Abstract
Aims/Introduction
To compare the treatment satisfaction of four classes of oral hypoglycemic agents (OHAs): dipeptidyl peptidase‐4 (DPP‐4) inhibitors, α‐glucosidase inhibitors (αGI), biguanides (BG) and sulfonylureas (SU), which are common initial treatments for type 2 diabetes mellitus patients in Japan, and to identify the best oral hypoglycemic agent in terms of treatment satisfaction.
Materials and Methods
In this 12‐week, randomized, controlled, open‐label study, Japanese outpatients with type 2 diabetes mellitus who were naïve to pharmacological treatment were randomly assigned a DPP‐4 inhibitor, a BG., an αGI or a SU. The primary end‐point was the Oral Hypoglycemic Agent Questionnaire (OHA‐Q) total and subscale scores (treatment convenience, somatic symptoms and satisfaction) at week 4. Adherence, glycated hemoglobin (HbA1c) level and safety were also evaluated.
Results
The DPP‐4 inhibitor group scored highest in the OHA‐Q total and all subscale scores at week 4. The total score was significantly higher in the DPP‐4 inhibitor group than in the BG or αGI groups (P = 0.0084 and 0.0147, respectively). The mean total score at week 12 was also highest in the DPP‐4 inhibitor group, with a significant difference compared with the αGI group (P = 0.0293). The mean HbA1c decreased from baseline to week 12 in all groups. The DPP‐4 inhibitor group had the highest adherence at weeks 4 and 12. A total of 11 patients reported adverse events, including one hypoglycemic event in the SU group.
Conclusions
The DPP‐4 inhibitor was the most preferable option in terms of treatment satisfaction.
Keywords: Oral hypoglycemic agents, Randomized controlled study, Treatment satisfaction
Introduction
For the management of type 2 diabetes mellitus, various oral hypoglycemic agents (OHAs) are available, such as dipeptidyl peptidase‐4 (DPP‐4) inhibitors, α‐glucosidase inhibitors (αGI), biguanides (BG), sulfonylureas (SU), thiazolidine insulin sensitizers and glinides, which differ in dosage, administration, side‐effects and cost1, 2. Of this widening array of options, the American Diabetes Association and the European Association for the Study of Diabetes guidelines designate metformin as the first‐line treatment for type 2 diabetes1. Conversely, the Japan Diabetes Society does not specify an OHA as the first‐line drug, but recommends that the treatment be selected according to the pathophysiological condition of each patient3.
Given the heterogeneity of type 2 diabetes and of patients themselves, the most appropriate OHA should be selected on a case‐by‐case basis taking into consideration the advantages and disadvantages of each option, the practical aspects of the treatment and disease conditions, and the patient's preference1, 4. Treatment satisfaction is an important factor that should be considered during treatment selection. Type 2 diabetes management requires long‐term and complex self‐management, which has a great impact on a patient's daily life; therefore, successful treatment heavily depends on patient adherence. Patients who are dissatisfied with their treatment are less likely to adhere to that treatment5, 6, and non‐adherence to treatments for type 2 diabetes can result in poor glycemic control7, 8, 9, 10, which increases the risk of complications and can lead to disease deterioration. Treatment selection should not solely stand on an objective efficacy assessment or disease conditions, but rather it should be a comprehensive consideration that encompasses the patient's view. Therefore, treatment satisfaction should be involved in the decision for selection of the optimal OHA.
To determine the treatment satisfaction with currently available OHAs, we developed and validated the patient‐administered Oral Hypoglycemic Agent Questionnaire (OHA‐Q). The OHA‐Q assesses treatment satisfaction with adequate reproducibility and validity, specifically for OHAs for type 2 diabetes patients11, 12. Using the OHA‐Q, this randomized, controlled, open‐label study aimed to compare the treatment satisfaction among four OHAs (PREFERENCE 4 study) that are widely prescribed as an initial treatment for type 2 diabetes in Japan (DPP‐4 inhibitors, BGs, αGIs and SUs). We also aimed to identify the best OHA in terms of treatment satisfaction. Considering the lack of studies that directly compare the treatment satisfaction of major OHAs, we expect that the present study will contribute to improving patient‐centered drug selection for type 2 diabetes patients.
