Abstract
Aims/Introduction
A soluble form of the leptin receptor (soluble Ob‐R) in the circulation regulates leptin's bioactivity, and is inversely associated with body adiposity and circulating leptin levels. However, no study has examined the clinical impact of soluble Ob‐R on glucose metabolism in diabetes. The present study aimed to investigate the association of plasma soluble Ob‐R levels with insulin resistance and pancreatic β‐cell function in patients with type 2 diabetes.
Materials and Methods
A total of 289 Japanese patients with type 2 diabetes were included in the present study. Fasting plasma soluble Ob‐R levels and plasma leptin levels were measured by enzyme‐linked immunosorbent assay. Insulin resistance and pancreatic β‐cell function were estimated by homeostasis model assessment of insulin resistance, homeostasis model assessment of β‐cell function and fasting C‐peptide index.
Results
The median plasma soluble Ob‐R level and plasma leptin level were 3.4 ng/mL and 23.6 ng/mL, respectively. Plasma soluble Ob‐R levels were negatively correlated with homeostasis model assessment of insulin resistance, homeostasis model assessment of β‐cell function and the C‐peptide index, whereas plasma leptin levels were positively correlated with each index in univariate analyses. Multivariate analyses including plasma soluble Ob‐R levels, plasma leptin levels and use of sulfonylureas, along with age, sex, body mass index and other covariates, showed that soluble Ob‐R levels were independently and negatively associated with homeostasis model assessment of β‐cell function and the C‐peptide index, but not significantly associated with homeostasis model assessment of insulin resistance.
Conclusions
Plasma soluble Ob‐R levels are independently associated with pancreatic β‐cell function, but not with insulin resistance, in patients with type 2 diabetes. The present study implicates the role of soluble Ob‐R in pancreatic β‐cell dysfunction in type 2 diabetes.
Keywords: β‐Cell function, Leptin, Soluble leptin receptor
Introduction
Leptin is produced by adipose tissue, and plays a pivotal role in the regulation of bodyweight by suppressing food intake and stimulating energy expenditure1, 2. Several lines of evidence indicate that leptin also plays a primary role in the regulation of glucose metabolism, independent of its actions on bodyweight control3. Leptin receptors are widely expressed in both the central nervous system and peripheral tissues, including the endocrine pancreas, liver, skeletal muscle and adipose tissue2. Thus, neurally‐mediated pathways4 and direct action of leptin on peripheral tissues contribute to the regulation of glucose homeostasis3.
Although the long form of the leptin receptor (Ob‐R), or Ob‐Rb, is essential in mediating leptin's weight‐reducing effects through the hypothalamus, other Ob‐R isoforms are also involved in mediating leptin action in vivo 2, 5. Ob‐Re is a soluble isoform that lacks a transmembrane domain, and is responsible for most leptin‐binding activity in the circulation6, 7. Specifically, in vitro research showed that soluble Ob‐R suppresses leptin‐stimulated Ob‐Rb signal transduction8, 9, and several experimental studies showed that soluble Ob‐R delays leptin clearance and stabilizes leptin in the circulation to regulate its bioavailability and physiological activity in vivo 8, 9, 10. Prior studies in humans consistently showed that the majority of leptin circulates in the receptor‐bound form in lean individuals, whereas the majority of leptin circulates in free form in obese individuals7, 11, 12. Furthermore, a number of clinical studies showed that circulating soluble Ob‐R levels were lower in obese individuals than that in lean individuals, and were inversely correlated with circulating leptin levels, the degree of obesity13, 14, 15, insulin resistance and other metabolic risk factors13, 14, 16, 17.
However, there are few studies on the association between soluble Ob‐R and abnormal glucose metabolism in relation to diabetes in humans13, 14, 18, 19. In prospective studies, baseline soluble Ob‐R levels predicted a low risk of type 2 diabetes in non‐diabetic women19, and negatively predicted fasting glucose and body adiposity in healthy young men13. In cross‐sectional studies, soluble Ob‐R levels were negatively correlated with insulin resistance index in Japanese patients with type 2 diabetes14, whereas they were positively correlated with the presence of diabetes, but not with fasting glucose, insulin levels or insulin resistance index, in morbidly obese Americans requiring bariatric surgery18. To date, it remains unclear whether soluble Ob‐R is implicated in the pathogenesis of type 2 diabetes, as no study has assessed the impact of soluble Ob‐R levels on both insulin resistance and β‐cell dysfunction in humans.
The present study aimed to explore whether soluble Ob‐R is involved in abnormal glucose metabolism, namely insulin resistance and β‐cell dysfunction, in patients with type 2 diabetes. For this purpose, we measured fasting plasma levels of soluble Ob‐R and leptin, estimated insulin resistance by homeostasis model assessment of insulin resistance (HOMA‐IR)20 and β‐cell function by HOMA of β‐cell function (HOMA‐β)20, 21, and calculated the C‐peptide index from fasting C‐peptide and fasting glucose22, 23. We then assessed the cross‐sectional associations between plasma soluble Ob‐R levels or plasma leptin levels and HOMA‐IR, HOMA‐β, and the C‐peptide index in patients with type 2 diabetes.
