Abstract
Aims/Introduction
We compared the satisfaction levels of patients with type 2 diabetes undergoing combination therapy with lixisenatide (LIX) and basal insulin with that of patients undergoing multiple daily insulin injection (MDI) therapy.
Materials and Methods
The study was a 12‐week open‐label, randomized, multicenter, controlled trial. Participants were Japanese patients with type 2 diabetes receiving MDI for >3 months. Patients were randomly assigned to each treatment cohort: (i) a group that continued MDI (MDI group); and (ii) a group that switched from MDI to combination therapy with LIX and basal insulin (LIX group). The primary outcome was change in Diabetes Treatment Satisfaction Questionnaire scores from baseline to 12 weeks between these two groups. Key secondary outcomes were glycated hemoglobin and body weight changes.
Results
A total of 31 patients were initially enrolled in the study, and 26 of them completed the study. The change in Diabetes Treatment Satisfaction Questionnaire scores in the LIX group was significantly greater compared with that in the MDI group. Mean changes in glycated hemoglobin levels were −0.05 ± 0.37% in the MDI group and 0.04 ± 0.38% in the LIX group (P = 0.36). Mean changes in body weight were +0.6 ± 1.8 kg in the MDI group and −2.5 ± 1.8 kg in the LIX group (P < 0.01).
Conclusions
Switching from MDI to combination therapy with LIX and basal insulin improved satisfaction levels while maintaining glycemic control in Japanese patients with type 2 diabetes.
Keywords: Lixisenatide, Treatment satisfaction, Type 2 diabetes
Introduction
The primary objective of diabetes treatment is to help patients live a long lifespan as healthy individuals by preventing the onset and worsening of diabetes‐related micro and macrovascular complications1. To achieve this objective, patients need to maintain good glycemic control with diet, exercise and medication therapy over a long period of time2. Multiple daily insulin injection (MDI) therapy is more effective than oral antidiabetic treatment for maintaining glycemic control over a long period in patients with type 2 diabetes3, 4, 5. However, the increased injection frequency results in decreased patient satisfaction, which can be associated with poor treatment adherence6. Poor treatment satisfaction results can also lead to treatment interruption, which consequently leads to the onset and worsening of complications. Nevertheless, diabetes treatment is widely expected to preserve the patient's quality‐of‐life as continuously as possible while being safe and tolerable.
Glucagon‐like peptide‐1 (GLP‐1) analogs represent one class of antidiabetic medications. Compared with conventional agents, such as sulfonylurea and insulin injection therapy, they have a lower incidence of hypoglycemia through their glucose‐dependent enhanced insulin secretion7. Additionally, weight loss is common among patients receiving GLP‐1 analogs8, 9. Lixisenatide is a once‐daily GLP‐1 analog approved as add‐on treatment to basal insulin for the treatment of type 2 diabetes. It has been reported that combination therapy with lixisenatide once‐daily and basal insulin improved glycemic control10. However, little has been known about the effect of combination therapy with lixisenatide once‐daily and basal insulin on patient‐reported outcomes. In the present study, we compared the satisfaction levels of patients with type 2 diabetes undergoing this regimen with that of patients undergoing MDI therapy.
Methods
Protocol
The study was a 12‐week, open‐label, randomized controlled trial carried out at five medical institutions (Hokkaido University Hospital, Kushiro Red Cross Hospital, Sapporo Medical Center NTT EC, Kurihara Clinic and Aoki Clinic). Participants were Japanese patients with type 2 diabetes treated at these centers.
The clinical examination consisted of a medical history, physical examination and anthropometric measurements. Patients receiving MDI were randomly assigned to each treatment group: (i) a group that continued MDI (MDI group); and (ii) a group that switched from MDI to combination therapy with lixisenatide (Lyxumia(R); Sanofi‐Aventis, Paris, France) and basal insulin glargine (Lantus(R); Sanofi‐Aventis) once‐daily before breakfast (lixisenatide [LIX] group). Randomization of patients and allocation to each treatment group were carried out using a central computer‐based randomization. Patients were stratified by screening age (<65 years, ≥65 years), values of glycated hemoglobin (HbA1c; <7.5%, ≥7.5%) and body mass index (BMI; <25 kg/m2, ≥25 kg/m2).
