitus (DM) group (n = 16 for each group). The data are expressed as mean ± SD (n = 8–16). **P < 0.01. NC, normal control group; SAH, subcutaneous administration of H2 group.
Discussion
Previous studies showed that H2‐water is able to attenuate oxidative stress in patients with type 2 diabetes mellitus and metabolic syndrome20, 21, 22. Although ingesting H2‐water is a convenient route of administration, the absorptive dose of H2 is extremely limited because of low saturation (0.8 mmol/L at atmospheric pressure). In the present study, we showed for the first time that subcutaneous injection of H2, which is locally stored in tissue and subsequently stably diffused, has significant effects on type 2 diabetes mellitus‐associated disease features.
In preliminary experiments, we found that it took approximately 1 week to completely absorb 1 mL of subcutaneously injected H2, and these observations were used to design the H2 administration schedule. Based on the H2 solubility coefficient (0.0182 at 20°C) and international standard atmosphere (101.3 kPa), 55 mL of water is required to dissolve 1 mL of H2. At an ingestion rate of 5–7 mL/mouse/day, it would require 8 days to consume 1 mL of H2. In the digestive tract, the absorption of H2 into the body is even less efficient. Furthermore, injection of saturated‐H2 saline at a dose of 0.6 mL/mouse/day would require approximately 91 days to consume 1 mL of H2. Although previously reported H2 administration routes have been shown to have significant anti‐oxidative effects9, 22, 23, our studies suggest that subcutaneous H2 injection is an efficient way to enhance H2 absorption by tissues and thereby improve therapeutic efficacy.
Hyperlipemia is a typical clinical feature of metabolic syndrome induced by diabetes. Other studies showed that the plasma TG level of diabetic mice12 and the plasma LDL, especially oxidized LDL, of type 2 diabetes patients, were suppressed by drinking H2‐water20. However, this improvement in hyperlipemia by drinking H2‐water was not observed in type 2 diabetic mice by Haruka10. The present experiments show that subcutaneous administration of H2 for 4 weeks (1 mL/week) in diabetic mice significantly decreased the serum levels of LDL and TG. In particular, we showed the high‐density lipoprotein content was increased by subcutaneous injection of H2, which was not previously reported. The results show that subcutaneous administration is a more effective method for H2 treatment.
Another important finding of the present study was that subcutaneous administration of H2 ameliorated hyperglycemia. Furthermore, the data of GTT and ITT showed that glucose homeostasis and insulin sensitivity in diabetic mice were also significantly improved by subcutaneous H2 treatment. However, these effects were not stably achieved through ingestion of H2 water. Haruka et al.10 reported that hyperglycemia attenuation from drinking H2 water was effective only for type 1, but not type 2, diabetic mice.
Oxidative stress, involved in the pathogenesis of various diseases23, 24, 25, 26, 27, 28, 29, is a process of imbalance between increased ROS and impaired anti‐oxidant defenses to induce cellular injury. Most of the superoxide anion radical () is produced by electron leakage from the electron transport chain and the Krebs cycle. SOD converts into hydrogen peroxide (H2O2), which is detoxified into H2O by either glutathione peroxidase or CAT. Excessive reduces transition metal ions, such as Fe3+ and Cu2+, which in turn react with H2O2 to produce hydroxyl radicals () by the Fenton reaction. , the strongest of the oxidant species without an especially targeted detoxification system, reacts easily with nucleic acids, lipids and proteins. Therefore, scavenging is a critical anti‐oxidant process. Oxidative stress is an important mechanism underlying diabetes mellitus24, 30, 31, which impacts millions of people worldwide. Oxidative stress can cause pancreatic β‐cell damage32, and induce insulin resistance in fat cells and liver cells33, 34, thus affecting glycolipid metabolism. As an anti‐oxidant gas, H2 can reduce oxidative damage in multiple tissues and organs. Previous studies have shown that H2 can improve diabetes and diabetes‐associated complications1, 35. Therefore, we believe that H2 improves glucose and lipid metabolism, likely through reducing oxidative damage in the liver, adipose tissue and pancreatic β‐cells.