Methods
Study population
We enrolled 289 participants with type 2 diabetes (173 men and 116 women) who were admitted to the Diabetes Center of the Osaka City University Hospital, Osaka, Japan, for the purposes of glycemic control, education and/or evaluation of diabetic complications, between March 2005 and August 2012. Type 2 diabetes was diagnosed based on the American Diabetes Association criteria24. Patients who met the following criteria were not included in the present study: those with type 1 diabetes or other types of diabetes, those receiving insulin therapy or those with renal impairment (defined by our laboratory as serum creatinine ≥1.1 mg/dL). The patients whose fasting glucose levels were extremely low (<4 mmol/L, n = 2) or high (≥13 mmol/L, n = 4) were excluded from analyses, according to the original report on HOMA index by Matthews et al.20 The remaining 283 participants were subjected to analyses.
This study was carried out in accordance with the Declaration of Helsinki (1975, as revised in 2013). The study protocol was approved by the ethics committee of our institution (no. 164). All study participants provided written informed consent.
Assay of soluble leptin receptor levels and leptin levels in plasma
Plasma soluble Ob‐R levels and leptin levels were measured with commercial enzyme‐linked immunosorbent assay kits (R&D Systems, Inc., Minneapolis, MN, USA), as described previously25. The minimum detectable levels of soluble Ob‐R and leptin were 0.057 ng/mL and 0.16 ng/mL, respectively. The intra‐ and interassay coefficients of variation were 3.5 and 6.7%, respectively, for soluble Ob‐R, and 3.2 and 4.9%, respectively, for leptin13, 25, 26.
Laboratory measurements
Blood was drawn after an overnight fast, and biochemical parameters were analyzed by means of a standard laboratory method as previously described25, 26. Glycated hemoglobin A1c (HbA1c) levels were estimated as National Glycohemoglobin Standardization Program equivalent values (%) by using the conversion formula established by the Japan Diabetes Society27. Serum immunoreactive insulin (IRI) levels and serum C‐peptide levels were measured by electrochemiluminescence immunoassay (Roche Diagnostics K.K., Tokyo, Japan). HOMA‐IR and HOMA‐β were calculated according to the following formulae: fasting IRI (μU/mL) × fasting glucose (mg/dL) / 405 and 360 × fasting IRI (μU/mL) / (fasting glucose [mg/dL] − 63) (%), respectively20. The C‐peptide index was calculated as follows: fasting C‐peptide (ng/mL) / fasting glucose (mg/dL) × 10028.
Statistical analysis
Data are expressed as number (%), mean ± standard deviation or median (interquartile range), as appropriate. Correlations were examined by using simple linear regression analyses. Skewed parameters, such as IRI, triglycerides, plasma soluble Ob‐R levels and plasma leptin levels, were logarithmically transformed before regression analyses. Factors associated with plasma leptin level, plasma soluble Ob‐R level, HOMA‐IR, HOMA‐β or C‐peptide index were analyzed using multiple regression analyses after adjusting for potential confounders. A P‐value of <0.05 was considered significant. Statistical analyses were carried out using the JMP version 10 software (SAS Institute Inc., Cary, NC, USA).
Results
Clinical characteristics, plasma leptin levels and plasma soluble Ob‐R levels of the participants
The clinical characteristics of the total study population are shown in Table 1. The mean age, median duration of diabetes and mean body mass index (BMI) were 61 years, 5 years and 25.4 kg/m2, respectively. A total of 88 participants (31%) were treated with dietary therapy alone, 125 (44%) with sulfonylureas, 76 (27%) with biguanides, 47 (17%) with α‐glucosidase inhibitors, 31 (11%) with thiazolidinediones, 23 (8%) with dipeptidyl peptidase‐4 inhibitors, 13 (5%) with meglitinides and six (2%) with glucagon‐like peptide‐1 analogues. A total of 99 participants (35%) were treated with statins for dyslipidemia, and 97 (34%) participants were treated with renin–angiotensin system inhibitors for hypertension.
Table 1.