Study population
Japanese outpatients with type 2 diabetes aged >20 years who were treated with MDI for at least 3 months before screening were recruited into the present study between September 2013 and September 2015. The inclusion criteria were as follows: diagnosis of type 2 diabetes, HbA1c level ≥6.0% and <9.0% despite of receiving MDI, and a total daily insulin dose ≤30 units. The exclusion criteria were type 1 diabetes and impaired insulin secretion with fasting serum C‐peptide <0.5 ng/mL. Other exclusion criteria were patients with diabetic ketosis or in a coma, with serious infection or trauma, history of pancreatitis or cancer, receiving steroid therapy, severe liver dysfunction, hypersensitivity to lixisenatide or glargine, receiving incretin‐based drugs such as GLP‐1 analogs or dipeptidyl peptidase 4 inhibitors within 3 months prior to the study entry, pregnancy or lactation, and patients scheduled to undergo surgery during the study.
The MDI group continued MDI throughout the study. In the LIX group, patients discontinued rapid‐acting insulin and started lixisenatide at 10 μg, once‐daily before breakfast with fortnightly increments of 5 μg, reaching a final daily dose of 20 μg by the end of week 4. The lixisenatide dose was then fixed for 8 weeks. Insulin glargine once‐daily before breakfast was continued. In the case of patients receiving premixed insulin injection therapy, an equivalent basal insulin (insulin glargine) once‐daily before breakfast was prescribed when switching from MDI. In each group, concomitant medication with oral hypoglycemic agents was continued at the same dose. The basal insulin doses were adjusted at every clinic visit by the attending physician based on self‐measured fasting blood glucose (FBG). The target FBG was 110 mg/dL. In the MDI group, the prandial insulin doses were changed based on self‐measured preprandial blood glucose. During the study, diet and exercise therapy were controlled by each physician.
The primary outcome was a change in the Diabetes Treatment Satisfaction Questionnaire (DTSQ) scores from randomization (baseline) to week 12, which was compared between the two groups. The DTSQ is a self‐administered questionnaire assessing patient‐reported outcomes11. The DTSQ includes eight items, and responses are scored on a seven‐point scale, from +6 to 0. The scores of the six items of the DTSQ (current treatment, convenience, flexibility, understanding, recommend and continue) were summed as the overall treatment satisfaction score, ranging from +36 to 0, with higher scores denoting greater treatment satisfaction. Additionally, perceived frequency of hyperglycemia and hypoglycemia were assessed in the DTSQ, which were rated on a scale of +6 (“most of the time”) to 0 (“never”). Patients completed a Japanese version of the DTSQ at baseline and week 1212. Key secondary outcomes were the changes in HbA1c, body weight and BMI changes, and the daily profile of blood glucose. Adverse effects, such as hypoglycemia and gastrointestinal symptoms, were monitored during the trial. Hypoglycemia was defined as blood glucose of <70 mg/dL or the presence of hypoglycemic symptoms. The M‐value, which is a marker of daily blood glucose variability, was calculated from daily profiles of blood glucose carried out at baseline and at week 12.
Statistical analysis
Based on a previous study, which compared the change in DTSQ between patients treated twice‐daily with premixed insulin plus sitagliptin and those treated with once‐daily basal insulin plus sitagliptin13, power calculations determined that a sample size of 15 individuals per group was required to have at least a 90% power to detect a difference between treatments. Statistical significance was assumed at the 5% level. All tests were two‐sided. Assuming a dropout rate of 10%, the sample size was set at 17 patients per group. The results were expressed as the means ± standard deviation. Differences between the two groups were analyzed for statistical significance using the Mann–Whitney U‐test. The correlation coefficients were calculated using a Spearman's rank‐order correlation. We carried out the statistical analyses using JMP 11 (SAS Institute Inc., Cary, NC, USA) and Microsoft Excel Statistics 2011 for Mac (SSRI Co. Ltd, Tokyo, Japan).
Ethics statement
The trial was registered at the University Hospital Medical Information Network (UMIN) Center under the identifier UMIN 000012697. The protocol for this research was approved by the institutional review board of Hokkaido University Hospital Clinical Research and Medical Innovation Center (013‐0217), and conformed to the provisions of the Declaration of Helsinki. Signed informed consent was obtained from all the participants.