H2 is a promising scavenger of reactive oxygen species, and shows remarkable protective effects in various diseases5, 7. Ohsawa et al 5 showed that H2 is a novel anti‐oxidative gas molecule to selectively address . In the present study, we examined oxidative stress‐related parameters including T‐SOD, CAT and MDA levels in plasma, and found that subcutaneous injection of H2 strikingly alleviated the oxidative stress of diabetes, which is consistent with previous studies35. Here, we also showed that subcutaneous injection of H2 improved diabetic nephropathy (DN) through anti‐oxidative stress. DN is one of the most common complications of diabetes. We examined multiple parameters to evaluate anti‐DN effects of subcutaneous injection of H2 including 24‐h urine volume, urinary total protein and urine β2‐microglobulin, which is a sensitive diagnostic feature for DN36. DN was successfully induced by the high‐fat diet and low‐dose STZ in the present study, and was strikingly improved by subcutaneous injection of H2. Fibrosis, a typical pathological change of DN37, observed in diabetic mice by Masson staining of kidney tissue sections, was consistently alleviated by subcutaneous injection of H2. Oxidative stress is a critical factor in diabetic kidney damages30, and H2 was previously reported to prevent chronic allograft nephropathy through anti‐oxidation properties38. The present study showed that DN was effectively inhibited by subcutaneous injection of H2.
In conclusion, the present study showed for the first time that subcutaneous injection of H2 confers beneficial effects on lipid and glucose metabolism, and DN in diabetic mice by providing protection against oxidative stress. These observations support the potential clinical application of this novel route of H2 administration for the treatment of type 2 diabetes mellitus.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We gratefully acknowledge Xiaoyi Qin and Youpei Wang (Department of Pathology, Eye Hospital of Wenzhou Medical University) for instruction in pathological examination. This work was supported by the Zhejiang Province Natural Science Foundation of China (NO. LY14H070005), Zhejiang Province Medical Science and Technology Project of China (NO. 2013C37005). The funders are not involved in study design, data collection and analysis, and manuscript preparation.
J Diabetes Investig 2018; 9: 83–90
[Correction added on 2 June 2017, after first online publication: The affiliation for the first author has been amended.]
References
- 1. Brem H, Tomic‐Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007; 117: 1219–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Death rates fall for 8 of 10 top causes; Alzheimer's up to no. 6. Hosp Health Netw 2008; 82: 127. [PubMed] [Google Scholar]
- 3. Mansor LS, Gonzalez ER, Cole MA, et al Cardiac metabolism in a new rat model of type 2 diabetes using high‐fat diet with low dose streptozotocin. Cardiovasc Diabetol 2013; 12: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. L'Heveder R, Nolan T. International Diabetes Federation. Diabetes Res Clin Pract 2013; 101: 349–351. [DOI] [PubMed] [Google Scholar]
- 5. Ohsawa I, Ishikawa M, Takahashi K, et al Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007; 13: 688–694. [DOI] [PubMed] [Google Scholar]
- 6. Wood KC, Gladwin MT. The hydrogen highway to reperfusion therapy. Nat Med 2007; 13: 673–674. [DOI] [PubMed] [Google Scholar]
- 7. Buchholz BM, Kaczorowski DJ, Sugimoto R, et al Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant 2008; 8: 2015–2024. [DOI] [PubMed] [Google Scholar]
- 8. Xie K, Yu Y, Pei Y, et al Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock 2010; 34: 90–97. [DOI] [PubMed] [Google Scholar]
- 9. Xie K, Yu Y, Huang Y, et al Molecular hydrogen ameliorates lipopolysaccharide‐induced acute lung injury in mice through reducing inflammation and apoptosis. Shock 2012; 37: 548–555. [DOI] [PubMed] [Google Scholar]
- 10. Amitani H, Asakawa A, Cheng K, et al Hydrogen improves glycemic control in type1 diabetic animal model by promoting glucose uptake into skeletal muscle. PLoS One 2013; 8: e53913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shirahata S, Hamasaki T, Haramaki K, et al Anti‐diabetes effect of water containing hydrogen molecule and Pt nanoparticles. BMC Proc 2011; 5(Suppl 8): P18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamimura N, Nishimaki K, Ohsawa I, et al Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity (Silver Spring) 2011; 19: 1396–1403. [DOI] [PubMed] [Google Scholar]