Clinical characteristics, plasma leptin levels and plasma soluble leptin receptor levels in participants with type 2 diabetes
| All participants | |
|---|---|
| n, (male %) | 283 (60.1) |
| Age (years) | 61 ± 12 |
| Duration of diabetes (years) | 5 (2–12) |
| BMI (kg/m2) | 25.4 ± 4.7 |
| Systolic blood pressure (mmHg) | 128 ± 19 |
| Diastolic blood pressure (mmHg) | 75 ± 10 |
| Antihyperglycemic agents, n (%) | |
| None | 88 (31) |
| Sulfonylureas | 125 (44) |
| Biguanides | 76 (27) |
| α‐Glucosidase inhibitors | 47 (17) |
| Thiazolidinediones | 31 (11) |
| Dipeptidyl peptidase‐4 inhibitors | 23 (8) |
| Meglitinides | 13 (5) |
| Glucagon‐like peptide 1 analogues | 6 (2) |
| Serum creatinine (mg/dL) | 0.73 ± 0.16 |
| Fasting glucose (mg/dL) | 128 ± 29 |
| HbA1c (%) | 8.1 ± 1.5 |
| IRI (μU/mL) | 6.4 (4.4–9.1) |
| C‐peptide (ng/mL) | 1.9 ± 0.8 |
| HOMA‐IR | 2.0 (1.3–2.7) |
| HOMA‐β (%) | 38.1 (24.0–64.8) |
| C‐peptide index | 1.6 ± 0.7 |
| Uric acid (mg/dL) | 5.7 ± 1.4 |
| Triglycerides (mg/dL) | 119 (94–157) |
| HDL cholesterol (mg/dL) | 44 ± 11 |
| LDL cholesterol (mg/dL) | 117 ± 34 |
| Leptin (ng/mL) | 3.4 (1.9–6.5) |
| Soluble Ob‐R (ng/mL) | 23.6 (19.7–28.3) |
Data are expressed as n (%), mean ± standard deviation or median (interquartile range) as appropriate. BMI, body mass index; HbA1c, glycated hemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; IRI, immunoreactive insulin; LDL, low‐density lipoprotein; Ob‐R, leptin receptor.
The median of HOMA‐IR and HOMA‐β indices were 2.0 (range 0.27–33.0) and 38.1% (range 4.5–380%), respectively. The mean C‐peptide index was 1.6 (range 0.4–4.9). The median plasma leptin level and plasma soluble Ob‐R level were 3.4 ng/mL (range 0.2–32.8 ng/mL) and 23.6 ng/mL (range 10.2–47.1 ng/mL), respectively.
Factors associated with plasma leptin levels or plasma soluble Ob‐R levels
We first examined the factors associated with plasma leptin levels or plasma soluble Ob‐R levels by multiple regression analyses after adjusting for age, sex, BMI, systolic blood pressure, HbA1c, IRI, serum creatinine, triglycerides, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein cholesterol, and plasma soluble Ob‐R levels or plasma leptin levels (Table 2). As expected, plasma leptin levels were positively associated with female sex, BMI, IRI and triglycerides levels, whereas plasma soluble Ob‐R levels were negatively associated with BMI, and positively associated with HDL cholesterol. The results also showed statistically significant correlations between plasma soluble Ob‐R levels and systolic blood pressure, triglycerides, and low‐density lipoprotein cholesterol, unlikely implying any causal relationships between them. Although an inverse correlation was found between plasma soluble leptin receptor levels and plasma leptin levels in an unadjusted analysis (r = −0.293, P < 0.001), the association was not significant after adjustment for multiple factors (Table 2). Notably, unlike plasma leptin levels, plasma soluble Ob‐R levels were positively associated with HbA1c.
Table 2.
Multivariate analyses for the factors associated with plasma leptin levels or plasma soluble leptin receptor levels in participants with type 2 diabetes
| Log (leptin) | Log (soluble Ob‐R) | |||
|---|---|---|---|---|
| β | P | β | P | |
| Age (years) | 0.038 | 0.308 | −0.093 | 0.114 |
| Sex (male = 1, female = 0) | −0.506 | <0.001 | −0.030 | 0.705 |
| BMI (kg/m2) | 0.360 | <0.001 | −0.193 | 0.009 |
| Systolic blood pressure (mmHg) | 0.006 | 0.844 | 0.126 | 0.011 |
| HbA1c (%) | −0.047 | 0.132 | 0.158 | 0.001 |
| Log, IRI (μU/mL) | 0.375 | <0.001 | −0.118 | 0.098 |
| Serum creatinine (mg/dL) | 0.030 | 0.446 | −0.128 | 0.040 |
| Log, triglycerides (mg/dL) | 0.098 | 0.005 | 0.171 | 0.002 |
| HDL cholesterol (mg/dL) | −0.025 | 0.505 | 0.373 | <0.001 |
| LDL cholesterol (mg/dL) | 0.025 | 0.439 | 0.154 | 0.003 |
| Log, soluble Ob‐R (ng/mL) | −0.058 | 0.129 | – | – |
| Log, leptin (ng/mL) | – | – | −0.148 | 0.129 |
| R 2 | 0.759 | <0.001 | 0.382 | <0.001 |
β, standardized regression coefficients determined by multiple regression analysis; BMI, body mass index; HbA1c, glycated hemoglobin A1c; HDL, high‐density lipoprotein; IRI, immunoreactive insulin; LDL, low‐density lipoprotein; Ob‐R, leptin receptor; R 2, coefficients of determination.
Correlations between plasma leptin levels or plasma soluble Ob‐R levels and the index of insulin resistance or β‐cell function
We next examined the correlations between plasma leptin levels or plasma soluble‐Ob‐R levels and the indices of insulin resistance and β‐cell function by simple regression analysis (Figure 1). Plasma leptin levels were strongly and positively correlated with HOMA‐IR, HOMA‐β and the C‐peptide index (Figure 1a,c,e). In contrast, plasma soluble Ob‐R levels were strongly and negatively correlated with HOMA‐β and the C‐peptide index (Figure 1d,f), but were only weakly correlated with HOMA‐IR (Figure 1b).