Results
Patients' enrollment and baseline characteristics
A total of 31 patients (18 men and 13 women) were initially enrolled in the study. Every patient was randomly assigned to the MDI group or the LIX group, and 26 patients completed the study (MDI group: 15 patients; LIX group: 11 patients). Lixisenatide treatment was discontinued in two patients because of withdrawal of their consent before the study was started. Two patients discontinued treatment prematurely because of nausea, and another patient stopped treatment for unrelated otological surgery after 4 weeks of follow up (Figure 1). None of the patients dropped out because of their worsening glucose levels. The final follow‐up rate was 83.9%. The baseline clinical and metabolic characteristics of both groups are shown in Table 1. No statistically significant differences in the baseline characteristics, such as age, BMI, HbA1c, disease duration, fasting serum C‐peptide or total daily insulin dose, were observed between the two groups. Information on concomitant medications of oral hypoglycemic agents is also shown in Table 1.
Figure 1.
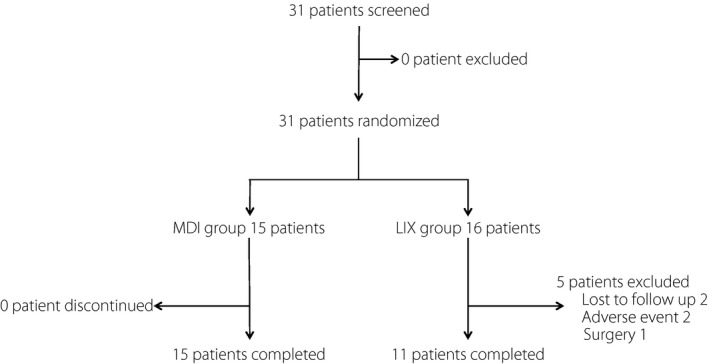
Flow chart of study participants throughout the trial. LIX group, the group that switched from multiple insulin therapy to combination therapy with lixisenatide and basal insulin; MDI group, the group that continued multiple daily insulin injection therapy.
Table 1.
Baseline participant characteristics
| Total | MDI group | LIX group | P | |
|---|---|---|---|---|
| n | 26 | 15 | 11 | |
| Age (years) | 62.3 ± 11.4 | 59.6 ± 12.8 | 66.0 ± 8.4 | 0.15 |
| Sex (male/female) | 14/12 | 9/6 | 5/6 | 0.46 |
| Body weight (kg) | 68.9 ± 18.1 | 68.9 ± 22.3 | 69.0 ± 11.1 | 0.80 |
| Duration of diabetes (years) | 20.2 ± 11.3 | 21.4 ± 10.8 | 18.6 ± 12.3 | 0.36 |
| Fasting plasma glucose (mg/dL) | 132.5 ± 32.7 | 125.5 ± 28.7 | 142.1 ± 36.6 | 0.23 |
| HbA1c (%) | 7.2 ± 0.7 | 7.2 ± 0.9 | 7.1 ± 0.5 | 0.96 |
| BMI (kg/m2) | 26.8 ± 5.6 | 26.4 ± 6.4 | 27.2 ± 4.2 | 0.48 |
| Total insulin (units/day) | 23.3 ± 5.6 | 24.5 ± 5.7 | 21.6 ± 5.2 | 0.20 |
| Total insulin (units/kg/day) | 0.35 ± 0.10 | 0.38 ± 0.10 | 0.32 ± 0.10 | 0.31 |
| Oral hypoglycemic agents | ||||
| Sulfonylurea (n) | 1 | 1 | 0 | 0.38 |
| Glinide (n) | 0 | 0 | 0 | 1.00 |
| Biguanide (n) | 8 | 3 | 5 | 0.16 |
| Thiazolidinediones (n) | 1 | 1 | 0 | 0.38 |
| α‐Glucosidase inhibitor (n) | 2 | 1 | 1 | 0.82 |
| Fasting C‐peptide (ng/mL) | 1.6 ± 0.9 | 1.7 ± 0.9 | 1.4 ± 0.9 | 0.31 |
| Fasting C‐peptide index | 1.2 ± 0.7 | 1.4 ± 0.7 | 1.0 ± 0.6 | 0.14 |
| M‐value | 53.8 ± 39.3 | 55.1 ± 38.9 | 51.9 ± 41.6 | 0.80 |
| Week 0 DTSQ score | ||||
| Treatment satisfaction | 23.0 ± 6.9 | 22.3 ± 7.0 | 23.9 ± 6.9 | 0.64 |
| Frequency of hyperglycemia and hypoglycemia | 4.0 ± 1.4 | 4.2 ± 1.4 | 3.7 ± 1.5 | 0.83 |
Values are expressed as mean ± standard deviation. Parameters described as mean ± standard deviation at baseline in each group were analyzed using a Mann–Whitney U‐test. BMI, body mass index; DTSQ, Diabetes Treatment Satisfaction Questionnaire; HbA1c, glycated hemoglobin; LIX group, the group that switched from multiple daily insulin injection therapy to combination therapy with lixisenatide and basal insulin; MDI group, the group that continued multiple daily insulin injection therapy.