- 13. Fukuda K, Asoh S, Ishikawa M, et al Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun 2007; 361: 670–674. [DOI] [PubMed] [Google Scholar]
- 14. Xiang L, Tan JW, Huang LJ, et al Inhalation of hydrogen gas reduces liver injury during major hepatotectomy in swine. World J Gastroenterol 2012; 18: 5197–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kajiya M, Sato K, Silva MJ, et al Hydrogen from intestinal bacteria is protective for Concanavalin A‐induced hepatitis. Biochem Biophys Res Commun 2009; 386: 316–321. [DOI] [PubMed] [Google Scholar]
- 16. Xue J, Ding W, Liu Y. Anti‐diabetic effects of emodin involved in the activation of PPARgamma on high‐fat diet‐fed and low dose of streptozotocin‐induced diabetic mice. Fitoterapia 2010; 81: 173–177. [DOI] [PubMed] [Google Scholar]
- 17. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–358. [DOI] [PubMed] [Google Scholar]
- 18. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972; 247: 3170–3175. [PubMed] [Google Scholar]
- 19. Aebi H. Catalase in vitro . Methods Enzymol 1984; 105: 121–126. [DOI] [PubMed] [Google Scholar]
- 20. Kajiyama S, Hasegawa G, Asano M, et al Supplementation of hydrogen‐rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res 2008; 28: 137–143. [DOI] [PubMed] [Google Scholar]
- 21. Nakao A, Toyoda Y, Sharma P, et al Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome‐an open label pilot study. J Clin Biochem Nutr 2010; 46: 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eo H, Lee HJ, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper‐inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophys Res Commun 2016; 478: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 23. Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 1997; 272: 20313–20316. [DOI] [PubMed] [Google Scholar]
- 24. Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 1993; 49: 642–652. [DOI] [PubMed] [Google Scholar]
- 25. Dong K, Ni H, Wu M, et al ROS‐mediated glucose metabolic reprogram induces insulin resistance in type 2 diabetes. Biochem Biophys Res Commun 2016; 476: 204–211. [DOI] [PubMed] [Google Scholar]
- 26. Araki E, Nishikawa T. Oxidative stress: a cause and therapeutic target of diabetic complications. J Diabetes Investig 2010; 1: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. J Diabetes Investig 2014; 5: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siewert S, Gonzalez II, Lucero RO, et al Association of cholesteryl ester transfer protein genotypes with paraoxonase‐1 activity, lipid profile and oxidative stress in type 2 diabetes mellitus: a study in San Luis. Argentina. J Diabetes Investig 2015; 6: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsu YC, Lee PH, Lei CC, et al Nitric oxide donors rescue diabetic nephropathy through oxidative‐stress‐and nitrosative‐stress‐mediated Wnt signaling pathways. J Diabetes Investig 2015; 6: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991; 40: 405–412. [DOI] [PubMed] [Google Scholar]
- 31. West IC. Radicals and oxidative stress in diabetes. Diabet Med 2000; 17: 171–180. [DOI] [PubMed] [Google Scholar]
- 32. Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in beta‐cell dysfunction in diabetes. J Mol Endocrinol 2016; 56: R33–R54. [DOI] [PubMed] [Google Scholar]
- 33. Manna P, Jain SK. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: causes and Therapeutic Strategies. Metab Syndr Relat Disord 2015; 13: 423–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 2015; 6: 456–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fan M, Xu X, He X, et al Protective effects of hydrogen‐rich saline against erectile dysfunction in a streptozotocin induced diabetic rat model. J Urol 2013; 190: 350–356. [DOI] [PubMed] [Google Scholar]
- 36. Viberti GC, Hill RD, Jarrett RJ, et al Microalbuminuria as a predictor of clinical nephropathy in insulin‐dependent diabetes mellitus. Lancet 1982; 1: 1430–1432. [DOI] [PubMed] [Google Scholar]
- 37. Sugimoto H, Grahovac G, Zeisberg M, et al Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein‐7 and advanced glycation end product inhibitors. Diabetes 2007; 56: 1825–1833. [DOI] [PubMed] [Google Scholar]
- 38. Cardinal JS, Zhan J, Wang Y, et al Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int 2010; 77: 101–109. [DOI] [PubMed] [Google Scholar]