Figure 1.
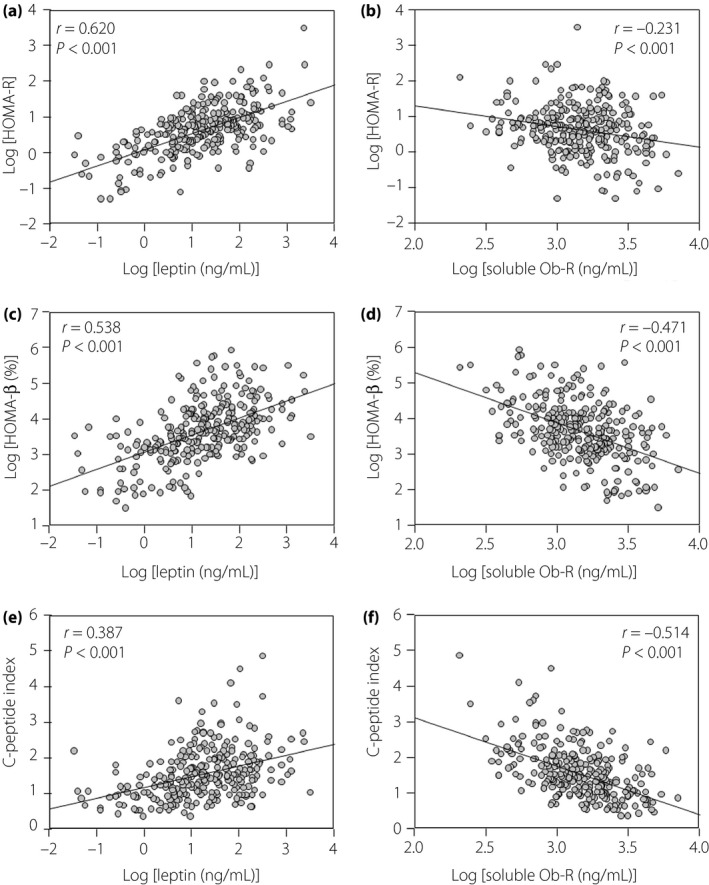
Association of plasma levels of (a,c,e) leptin or (b,d,f) soluble leptin receptor (Ob‐R) with (a,b) homeostasis model assessment of insulin resistance (HOMA‐IR), (c, d) homeostasis model assessment of β‐cell function (HOMA‐β) and (e,f) the C‐peptide index in participants with type 2 diabetes. HOMA‐IR, HOMA‐β, plasma leptin levels and plasma soluble Ob‐R levels are logarithmically transformed. r, Correlation coefficient by simple regression analysis.
Multivariate analyses of the determinants for the index of insulin resistance or β‐cell function
Finally, to explore the impact of plasma leptin levels or plasma soluble Ob‐R levels on HOMA‐IR, HOMA‐β and the C‐peptide index, we carried out multiple regression analyses. We included plasma leptin levels and soluble Ob‐R levels, along with age, sex, BMI, systolic blood pressure, HbA1c, serum creatinine, triglycerides, HDL cholesterol, low‐density lipoprotein cholesterol and use of sulfonylureas in our models (Table 3). Plasma leptin levels were independently and positively associated with each HOMA‐IR, HOMA‐β and C‐peptide index. In contrast, plasma soluble Ob‐R levels were independently and negatively associated with HOMA‐β and the C‐peptide index, but not independently associated with HOMA‐IR. The presence of treatment with sulfonylureas did not significantly affect HOMA‐IR, HOMA‐β or the C‐peptide index (Table 3).
Table 3.
Associations of plasma leptin levels or soluble leptin receptor levels with the indices of insulin resistance and β‐cell function in participants with type 2 diabetes
| Log (HOMA‐IR) | Log (HOMA‐β) | C‐peptide index | ||||
|---|---|---|---|---|---|---|
| β | P | β | P | β | P | |
| Log, leptin (ng/mL) | 0.680 | <0.001 | 0.488 | <0.001 | 0.356 | <0.001 |
| Log, soluble Ob‐R (ng/mL) | −0.005 | 0.931 | −0.200 | <0.001 | −0.344 | <0.001 |
| Sulfonylureas (yes = 1, no = 0) | 0.006 | 0.899 | −0.029 | 0.546 | −0.048 | 0.360 |
For all participants, log leptin (ng/mL) and log soluble Ob‐R (ng/mL) were set as independent variables along with age (years), sex (male = 1, female = 0), body mass index (kg/m2), systolic blood pressure (mmHg), glycated hemoglobin A1c (%), serum creatinine (mg/dL), log triglycerides (mg/dL), high‐density lipoprotein cholesterol (mg/dL), low‐density lipoprotein cholesterol (mg/dL) and use of sulfonylureas (yes = 1, no = 0). β, standard regression coefficient determined by multiple regression analysis; HOMA‐β, homeostasis model assessment of β‐cell function; HOMA‐IR, homeostasis model assessment of insulin resistance; Ob‐R, leptin receptor.