Change in DTSQ scores
Patient‐reported outcome measures were assessed in all 26 patients (15 and 11 patients in the MDI and LIX groups, respectively). As shown in Table 2, changes in overall treatment satisfaction score evaluated by DTSQ in the LIX group were significantly greater compared with changes in the MDI group at week 12 (+5.7 ± 10.7 in the MDI group vs +12.7 ± 6.4 in the LIX group; P = 0.01). Scores for the three items of the “Current treatment,” “Flexibility” and “Continue” were significantly greater in the LIX group than those in the MDI group. Changes in scores for frequency of hyperglycemia and hypoglycemia tended to be lower in the LIX group than those in the MDI group (+1.5 ± 2.2 in the MDI group vs −0.4 ± 3.5 in the LIX group; P = 0.07), but the differences were not significant.
Table 2.
Changes in Diabetes Treatment Satisfaction Questionnaire from baseline to week 12
| MDI group | LIX group | P | |
|---|---|---|---|
| Treatment satisfaction | +5.7 ± 10.7 | +12.7 ± 6.4 | 0.01 |
| Current treatment | +0.9 ± 2.2 | +2.3 ± 1.2 | 0.04 |
| Convenience | +0.9 ± 2.3 | +2.4 ± 1.2 | 0.06 |
| Flexibility | +0.6 ± 2.0 | +2.4 ± 0.9 | <0.01 |
| Understanding | +1.2 ± 2.0 | +1.6 ± 1.4 | 0.35 |
| Recommend | +1.3 ± 1.8 | +1.9 ± 1.2 | 0.13 |
| Continue | +0.7 ± 2.1 | +2.2 ± 1.3 | 0.02 |
| Frequency of hyperglycemia and hypoglycemia | +1.5 ± 2.2 | –0.4 ± 3.5 | 0.07 |
Values are expressed as mean ± standard deviation. Parameters described as mean ± standard deviation at baseline in each group were analyzed using a Mann–Whitney U‐test. DTSQ, Diabetes Treatment Satisfaction Questionnaire; LIX group, the group that switched from multiple daily insulin injection therapy to combination therapy with lixisenatide and basal insulin; MDI group, the group that continued multiple daily insulin injection therapy. Values are expressed as mean ± standard deviation.
Metabolic parameters
As shown in Table 3, mean changes in HbA1c levels from week 0 to the end of week 12 were −0.05 ± 0.37% in the MDI group and +0.04 ± 0.38% in the LIX group (P = 0.36). From week 0 to the end of week 12, no significant differences in the mean changes in M‐value for the assessment of the daily blood glucose profile were observed between the two groups (+2.3 ± 32.2 in the MDI group vs −6.8 ± 49.7 in the LIX group; P = 0.59). These results indicated that combination therapy with lixisenatide and basal insulin showed comparable glycemic control to MDI. Mean changes in body weight from week 0 to the end of week 12 were significantly decreased in the LIX group (+0.6 ± 1.8 kg in the MDI group vs −2.5 ± 1.8 kg in the LIX group; P < 0.01). Furthermore, the average rates of change in BMI were significantly decreased in the LIX group (+0.8 ± 1.1% in the MDI group vs −3.7 ± 3.3% in the LIX group; P < 0.01). Hypoglycemia (one patient [6.7%] in the MDI group vs no patients [0%] in the LIX group) and mild gastrointestinal symptoms (no patients [0%] in the MDI group vs six patients [37.5%] in the LIX group) were observed during the 12 weeks. Cases of severe hypoglycemia were not observed in either group during the trial.
Table 3.