Methods
This was a 12‐week, prospective, randomized, controlled, open‐label, multicenter study, carried out from July 2012 to March 2015 at 19 sites in Japan. The protocol was approved by the ethics committee of each participating site.
Patients
Inclusion criteria were as follows: Japanese type 2 diabetes outpatients (aged 20–79 years) who were naïve to pharmacological treatments (including OHAs), and had suboptimal glycemic control (6.9–9.4%) after ≥4‐week diet and exercise therapy. The exclusion criteria included a history of severe ketosis, diabetic coma, liver dysfunction (alanine aminotransferase >3 times the upper limit of normal), pregnancy/possibility of pregnancy, history/presence of cancer, or the judgment by the attending physicians that participation for this study was inappropriate based on medical evidence. Written informed consent was obtained from all patients before study participation.
Randomization and treatment
Patients were randomly assigned at a 1:1:1:1 ratio to receive either a DPP‐4 inhibitor, a BG, an αGI or an SU by a central registration system using minimization methods to ensure a well‐balanced allocation in terms of age, sex, body mass index, disease duration and glycated hemoglobin (HbA1c) at baseline.
The four OHAs were selected according to the typical prescriptions in Japan. The assigned single OHA was administered, and the doses were adjusted at the physician's discretion. Changes in diet and exercise therapy, and concomitant drugs were not permitted.
Assessment
Treatment satisfaction with the assigned OHA was assessed using the OHA‐Q at weeks 4 and 12. In addition, adherence to the assigned OHA, HbA1c levels and dosages were assessed at the same assessment points. HbA1c was also assessed at baseline. Data on adverse events (AEs) were also recorded.
The primary end‐point was OHA‐Q total and subscale scores at week 4. The OHA‐Q consists of 20 items categorized into three subscales: treatment convenience (9 items), somatic symptoms (8 items) and satisfaction (3 items). Each item score ranges from 0 (worst) to 3 points (best)11. Patients received the questionnaire at weeks 4 and 12, and sent the completed questionnaire back by post.
The level of adherence to the assigned OHA was assessed through interviews by physicians. Patients stated their level of adherence by describing how frequently they missed a dose from the following four options: (i) never; (ii) once a month; (iii) once a week; or (iv) more than once a week.
Statistical analysis
OHA‐Q scores and HbA1c levels were analyzed for patients who received the assigned OHA and completed the OHA‐Q at week 4. Baseline characteristics, adherence and safety data were summarized for patients who received the assigned OHA at least once.
OHA‐Q total and subscale scores were analyzed using a pairwise comparison of the least square means of the OHA‐Q scores with the corresponding 95% confidence intervals (CIs). The scores were calculated under the assumption of an equal interval between the response choices. Descriptive statistics were used to summarize the baseline demographics, adherence, HbA1c levels, dosages and safety data by OHA group. HbA1c values at weeks 4 and 12 were evaluated by pairwise comparison of the least square means for the OHA groups.
In the present study, missing data were not imputed. The analysis was not adjusted for multiplicity because of the exploratory nature of this study. Statistical analyses were carried out using SAS 9.3 (SAS Institute Inc., Cary, North Carolina, USA). All statistical tests were two‐tailed, and the significance level was set at 0.05. We did not carry out a sample size calculation for this study.
Results
A total of 64 patients were randomized to four groups (16 patients per group; Figure 1). After randomization, four patients withdrew from the study (one in the DPP‐4 inhibitor group withdrew consent; and two patients in the BG group and one patient in the SU group were withdrawn for not taking the assigned OHA). The remaining 60 patients received the assigned OHAs. Eight patients were excluded for not completing the OHA‐Q at week 4 (3, 1, 3, and 1 patient in the DPP‐4 inhibitor, BG, αGI and SU groups, respectively), leaving 52 patients for inclusion in the OHA‐Q and HbA1c analyses.
Figure 1.