Additionally, the impact of plasma soluble Ob‐R levels on HOMA‐β and the C‐peptide index was analyzed by stratifying the participants according to the use of sulfonylureas after adjustment for age, sex, BMI, HbA1c, serum creatinine, triglycerides, HDL cholesterol and plasma leptin levels. The results showed that plasma soluble Ob‐R levels were independently and negatively associated with HOMA‐β (users, β = −0.222, P = 0.009; non‐users, β = −0.187, P = 0.011) and C‐peptide index (users, β = −0.408, P < 0.001; non‐users, β = −0.267, P = 0.002) in both users and non‐users of sulfonylureas.
Discussion
In the present study, we measured plasma soluble Ob‐R levels and investigated the impact of soluble Ob‐R on glucose metabolism, namely insulin sensitivity and β‐cell function, in patients with type 2 diabetes. The results of the present study showed that plasma soluble Ob‐R levels were associated with decreased β‐cell function, as estimated by HOMA‐β and the C‐peptide index. The results further revealed that the association between soluble Ob‐R levels and β‐cell function was independent of plasma leptin level, which is an independent determinant of HOMA‐IR, HOMA‐β and the C‐peptide index.
The present study clearly demonstrated that plasma soluble Ob‐R levels were associated with decreased HOMA‐β and C‐peptide index in patients with type 2 diabetes. Importantly, these associations were independent of other parameters, including BMI and plasma leptin levels, indicating a close link between soluble Ob‐R and β‐cell dysfunction. The present results are in contrast to those of the prospective cohort studies that showed an inverse relationship between soluble Ob‐R levels and a risk of type 2 diabetes19 or fasting glucose levels13. In non‐diabetic American women, baseline plasma soluble Ob‐R levels, but not plasma leptin levels, were associated with a decreased risk of developing type 2 diabetes, independent of BMI and adiponectin levels19. In another study carried out in young healthy men, baseline soluble Ob‐R levels negatively predicted fasting glucose, adiposity and metabolic syndrome score at 2‐year follow up after adjustment for age, smoking and waist‐to‐hip ratio13. There could be a couple of explanations for the discrepancy between the previous studies and our current study. Most importantly, our study did not evaluate a longitudinal effect of soluble Ob‐R on β‐cell function in diabetes patients; therefore, our results cannot be simply compared with those from the prior prospective studies. Second, participants in the study by Sun et al.19 were American women with obesity and insulin resistance that were much greater than those of our Japanese participants. As soluble Ob‐R levels are inversely related to insulin resistance13, 14, 16, 17, it is possible that the beneficial effect of soluble Ob‐R on insulin sensitivity was greater than its negative effect on β‐cell function, thereby a favorable relationship was found between soluble Ob‐R and the risk of diabetes19. Third, participants in the prior studies were non‐diabetic women19 or young healthy men13, whereas our participants were type 2 diabetes patients with poor glycemic control and presumably diminished β‐cell function. Therefore, the impact of soluble Ob‐R levels on β‐cell function could be different according to the degree of β‐cell function or diabetic status of the study population.
Besides the longitudinal studies, there was one cross‐sectional study18 carried out in morbidly obese Americans that showed a positive correlation between soluble Ob‐R levels and the presence of type 2 diabetes. However, the link between soluble Ob‐R and insulin resistance or β‐cell dysfunction was unclear, because significant correlations between soluble Ob‐R levels and fasting glucose, insulin or HOMA‐IR were not found in that study18. As none of the abovementioned previous studies evaluated insulin secretory function in the participants13, 18, 19, the present study is the first to focus on the association of soluble Ob‐R levels with pancreatic β‐cell function in patients with type 2 diabetes.
There are a few possible explanations for the negative impact of plasma soluble Ob‐R levels on β‐cell function observed in our participants with type 2 diabetes. First, the plasma soluble Ob‐R level reflects the cell surface expression of Ob‐R29, and might indicate whole‐body leptin sensitivity30. Evidence from experimental studies shows that circulating soluble Ob‐R is generated by ectodomain shedding of membrane‐anchored receptors, and regulates the bioactivity and circulating levels of leptin in vivo 29, 31, 32. The clinical significance of soluble Ob‐R levels as a marker of leptin sensitivity has been further supported by several studies showing that circulating soluble Ob‐R levels are inversely correlated with bodyweight and adiposity15, and increased after weight loss by a low‐calorie diet15 or gastric banding11. Second, several lines of evidence from experimental studies show that leptin inhibits insulin secretion and biosynthesis through its direct action on pancreatic β‐cells3, 33. The inhibitory effect of leptin on β‐cell insulin secretion was suggested by several animal studies utilizing acute disruption of leptin signaling in wild‐type mice through leptin antagonists34 or genetic disruption of Ob‐Rb signaling selectively in β‐cells35, 36. In these studies, mice with disrupted leptin action exhibited fasting hyperinsulinemia with hypoglycemia35, 36, 37 and/or enhanced glucose‐induced insulin secretion34, 36, 38 compared with the control mice. Although a direct effect of leptin on β‐cell insulin secretory function remains to be clarified in human subjects, it is speculated that individuals with abundant plasma soluble Ob‐R levels are sensitive to leptin action, not only for reducing bodyweight/adiposity, but also for limiting insulin secretion from pancreatic β‐cells. The notion that soluble Ob‐R is related to β‐cell dysfunction is supported by our finding that plasma soluble Ob‐R levels were independently associated with elevated HbA1c levels in patients with type 2 diabetes (Table 2).