Changes in secondary outcomes from baseline to week 12
| MDI group | LIX group | P | |
|---|---|---|---|
| HbA1c (%) | −0.05 ± 0.37 | +0.04 ± 0.38 | 0.36 |
| M‐value | +2.3 ± 32.2 | −6.8 ± 49.7 | 0.59 |
| Body weight (kg) | +0.6 ± 1.8 | −2.5 ± 1.8 | <0.01 |
Values are expressed as mean ± standard deviation. Changes in parameters described as mean ± standard deviation between 0 and 12 weeks were analyzed using a Mann–Whitney U‐test. HbA1c, glycated hemoglobin; LIX group, the group that switched from multiple daily insulin injection therapy to combination therapy with lixisenatide and basal insulin; MDI group, the group that continued multiple daily insulin injection therapy.
In each group, the basal insulin dose was titrated aiming to achieve a target FBG value of 110 mg/dL. No statistically significant difference in the attained average FBG at week 12 was observed between the two groups (130.1 ± 33.3 mg/dL in the MDI group vs 128.4 ± 20.2 mg/dL in the LIX group; P = 1.00). In the MDI group, 46.7% of patients received a titrating dose of basal insulin (mean, +1.2 units per day); 27.3% of patients in the LIX group received a titrating dose of basal insulin (mean, +0.36 units per day). In the LIX group, the dose of insulin was reduced by 12.7 units per day (59.9%) at week 0 according to the protocol for switching to combination therapy with lixisenatide and basal insulin. Therefore, the mean change in the total daily dose of insulin in the LIX group was significantly decreased compared with that in the MDI group at week 12 (+0.7 ± 4.8 units per day in the MDI group vs –12.7 ± 3.4 units per day in the LIX group; P < 0.01).
Treatment satisfaction and the clinical characteristics
To reveal the factors influencing patient satisfaction, we examined the relationships between treatment satisfaction and the clinical characteristics of all the participants. Although no correlations were found between the treatment satisfaction score evaluated by DTSQ and mean changes in HbA1c levels, M‐value or baseline characteristics (i.e., age, disease duration or fasting serum C‐peptide), a significant negative correlation was observed between the treatment satisfaction score and the average rates of BMI change (ρ = −0.41, P = 0.04). Additionally, a significant negative correlation was observed between the treatment satisfaction score and the mean change of total daily dose of insulin (ρ = −0.56, P < 0.01) and the frequency of injection (ρ = –0.48, P = 0.01), as shown in Table 4.
Table 4.
Relationship between changes in the Diabetes Treatment Satisfaction Questionnaire and various items
| ρ | P | |
|---|---|---|
| Age (years) | −0.05 | 0.79 |
| Average rates of BMI change (%) | −0.41 | 0.04 |
| Mean changes in M‐value | 0.18 | 0.37 |
| Mean changes in HbA1c levels (%) | −0.01 | 0.97 |
| Disease duration (years) | −0.31 | 0.12 |
| Week 0 fasting serum C‐peptide (ng/mL) | −0.28 | 0.17 |
| Week 0 C‐peptide index | −0.32 | 0.11 |
| Frequency of injection (times) | −0.48 | 0.01 |
| Mean change of total insulin dose (units) | −0.56 | <0.01 |
The correlation coefficients were analyzed using a Spearman's rank‐order correlation. BMI, body mass index; DTSQ, Diabetes Treatment Satisfaction Questionnaire; HbA1c, glycated hemoglobin.
Discussion
To the best of our knowledge, the present study is the first to investigate treatment satisfaction, efficacy, and safety of switching to combination therapy with GLP‐1 receptor agonists and basal insulin in patients with type 2 diabetes receiving MDI. The main findings of the present study were the following: (i) switching to combination therapy with lixisenatide and basal insulin in patients with type 2 diabetes receiving MDI improved patient satisfaction, and reduced body weight, total insulin dose and frequency of injection without losing control of blood glucose; and (ii) treatment satisfaction was associated with changes in BMI, total insulin dose and frequency of injection, but not glycemic control. Furthermore, this is the first randomized controlled trial in which combination therapy with GLP‐1 receptor agonists and basal insulin improved patient satisfaction compared with MDI as the primary outcome. Several previous studies have investigated treatment satisfaction with GLP‐1 receptor agonists vs MDI in patients with type 2 diabetes. For example, in a trial including 82 patients with type 2 diabetes who switched from MDI to a single agent of liraglutide or insulin detemir plus sitagliptin, the treatment satisfaction (DTSQ score) was significantly improved in the liraglutide group after 24 weeks14. In these trials, patient satisfaction using GLP‐1 receptor agonists or MDI was assessed as a secondary outcome14, 15, 16.