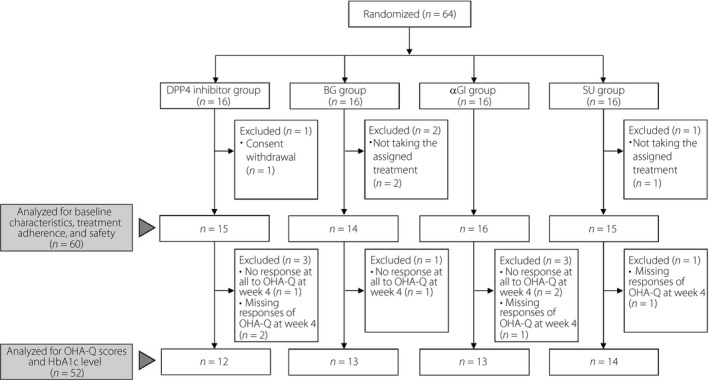
Patient disposition. αGI, α‐glucosidase inhibitor; BG, biguanide; DPP‐4, dipeptidyl peptidase‐4; HbA1c, glycated hemoglobin; OHA‐Q, Oral Hypoglycemic Agent Questionnaire; SU, sulfonylurea.
Demographic and baseline characteristics are summarized in Table 1. The four OHA groups did not differ notably. Overall, women accounted for 41.7%. The mean (SD) age, body mass index and duration of type 2 diabetes were 63.1 years (11.1 years), 25.8 kg/m2 (4.2 kg/m2) and 3.6 years (4.2 years), respectively.
Table 1.
Baseline demographic and clinical characteristics of the patients
| DPP‐4 inhibitor (n = 15) | BG (n = 14) | αGI (n = 16) | SU (n = 15) | Total (n = 60) | |
|---|---|---|---|---|---|
| Sex | |||||
| Men, n (%) | 9 (60.0) | 9 (64.3) | 9 (56.3) | 8 (53.3) | 35 (58.3) |
| Women, n (%) | 6 (40.0) | 5 (35.7) | 7 (43.8) | 7 (46.7) | 25 (41.7) |
| Mean age, years (SD) | 63.1 (12.1) | 63.6 (13.4) | 64.4 (9) | 61.2 (10.7) | 63.1 (11.1) |
| Mean weight, kg (SD) | 65.4 (15.5) | 67.9 (11.6) | 64.0 (12.1) | 70.6 (10.6) | 66.9 (12.5) |
| Mean body mass index, kg/m2 (SD) | 25.1 (4.8) | 26.0 (3.9) | 25.4 (3.7) | 26.7 (4.7) | 25.8 (4.2) |
| Mea duration of disease, years (SD) | 3.5 (4.8) | 3.7 (4.1) | 3.4 (4.3) | 3.7 (4.1) | 3.6 (4.2) |
| Smoking, n (%) | 3 (20.0) | 5 (35.7) | 2 (12.5) | 4 (26.7) | 14 (23.3) |
| Drinking habit, n (%) | 8 (53.3) | 7 (50.0) | 8 (50.0) | 8 (53.3) | 31 (51.7) |
| Comorbidities, n (%) | 7 (46.7) | 7 (50.0) | 8 (50.0) | 10 (66.7) | 32 (53.3) |
| Hypertension | 4 (26.7) | 3 (21.4) | 5 (31.3) | 7 (46.7) | 19 (31.7) |
| Hyperlipidemia | 5 (33.3) | 6 (42.9) | 3 (18.8) | 6 (40.0) | 20 (33.3) |
| Fatty liver | 1 (6.7) | 2 (14.3) | 0 (0.0) | 0 (0.0) | 3 (5.0) |
| Retinopathy | 1 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Diabetic nephropathy | 1 (6.7) | 0 (0.0) | 0 (0.0) | 2 (13.3) | 3 (5.0) |
Data are presented as n (%) or mean (SD).
αGI, α‐glucosidase inhibitors; BG, biguanides; DPP‐4, dipeptidyl peptidase‐4; SU, sulfonylureas.