Unlike the indices of β‐cell function, plasma soluble Ob‐R levels were not significantly associated with HOMA‐IR after adjustment for age, sex, BMI, plasma leptin levels and other covariates. The present results are not in agreement with previous studies showing a negative correlation between soluble Ob‐R levels and HOMA‐IR, independent of age, and BMI, in non‐diabetic healthy subjects14, 17 and type 2 diabetic men14. The diabetic patients in the present study had poor glycemic control (mean HbA1c 8.1%), and therefore possibly had impaired insulin secretory function compared with those in the prior studies who were non‐diabetic14, 17 or diabetic with better glycemic control than the patients in the present study (mean HbA1c 6.9%)14. Therefore, it can be speculated that the association of soluble Ob‐R with insulin resistance was complicated by the advanced β‐cell dysfunction in our participants with diabetes.
Consistent with earlier studies39, 40, 41, the present study showed that plasma leptin levels were positively associated with all the indices of insulin resistance and β‐cell function; that is, HOMA‐IR, HOMA‐β and the C‐peptide index, independent of other covariates in patients with type 2 diabetes. These findings suggest an association of plasma leptin levels with insulin resistance and hyperinsulinemia, independent of obesity, in our participants with type 2 diabetes. Of importance, the association of soluble Ob‐R with β‐cell dysfunction was independent of plasma leptin levels in the present study. A prior study also found an association between plasma soluble Ob‐R levels and a risk of developing type 2 diabetes, irrespective of plasma leptin levels19. Although a detailed mechanism remains unclear, these findings, including ours, suggest that soluble Ob‐R levels affect pancreatic β‐cell function, and leptin levels affect insulin sensitivity through independent mechanisms, which impact glucose tolerance in humans.
In the present study, we estimated pancreatic β‐cell function using HOMA‐β and the C‐peptide index based on a single measurement of fasting glucose, IRI, and C‐peptide. One limitation of the present study was that we were unable to measure β‐cell function by more accurate methods, such as glucose or meal challenge tests, intravenous glucose tolerance tests or hyperglycemic clamps. However, HOMA‐β is established as a surrogate estimate of pancreatic β‐cell function that is well correlated with the estimates derived from the hyperglycemic clamp and intravenous glucose tolerance test20. Furthermore, HOMA‐β has been widely used in longitudinal studies showing progressive loss of insulin secretary function over the natural history of type 2 diabetes21. Several studies showed the clinical utility of the HOMA‐β index even in diabetes patients with fasting hyperglycemia (≥8–9 mmol/L)42, 43, 44 and/or receiving sulfonylureas43, in which HOMA‐β was significantly increased along with the improvement of glycemic control, postprandial insulin secretion44 and hyperglycemic clamp‐measures of β‐cell function42 after administration of antihyperglycemic agents. In addition to HOMA‐β, we utilized the C‐peptide index as an estimate of β‐cell function in the present study. As C‐peptide is not extracted by the liver and its half‐life in blood is longer than that of insulin, the C‐peptide level is believed to reflect endogenous insulin secretion more accurately than the insulin level28. The clinical utility of fasting C‐peptide divided by glucose (C‐peptide index) as a predictor of future requirement for insulin therapy has been recently shown in Japanese patients with type 2 diabetes22, 23. The present study confirmed the impact of soluble Ob‐R levels on β‐cell dysfunction by two different indices in a relatively large number of subjects with type 2 diabetes, and provides clinical evidence of the relationship between soluble Ob‐R and pancreatic β‐cell function in type 2 diabetes.
The present study had several other limitations. First, this was a cross‐sectional study; therefore, a causal relationship remains to be clarified between soluble Ob‐R levels and β‐cell function. Second, our participants were treated with various kinds of antihyperglycemic agents that might modulate insulinemia or insulin secretory function. However, data from the multivariate analyses showed that the association of soluble Ob‐R with HOMA‐β and the C‐peptide index were independent of the use of sulfonylureas. The stratified analysis further showed the inverse correlation between soluble Ob‐R and HOMA‐β and the C‐peptide index in both users and non‐users of sulfonylureas, indicating that the use of sulfonylureas does not virtually affect the association between soluble Ob‐R and the indices of β‐cell function. Third, the present participants were inpatients in a university hospital with poor glycemic control. Furthermore, patients receiving insulin therapy and those with renal impairment were not included in the present study. Therefore, the results from the present study cannot be generalized to the entire population of type 2 diabetes patients.