In accordance with previous studies, the present study showed that less frequent injection and reduction of insulin dose, and decreased body weight were significantly associated with patient satisfaction17, 18, 19. For example, once‐weekly exenatide treatment improved treatment satisfaction compared with twice‐daily exenatide treatment17. In a previous study, the addition of GLP‐1 receptor agonists to insulin therapy resulted in reducing the insulin dose along with improvement of the DTSQ score20. As reported recently, weight gain leads to significant exacerbation of scores of Diabetes Therapy‐Related QOL in Japanese patients with diabetes21. The beneficial weight effects of short‐acting GLP‐1 receptor agonists have been observed clinically, as treatment with short‐acting GLP‐1 receptor agonists has stronger effects on decreased food intake, slowed gastric emptying and promotion of weight loss than long‐acting GLP‐1 receptor agonists8, 9, 22, 23. Furthermore, a weight loss effect has been shown by combination therapy with lixisenatide and basal insulin23, 24, 25. Therefore, we consider that the lixisenatide‐induced weight loss effect is associated with the improvement in patient satisfaction.
In the present study, the change in HbA1c was not associated with improvement of DTSQ score (ρ = –0.01, P = 0.97), as shown in Table 4. It has also been previously reported that patient satisfaction does not have any relationship with change in HbA1c value14, 26. For patients with type 1 diabetes or insulin secretory deficiency, MDI therapy is absolutely imperative. Patients whose fasting serum C‐peptide level was <0.5 ng/mL were excluded from the present study, because switching to combination therapy from MDI might result in treatment failure for patients with diabetes whose insulin secretion is depleted. There is no consensus regarding the fasting serum C‐peptide level cut‐off value; thus, the appropriate value for switching from MDI to combination therapy with lixisenatide and basal insulin remains to be discussed. As shown in the present study, patients with type 2 diabetes that required MDI with a relatively low insulin dose were able to maintain moderate glycemic control after switching to combination therapy. Furthermore, switching to combination therapy resulted in improved patient satisfaction levels without losing control of blood glucose. Switching to combination therapy would be effective for patients with type 2 diabetes having insulin‐induced weight gain and for patients who inject a relatively low insulin dose many times a day. For the appropriate selection of treatment, it is not only critical to obtain superior glycemic control, but also to ensure patient satisfaction. The reason is that patient satisfaction is expected to improve treatment adherence, associated with less treatment interruption, thereby preventing the onset and worsening of complications. The present results show that combination therapy with lixisenatide and basal insulin once‐daily before breakfast is a simplified approach to treating type 2 diabetes that can be easily adopted by patients with diverse lifestyles.
Other associated factors with patient satisfaction might be related to the frequency of adverse events, such as hypoglycemia and gastrointestinal symptoms. In the present study, hypoglycemia was rare in both groups, whereas gastrointestinal symptoms occurred more frequently in the LIX group compared with the MDI group. As an adverse event of lixisenatide, nausea has been reported exclusively in Asian populations10, 27, 28. However, very few patients discontinued the study because of such symptoms. The combination therapy with lixisenatide and basal insulin once daily might provide benefits that outweigh adverse events, such as gastrointestinal symptoms, and thus, this combination might be chosen as a suitable approach for long‐term treatment, while effectively maintaining adequate glycemic control.
The present study had some limitations. First, we carried out an interim analysis for a limited study period and the incomplete data of five patients were excluded from analysis, although the power calculations determined that the number of patients enrolled in this study was sufficient for an exploratory observational study in clinical practice. Additional studies with larger sample sizes will be required to confirm and extend our current findings. Second, the present study was carried out in Japanese mildly obese patients with type 2 diabetes receiving MDI with a relatively low insulin dose. The mean baseline BMI of Japanese patients is relatively lower than that of individuals from Western countries. Thus, this difference in the BMI and total daily insulin dose among each ethnic group is likely the cause of the discrepancy when comparing the present results with those of previous studies29, 30.