OHA‐Q total and subscale scores at week 4
The mean OHA‐Q total score was highest in the DPP‐4 inhibitor group and lowest in the BG group (48.2, 95% CI: 44.1–52.3 and 40.4, 95% CI: 36.4–44.3, respectively). OHA‐Q total score was significantly different not only between the DPP‐4 inhibitor and BG groups (P = 0.0084), but also between the DPP‐4 inhibitor and αGI groups (P = 0.0147; Figure 2. The DPP‐4 inhibitor group also scored highest in the treatment convenience, somatic symptom and satisfaction subscales (23.6, 95% CI: 21.1–26.1; 18.4, 95% CI: 16.0–20.8; and 6.2, 95% CI: 5.4–6.9, respectively). The lowest scores among subscales were as follows: αGI (treatment convenience 19.6, 95% CI: 17.2–22.0), BG (somatic symptom 14.1, 95% CI: 11.8–16.4) and SU (satisfaction 5.0, 95% CI: 4.3–5.7). The scores in the DPP‐4 inhibitor group were significantly different from the groups that scored the lowest in the respective subscales (P = 0.0246, 0.0109 and 0.0232, respectively).
Figure 2.
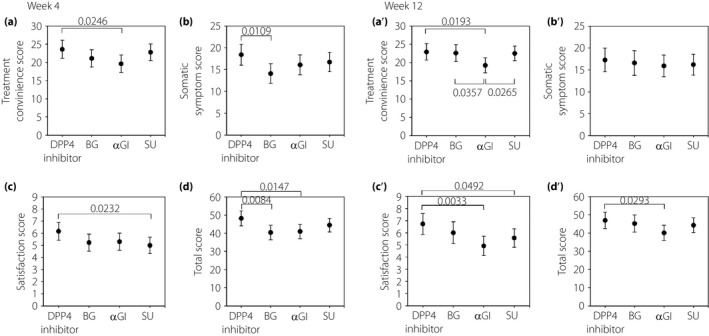
Mean OHA‐Q total and subscale scores at weeks 4 and 12. Pairwise comparison of least square means of Oral Hypoglycemic Agent Questionnaire scores with the corresponding 95% confidence intervals. The treatment convenience, somatic symptom, and satisfaction subscales consist of nine, eight and three items, respectively. Each item score ranges from 0 (worst) to 3 points (best); the higher score indicates better treatment satisfaction. αGI, α‐glucosidase inhibitor; BG, biguanide; DPP‐4, dipeptidyl peptidase‐4; OHA‐Q, Oral Hypoglycemic Agent Questionnaire; SU, sulfonylurea.
OHA‐Q item scores at week 4
Table 2 shows the mean OHA‐Q item scores at week 4. Compared with the scores of the DPP‐4 inhibitor group, the other groups scored lower by ≥0.5 points in the following items. For the treatment convenience subscale: BG ([2] difficulty swallowing, [7] compliance with treatment schedule and [8] number of doses) and αGI ([1] missed dose, [3] carrying and preparing for taking the agent, [7] compliance with treatment schedule, and [8] number of doses). For the somatic symptom subscale: BG ([11] rumbling stomach, [12] diarrhea, [13] constipation, [14] increased bodyweight and [15] tendency to become hungry easily), αGI ([11] rumbling stomach and [12] diarrhea) and SU ([14] increased bodyweight). For the satisfaction subscale: BG, SU ([19] glycemic control).
Table 2.