In conclusion, the present study shows that plasma soluble Ob‐R levels are independently and negatively associated with HOMA‐β and C‐peptide index in patients with type 2 diabetes. Our data suggest that soluble Ob‐R is one of the factors affecting the insulin secretary function of pancreatic β‐cells, independent of obesity and circulating leptin levels. To validate our findings, further studies should examine the effect of soluble Ob‐R levels on pancreatic insulin secretory function using estimates of β‐cell function other than HOMA‐β or the C‐peptide index. In addition, longitudinal studies are warranted to clarify whether soluble Ob‐R levels are predictive of future declines in β‐cell function; that is, initiation of insulin therapy or deterioration of glycemic control, in patients with type 2 diabetes.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors acknowledge the excellent technical assistance of Ms Setsuko Arita and Ms Mika Sakaki from the research laboratory at the Department of Metabolism, Endocrinology and Molecular Medicine, Osaka City University Graduate School of Medicine. This study was supported by a Grant‐in‐Aid for Scientific Research (no. 20591068) from the Japan Society for the Promotion of Science awarded to Masanori Emoto and Katsuhito Mori.
J Diabetes Investig 2018; 9: 55–62
References
- 1. Ahima RS, Flier JS. Leptin. Annu Rev Physiol 2000; 62: 413–437. [DOI] [PubMed] [Google Scholar]
- 2. Myers MG Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 2004; 59: 287–304. [DOI] [PubMed] [Google Scholar]
- 3. Denroche HC, Huynh FK, Kieffer TJ. The role of leptin in glucose homeostasis. J Diabetes Investig 2012; 3: 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev 2011; 91: 389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998; 395: 763–770. [DOI] [PubMed] [Google Scholar]
- 6. Lammert A, Kiess W, Bottner A, et al Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun 2001; 283: 982–988. [DOI] [PubMed] [Google Scholar]
- 7. Sinha MK, Opentanova I, Ohannesian JP, et al Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short‐term fasting. J Clin Invest 1996; 98: 1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang G, Ge H, Boucher A, et al Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol 2004; 18: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 9. Zastrow O, Seidel B, Kiess W, et al The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord 2003; 27: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 10. Lou PH, Yang G, Huang L, et al Reduced body weight and increased energy expenditure in transgenic mice over‐expressing soluble leptin receptor. PLoS ONE 2010; 5: e11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laimer M, Ebenbichler CF, Kaser S, et al Weight loss increases soluble leptin receptor levels and the soluble receptor bound fraction of leptin. Obes Res 2002; 10: 597–601. [DOI] [PubMed] [Google Scholar]
- 12. van Dielen FM, van 't Veer C, Buurman WA, et al Leptin and soluble leptin receptor levels in obese and weight‐losing individuals. J Clin Endocrinol Metab 2002; 87: 1708–1716. [DOI] [PubMed] [Google Scholar]
- 13. Hamnvik OP, Liu X, Petrou M, et al Soluble leptin receptor and leptin are associated with baseline adiposity and metabolic risk factors, and predict adiposity, metabolic syndrome, and glucose levels at 2‐year follow‐up: the Cyprus Metabolism Prospective Cohort Study. Metabolism 2011; 60: 987–993. [DOI] [PubMed] [Google Scholar]
- 14. Ogawa T, Hirose H, Yamamoto Y, et al Relationships between serum soluble leptin receptor level and serum leptin and adiponectin levels, insulin resistance index, lipid profile, and leptin receptor gene polymorphisms in the Japanese population. Metabolism 2004; 53: 879–885. [DOI] [PubMed] [Google Scholar]
- 15. Ogier V, Ziegler O, Mejean L, et al Obesity is associated with decreasing levels of the circulating soluble leptin receptor in humans. Int J Obes Relat Metab Disord 2002; 26: 496–503. [DOI] [PubMed] [Google Scholar]
- 16. Ingelsson E, Larson MG, Yin X, et al Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community‐based sample. J Clin Endocrinol Metab 2008; 93: 3149–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sandhofer A, Laimer M, Ebenbichler CF, et al Soluble leptin receptor and soluble receptor‐bound fraction of leptin in the metabolic syndrome. Obes Res 2003; 11: 760–768. [DOI] [PubMed] [Google Scholar]
- 18. Medici V, Ali MR, Seo S, et al Increased soluble leptin receptor levels in morbidly obese patients with insulin resistance and nonalcoholic fatty liver disease. Obesity 2010; 18: 2268–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun Q, van Dam RM, Meigs JB, et al Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in U.S. women: a prospective study. Diabetes 2010; 59: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 21. Reaven GM. HOMA‐beta in the UKPDS and ADOPT. Is the natural history of type 2 diabetes characterised by a progressive and inexorable loss of insulin secretory function? Maybe? Maybe not? Diab Vasc Dis Res 2009; 6: 133–138. [DOI] [PubMed] [Google Scholar]