In conclusion, switching from MDI to combination therapy with lixisenatide and basal insulin improved patient satisfaction levels, while maintaining the glycemic control in patients with type 2 diabetes.
Disclosure
AN has received honoraria for lectures from Sanofi. HM has received honoraria for lectures from Astellas Pharma Inc., AstraZeneca, Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly, Kissei, Mitsubishi Tanabe Pharma Co., MSD, Novartis Pharma, Novo Nordisk Pharma and Sanofi; and received research funding from Astellas Pharma Inc., AstraZeneca, Daiichi Sankyo, Eli Lilly, Mitsubishi Tanabe Pharma Co., MSD, Novo Nordisk Pharma, Sanofi, Takeda Pharmaceutical Co., Ltd. and Taisho Toyama Pharmaceutical Co., Ltd. YT has received honoraria for lectures from MSD, Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo, Mitsubishi Tanabe Pharma Co., Sanwa Kagaku Kenkyusho Co., Ltd., Novartis Pharma, Eli Lilly, Sanofi, Bayer Yakuhin, Ltd., Shionogi & Co., Ltd., AstraZeneca, Kowa Pharmaceutical Co., Ltd. and Novo Nordisk Pharma; and received research funding from MSD, Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Mitsubishi Tanabe Pharma Co., Sanwa Kagaku Kenkyusho Co., Ltd., Novartis Pharma, Eli Lilly, Sanofi, Bayer Yakuhin, Ltd., Dainippon Sumitomo Pharma Co., Ltd., Shionogi & Co., Ltd., AstraZeneca, Astellas Pharma Inc., Daiichi Sankyo, Novo Nordisk Pharma and Pfizer Inc. TA has received honoraria for lectures from Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Pfizer Inc. and AbbVie Inc.; and received research funding from Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo and Otsuka Pharmaceutical Co., Ltd. The other authors declare no conflict of interest.
Acknowledgment
The authors are deeply grateful to Professor Clare Bradley, Jonathan Gilbride and Alison Wilson for providing helpful comments and suggestions on the DTSQ. This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
J Diabetes Investig 2018; 9: 119–126
Clinical Trial Registry
UMIN Clinical Trials Registry System
UMIN 000012697
References
- 1. Prospective Diabetes Study Group . U.K. prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 1995; 44: 1249–1258. [PubMed] [Google Scholar]
- 2. United Kingdom Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 3. Holman RR, Farmer AJ, Davies MJ, et al Three‐year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009; 361: 1736–1747. [DOI] [PubMed] [Google Scholar]
- 4. Wright A, Burden AC, Paisey RB, et al Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002; 25: 330–336. [DOI] [PubMed] [Google Scholar]
- 5. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55: 1577–1596. [DOI] [PubMed] [Google Scholar]
- 6. Charpentier G, Fleury F, Dubroca I, et al Electronic pill‐boxes in the evaluation of oral hypoglycemic agent compliance. Diabetes Metab 2005; 31: 189–195. [DOI] [PubMed] [Google Scholar]
- 7. Garber A, Henry R, Ratner R, et al Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481. [DOI] [PubMed] [Google Scholar]
- 8. Holst JJ, Deacon CF, Vilsbøll T, et al Glucagon‐like peptide‐1, glucose homeostasis and diabetes. TrendsMed 2008; 14: 161–168. [DOI] [PubMed] [Google Scholar]
- 9. Russell‐Jones D. The safety and tolerability of GLP‐1 receptor agonists in the treatment of type‐2 diabetes. Int J Clin Pract 2010; 64: 1402–1414. [DOI] [PubMed] [Google Scholar]
- 10. Seino Y, Min KW, Niemoeller E, et al Randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab 2012; 14: 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradley C. The diabetes treatment satisfaction questionnaire: DTSQ In: Bradley C. (Ed). Handbook of Psychology and Diabetes: a Guide to Psychological Measurement in Diabetes Research and Practice. Chur, Switzerland: Harwood Academic Publishers, 1994; 111–132. [Google Scholar]
- 12. Ishii H, Bradley C, Riazi A, et al The Japanese version of the Diabetes Treatment Satisfaction Questionnaire (DTSQ): translation and clinical evaluation. J Clin Exp Med 2000; 192: 809–814 [in Japanese]. [Google Scholar]