Item scores for the OHA‐Q at week 4
| Subscales and items | DPP‐4 inhibitor | BG | αGI | SU |
|---|---|---|---|---|
| Treatment convenience subscale | ||||
| 1. Missed dose | 2.6 (0.8) | 2.3 (0.9) | 1.8 (0.9) | 2.7 (0.5) |
| 2. Difficulty swallowing | 3.0 (0.0) | 2.4 (0.8) | 2.8 (0.4) | 2.8 (0.4) |
| 3. Carrying and preparing for taking the agent | 2.8 (0.4) | 2.5 (0.7) | 2.0 (1.0) | 2.5 (0.9) |
| 4. People around the patient | 2.8 (0.6) | 2.8 (0.6) | 2.6 (0.5) | 2.6 (0.8) |
| 5. Following the meal schedule | 2.2 (0.8) | 2.3 (0.9) | 2.0 (0.7) | 2.2 (1.0) |
| 6. Interval between taking the agent and a meal | 2.2 (0.8) | 2.0 (0.9) | 2.1 (0.8) | 2.1 (0.9) |
| 7. Compliance with treatment schedule | 2.7 (0.7) | 2.2 (0.8) | 2.0 (0.8) | 2.4 (1.0) |
| 8. Number of doses | 2.8 (0.4) | 2.3 (0.9) | 1.8 (0.9) | 2.9 (0.4) |
| 9. Taking the agent at a place other than home | 2.6 (0.5) | 2.4 (0.7) | 2.4 (0.7) | 2.6 (0.9) |
| Somatic symptom subscale | ||||
| 11. Rumbling stomach | 2.3 (0.8) | 1.7 (1.1) | 1.7 (0.6) | 2.0 (0.9) |
| 12. Diarrhea | 2.6 (0.5) | 1.8 (1.0) | 1.9 (1.0) | 2.5 (0.7) |
| 13. Constipation | 2.5 (1.0) | 1.5 (1.2) | 2.2 (0.9) | 2.2 (0.9) |
| 14. Increased bodyweight | 1.9 (1.1) | 1.4 (1.1) | 1.7 (0.9) | 1.3 (0.7) |
| 15. Tendency to become hungry easily | 2.2 (0.7) | 1.7 (0.9) | 2.3 (0.5) | 1.8 (0.6) |
| 16. Nausea | 2.3 (0.8) | 1.9 (1.1) | 2.1 (1.0) | 2.5 (0.7) |
| 17. Bodily swelling | 2.5 (0.7) | 2.2 (1.1) | 2.2 (0.8) | 2.4 (0.8) |
| 18. Hypoglycemia | 2.3 (0.9) | 1.9 (1.1) | 2.1 (0.6) | 2.1 (0.9) |
| Satisfaction subscale | ||||
| 10. Desire to continue the treatment | 1.8 (1.0) | 1.5 (0.7) | 1.5 (0.5) | 1.5 (0.7) |
| 19. Glycemic control | 2.1 (0.5) | 1.6 (0.8) | 1.8 (0.4) | 1.6 (0.5) |
| 20. Satisfaction with the current agent | 2.3 (0.6) | 2.1 (0.5) | 1.9 (0.5) | 1.9 (0.4) |
Data are presented as mean (SD). Each item score ranges from 0 (worst) to 3 points (best); a higher score indicates better treatment satisfaction. αGI, α‐glucosidase inhibitors; BG, biguanides; DPP‐4, dipeptidyl peptidase‐4; OHA‐Q, Oral Hypoglycemic Agent Questionnaire; SU, sulfonylureas.
OHA‐Q total and subscale scores at week 12
Figure 2 shows the OHA‐Q scores at week 12 by group. The mean total score was highest in the DPP‐4 inhibitor group and lowest in the αGI group (46.9, 95% CI: 42.4–51.4 and 40.1, 95% CI: 35.9–44.2, respectively), with a significant difference between the groups (P = 0.0293). Scores for treatment convenience, somatic symptom and satisfaction were all highest in the DPP‐4 inhibitor group (22.9, 95% CI: 20.7–25.2; 17.3, 95% CI: 14.6–20.0; and 6.7, 95% CI: 5.9–7.6, respectively) and lowest in the αGI group (19.2, 95% CI: 17.2–21.3; 15.9 95% CI: 13.4–18.4; and 4.9 95% CI: 4.1–5.7, respectively). A statistically significant difference was detected between the following groups: αGI and DPP‐4 inhibitor, αGI and BG, and αGI and SU for treatment convenience subscale scores (P = 0.0193, 0.0357 and 0.0265, respectively). Significant differences were similarly found between DPP‐4 inhibitor and αGI, and DPP‐4 inhibitor and SU for satisfaction subscale scores (P = 0.0033 and 0.0492, respectively).