- 22. Funakoshi S, Fujimoto S, Hamasaki A, et al Utility of indices using C‐peptide levels for indication of insulin therapy to achieve good glycemic control in Japanese patients with type 2 diabetes. J Diabetes Investig 2011; 2: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goto A, Takaichi M, Kishimoto M, et al Body mass index, fasting plasma glucose levels, and C‐peptide levels as predictors of the future insulin use in Japanese type 2 diabetic patients. Endocr J 2010; 57: 237–244. [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes Association . Standards of medical care in diabetes–2014. Diabetes Care 2014; 37(Suppl 1): S14–S80. [DOI] [PubMed] [Google Scholar]
- 25. Yamazaki Y, Emoto M, Morioka T, et al Clinical impact of the leptin to soluble leptin receptor ratio on subclinical carotid atherosclerosis in patients with type 2 diabetes. J Atheroscler Thromb 2013; 20: 186–194. [DOI] [PubMed] [Google Scholar]
- 26. Morioka T, Emoto M, Yamazaki Y, et al Leptin is associated with vascular endothelial function in overweight patients with type 2 diabetes. Cardiovasc Diabetol 2014; 13: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus , Seino Y, Nanjo K, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saisho Y. Postprandial C‐peptide to glucose ratio as a marker of beta cell function: implication for the management of type 2 diabetes. Int J Mol Sci 2016; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maamra M, Bidlingmaier M, Postel‐Vinay MC, et al Generation of human soluble leptin receptor by proteolytic cleavage of membrane‐anchored receptors. Endocrinology 2001; 142: 4389–4393. [DOI] [PubMed] [Google Scholar]
- 30. Schaab M, Kausch H, Klammt J, et al Novel regulatory mechanisms for generation of the soluble leptin receptor: implications for leptin action. PLoS ONE 2012; 7: e34787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ge H, Huang L, Pourbahrami T, et al Generation of soluble leptin receptor by ectodomain shedding of membrane‐spanning receptors in vitro and in vivo. J Biol Chem 2002; 277: 45898–45903. [DOI] [PubMed] [Google Scholar]
- 32. Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem 2001; 276: 6343–6349. [DOI] [PubMed] [Google Scholar]
- 33. Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic beta‐cells. Am J Physiol Endocrinol Metab 2000; 278: E1–E14. [DOI] [PubMed] [Google Scholar]
- 34. Levi J, Gray SL, Speck M, et al Acute disruption of leptin signaling in vivo leads to increased insulin levels and insulin resistance. Endocrinology 2011; 152: 3385–3395. [DOI] [PubMed] [Google Scholar]
- 35. Covey SD, Wideman RD, McDonald C, et al The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 2006; 4: 291–302. [DOI] [PubMed] [Google Scholar]
- 36. Morioka T, Asilmaz E, Hu J, et al Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest 2007; 117: 2860–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gray SL, Donald C, Jetha A, et al Hyperinsulinemia precedes insulin resistance in mice lacking pancreatic beta‐cell leptin signaling. Endocrinology 2010; 151: 4178–4186. [DOI] [PubMed] [Google Scholar]
- 38. Morioka T, Dishinger JF, Reid KR, et al Enhanced GLP‐1‐ and sulfonylurea‐induced insulin secretion in islets lacking leptin signaling. Mol Endocrinol 2012; 26: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hattori A, Uemura K, Miura H, et al Gender‐related difference in relationship between insulin resistance and serum leptin level in Japanese type 2 diabetic and non‐diabetic subjects. Endocr J 2000; 47: 615–621. [DOI] [PubMed] [Google Scholar]
- 40. Kim‐Motoyama H, Yamaguchi T, Katakura T, et al Serum leptin levels are associated with hyperinsulinemia independent of body mass index but not with visceral obesity. Biochem Biophys Res Commun 1997; 239: 340–344. [DOI] [PubMed] [Google Scholar]
- 41. Larsson H, Elmstahl S, Ahren B. Plasma leptin levels correlate to islet function independently of body fat in postmenopausal women. Diabetes 1996; 45: 1580–1584. [DOI] [PubMed] [Google Scholar]
- 42. Derosa G, Franzetti IG, Querci F, et al Exenatide plus metformin compared with metformin alone on beta‐cell function in patients with Type 2 diabetes. Diabet Med 2012; 29: 1515–1523. [DOI] [PubMed] [Google Scholar]
- 43. Mu PW, Chen YM, Lu HY, et al Effects of a combination of oral anti‐diabetes drugs with basal insulin therapy on beta‐cell function and glycaemic control in patients with newly diagnosed type 2 diabetes. Diabetes Metab Res Rev 2012; 28: 236–240. [DOI] [PubMed] [Google Scholar]
- 44. Seino Y, Rasmussen MF, Zdravkovic M, et al Dose‐dependent improvement in glycemia with once‐daily liraglutide without hypoglycemia or weight gain: a double‐blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2008; 81: 161–168. [DOI] [PubMed] [Google Scholar]