- 13. Ono K, Nakamura A, Kawaguchi J, et al Next step of treatment in patients with inadequately controlled type 2 diabetes with twice‐daily premixed insulin. J Jpn Diabetes Soc 2013; 56(Suppl 1): S152 (Abstract) (in Japanese). [Google Scholar]
- 14. Inoue Y, Nakamura A, Kondo Y, et al A randomized controlled trial of liraglutide versus insulin detemir plus sitagliptin: effective switch from intensive insulin therapy to the once‐daily injection in patients with well‐controlled type 2 diabetes. J Clin Pharmacol 2015; 55: 831–838. [DOI] [PubMed] [Google Scholar]
- 15. Hirsch IB, Buse JB, Leahy J, et al Options for prandial glucose management in type 2 diabetes patients using basal insulin: addition of a short‐acting GLP‐1 analogue versus progression to basal‐bolus therapy. Diabetes Obes Metab 2014; 16: 206–214. [DOI] [PubMed] [Google Scholar]
- 16. Lind M, Hirsch IB, Tuomilehto J, et al Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: randomised clinical trial (MDI Liraglutide trial). BMJ 2015; 351: 5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Best JH, Boye KS, Rubin RR, et al Improved treatment satisfaction and weight‐related quality of life with exenatide once weekly or twice daily. Diabet Med 2009; 26: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hutchins V, Zhang B, Fleurence RL, et al A systematic review of adherence, treatment satisfaction and costs, in fixed‐dose combination regimens in type 2 diabetes. Curr Med Res Opin 2011; 27: 1157–1168. [DOI] [PubMed] [Google Scholar]
- 19. Bretzel RG, Nuber U, Landgraf W, et al Once‐daily basal insulin glargine versus thrice‐daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008; 371: 1073–1084. [DOI] [PubMed] [Google Scholar]
- 20. Lind M, Jendle J, Torffvit O, et al Glucagon‐like peptide 1 (GLP‐1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes 2012; 6: 41–46. [DOI] [PubMed] [Google Scholar]
- 21. Ishii H. Development and psychometric validation of the Diabetes Therapy‐Related QOL (DTR‐QOL) questionnaire. J Med Econ 2012; 15: 556–563. [DOI] [PubMed] [Google Scholar]
- 22. Werner U, Haschke G, Herling AW, et al Pharmacological profile of lixisenatide: a new GLP‐1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 2010; 164: 58–64. [DOI] [PubMed] [Google Scholar]
- 23. de Wit HM, Vervoort GM, Jansen HJ, et al Liraglutide reverses pronounced insulin‐associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT). Diabetologia 2014; 57: 1812–1819. [DOI] [PubMed] [Google Scholar]
- 24. Lane W, Weinrib S, Rappaport J, et al The effect of addition of liraglutide to high‐dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab 2014; 16: 827–832. [DOI] [PubMed] [Google Scholar]
- 25. Seino Y, Kaneko S, Fukuda S, et al Journal of Diabetes Investigation Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36‐week, randomized, double‐blind, parallel‐group trial. J Diabetes Investig 2016; 7: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobson AM, de Groot M, Samson JA. The evaluation of two measures of quality of life in patients with type I and type II diabetes. Diabetes Care 1994; 17: 267–274. [DOI] [PubMed] [Google Scholar]
- 27. Fonseca VA, Alvarado‐Ruiz R, Raccah D, et al Efficacy and safety of the once‐daily GLP‐1 receptor agonist lixisenatide in monotherapy: a randomized, double‐blind, placebo‐controlled trial in patients with type 2 diabetes (GetGoal‐Mono). Diabetes Care 2012; 35: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ratner RE, Rosenstock J, Boka G; DRI6012 Study Investigators . Dose‐dependent effects of the once‐daily GLP‐1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled trial. Diabet Med 2010; 27: 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheffield CA, Kane MP, Busch RS, et al Safety and efficacy of exenatide in combination with insulin in patients with type 2 diabetes mellitus. Endocr Pract 2008; 14: 285–292. [DOI] [PubMed] [Google Scholar]
- 30. Rosenstock J, Shenouda SK, Bergenstal RM, et al Baseline factors associated with glycemic control and weight loss when exenatide twice daily is added to optimized insulin glargine in patients with type 2 diabetes. Diabetes Care 2012; 35: 955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]