HbA1c values
The mean (±SD) HbA1c decreased from baseline to week 12 in all groups (at baseline, week 4 and week 12: 7.6 ± 0.5%, 7.2 ± 0.3% and 6.9 ± 0.7% in the DPP‐4 inhibitor group; 7.7 ± 0.9%, 7.3 ± 0.8% and 7.1 ± 0.8% in the BG group; 7.6 ± 0.5%, 7.5 ± 0.6%, and 7.1 ± 0.6% in the αGI group; and 7.5 ± 0.5%, 7.0 ± 0.6% and 6.7 ± 0.5% in the SU group, respectively). The mean HbA1c was lowest in the SU group at both weeks 4 and 12. There was a statistical difference between SU and αGI groups at week 4 (P = 0.0419), and no other statistical differences were found between groups at weeks 4 or 12. The mean change in HbA1c values from baseline to week 12 was −0.74% (95% CI: −1.38 to −0.11), −0.68% (95% CI: −0.91 to −0.44), −0.49% (95% CI: −0.79 to −0.20) and −0.74% (95% CI: −1.05 to −0.42) in the DPP‐4 inhibitor, BG, αGI, and SU groups, respectively.
Treatment adherence
A larger proportion of patients in the DPP‐4 inhibitor group took all the assigned medication, followed by the SU, αGI and BG groups at week 4 (92.9% [13/14], 86.7% [13/15], 64.3% [9/14] and 61.5% [8/13], respectively). Similarly, the DPP‐4 group had the highest proportion of patients who took all the assigned medication at week 12, followed by SU, BG and αGI groups (100% [14/14], 93.3% [14/15], 66.7% [8/12] and 64.3% [9/14], respectively). One patient in the DPP‐4 inhibitor group and two or more patients in the other groups missed their medication more than once a month.
Daily dosage
Of the 60 patients included in the analysis, 50 (83.3%) patients started the OHA treatment at or below the lowest standard dosage (Table 3)3. The dosage at week 12 was the same as the initial dosage for 49 patients (81.7%).
Table 3.
Dosages at baseline and week 12, and standard dosage
| Generic name | n | Dosage at baseline (mg/day) | Dosage at week 12 (mg/day) | Standard dosage (mg/day)3 |
|---|---|---|---|---|
| DPP‐4 inhibitor (n = 15) | ||||
| Sitagliptin | 1 | 25 | 50 | 50–100 |
| 8 | 50 | 50 | ||
| Teneligliptin | 1 | 10 | 10 | 20–40 |
| 1 | 20 | 20 | ||
| Linagliptin | 1 | 5 | 5 | 5 |
| Alogliptin | 3 | 25 | 25 | 25 |
| BG (n = 14) | ||||
| Metformin (Glycoran) | 2 | 500 | 500 | 500–750 |
| Metformin (Metgluco) | 1 | 250 | 250 | 500–1500 |
| 6 | 500 | 500 | ||
| 2 | 500 | 750 | ||
| 1 | 750 | 750 | ||
| 2 | 1000 | 1000 | ||
| αGI (n = 16) | ||||
| Miglitol | 2 | 50 | 50 | 150–225 |
| 1 | 100 | 150 | ||
| 1 | 150 | 100 | ||
| 4 | 150 | 150 | ||
| Voglibose | 4 | 0.6 | 0.6 | 0.6–0.9 |
| 4 | 0.9 | 0.9 | ||
| SU (n = 15) | ||||
| Glimepiride | 1 | 0.25 | 0.5 | 0.5–4 |
| 1 | 0.5 | 0.25 | ||
| 3 | 0.5 | 0.5 | ||
| 3 | 0.5 | 1 | ||
| 1 | 1 | 0.5 | ||
| 2 | 1 | 1 | ||
| Gliclazide | 1 | 10 | 10 | 20–120 |
| 3 | 20 | 20 | ||
αGI, α‐glucosidase inhibitors; BG, biguanides; DPP‐4, dipeptidyl peptidase‐4; SU, sulfonylureas.
Reported AEs
A total of 11 patients reported AEs as follows: abdominal distension and dry mouth (each n = 1) in the DPP‐4 inhibitor group; diarrhea, constipation, nausea and rash (each n = 1) in the BG group; diarrhea and abdominal distension (n = 3 and 1, respectively) in the αGI group; and cold sweat (n = 1) in the SU group. A total of 10 of the AEs were considered to be related to the assigned OHAs. Four patients discontinued the treatment because of OHA‐related AEs (abdominal distension and diarrhea each in one patient in the αGI group, dry mouth in one patient in the DPP‐4 inhibitor group, and rash in one patient in the BG group). No serious AEs were reported.
Discussion
In the present study, we compared the treatment satisfaction using the OHA‐Q between four classes of OHAs: DPP‐4 inhibitors, BGs, αGIs and SUs, which are commonly prescribed in Japan. The mean total and the three subscale scores at week 4 suggested that patients were most satisfied with the DPP‐4 inhibitor treatment. Furthermore, greater satisfaction sustained with high adherence, HbA1c improvement, and few AEs over 12 weeks reflect the actual popularity of DPP‐4 inhibitors in Japan for their ability to ameliorate β‐cell dysfunction with limited risk of hypoglycemia13, 14. Without previous studies that comprehensively compare several OHAs that are frequently prescribed, and that incorporate patient's perspectives, the present study could help to optimize the drug selection for patients with type 2 diabetes.
The findings support the well‐documented association among treatment satisfaction, treatment adherence5, 6, and the association between adherence and glycemic control7, 8, 9, 10. The ranking of the OHA‐Q total scores corresponded to the actual adherence level for each treatment. Furthermore, adherence level further corresponded to HbA1c level improvement. The DPP‐4 inhibitor might provide higher treatment satisfaction, because it is less frequently administered and causes less concern over AEs, which could motivate patients to adhere to the treatment, eventually leading to improved glycemic control. The previous report of the association between treatment adherence, glycemic control and quality of life15 might further suggest the possibility that DPP‐4 inhibitors could even improve the whole quality of life.
Alternatively, treatment that raises some concerns in patients might reduce satisfaction and weaken motivation for them to adhere, eventually falling short of successful glycemic control, as reflected in the BG and αGI groups. The BG and αGI groups scored significantly lower than the DPP‐4 inhibitor group in OHA‐Q total, and somatic symptoms and treatment convenience subscales, respectively. The item score showed that the BG group was particularly concerned regarding gastrointestinal AEs, and the αGI group regarding administration frequency, which are typical issues in BG2, 16, 17 and αGI, respectively. Such concerns might contribute to treatment dissatisfaction and eventually to smaller HbA1c improvement.
However, patients in the SU group were less satisfied with their treatment despite the HbA1c being maintained as low as in the DPP‐4 inhibitor group. OHA‐Q results suggest that patients in the SU group might have been particularly concerned about weight gain, which is a well‐known adverse effect of SUs1, 2, 16. This concern for SUs' influence on bodyweight might have surpassed the appreciation for glycemic improvement to suppress the overall satisfaction with SU.
Interestingly, from week 4 to 12, treatment satisfaction largely improved only in the BG group in terms of OHA‐Q total and somatic symptom subscale scores. Some of the adverse gastrointestinal symptoms might subside with continued treatment18. Alternatively, lower actual incidence of gastrointestinal AEs than expected during the early stage of the treatment could have resolved the pre‐existing anxiety.
Consistent with the present results, previous long‐term studies reported maintenance or further improvement in treatment satisfaction with DPP‐4 inhibitors over 26 and 52 weeks19, 20, 21. Furthermore, DPP‐4 inhibitors improved some aspects of quality of life including urinary frequency and paresthesia of the extremities22. These observations might suggest the DPP‐4 inhibitor as an option from patients' perspectives. Whereas, the more expensive cost of DPP‐4 inhibitors1, 3 is a concern for some patients. Given that lifelong self‐management in patients' daily lives is required for successful control of type 2 diabetes, patient‐centered care advocated as an approach for ‘providing care that is respectful of and responsive to individual patient preferences, needs and values, and ensuring that patient values guide all clinical decisions'23, 24 is imperative. Reflecting the patients’ perspectives in terms of self‐reported treatment satisfaction with each OHA, the present study adds important information for deciding the appropriate treatment based on patient‐centered care. Treatment should be selected by weighing a broad spectrum of important factors ranging from efficacy, safety, and patient pathophysiology to cost effectiveness and quality of life, in addition to the treatment satisfaction shown in the present study.
Some methodological limitations might require consideration on interpretation of the results. First, only a small number of patients were recruited in the present study (60 patients from 18 participating sites), which limits the generalizability of the findings. Larger‐scale studies are warranted to confirm the findings. Second, the study period of 12 weeks might be relatively short. However, based on the previous studies25, 26, 27, 28, 29, 30, we considered 12 weeks sufficient to detect the effect of newly‐initiated OHA treatment on OHA‐Q score. Third, the present study did not include all the OHAs currently available, and did not designate the dosages, as the aim lay in capturing the daily clinical practice. There might be the possibility that inclusion of other OHAs and at fixed doses could result in a different conclusion. Fourth, full OHA‐Q data could not be obtained for more than 10% of the recruited patients. Finally, we could not infer the relationships between OHA doses and OHA‐Q scores, as treatment was initiated at the lowest recommended dosage or lower3, and the initial dosages were continued until the end of the study for most of the patients, possibly driven by safety concerns of physicians. Nevertheless, the results show that even the low dosages can substantially improve HbA1c with few AEs.
In conclusion, among the four classes of OHAs in the present study, DPP‐4 inhibitors were the most preferable option in terms of treatment satisfaction. Patients might be satisfied with the DPP‐4 inhibitor treatment and thus be motivated to adhere to the treatment, which would likely result in better glycemic control. Patient‐centered treatment selection, including treatment satisfaction, as well as other important factors, could be a key driver for optimal selection of type 2 diabetes treatments.
Disclosure
HI has received lecture and/or consultant fees from Takeda Pharmaceutical Company, Ltd., Eli Lilly Japan K.K., Sanofi KK., Merck & Co., Inc., Astellas Pharma Inc., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Corporation., Daiichi Sankyo Company, Ltd., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Taisho Toyama Pharmaceutical Co., Ltd., SHIONOGI & CO., LTD., Kowa Pharmaceutical Co., Ltd., Boehringer Ingelheim, Novo Nordisk Pharma Ltd., Sumitomo Dainippon Pharma and Kyowa Hakko Kirin Co., Ltd. outside the submitted work. YH reports personal fees from Takeda Pharmaceutical Company, Ltd., Eli Lilly Japan K.K., Daiichi Sankyo Company, Ltd., Pfizer Japan Inc., Ono Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Astellas Pharma Inc. and Mitsubishi Tanabe Pharma Corporation outside the submitted work. YA received honorarium from Takeda Pharmaceutical Company, Ltd., Eli Lilly Japan K.K., Sanofi KK., Astellas Pharma Inc., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Company, Ltd., Ono Pharmaceutical Co., Ltd., AstraZeneca K.K., Taisho Toyama Pharmaceutical Co., Ltd., Boehringer Ingelheim., Novo Nordisk Pharma Ltd., Dainippon Sumitomo Pharma, Kyowa Hakko Kirin Co., Ltd, Chugai Pharmaceutical, Kissei Pharmaceutical and Merck & Co., Inc. outside the submitted work. MY reports personal fees from Mitsubishi Tanabe Pharma, AstraZeneca, Novo Nordisk, Sumitomo Dainippon Pharma, Eli Lilly Japan, Sanofi, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical, Ono Pharmaceutical, Novartis, Boehringer Ingelheim, Chugai Pharmaceutical, Kissei Pharmaceutical, Kowa Pharmaceutical, Kyowa Hakko Kirin, Shionogi, MSD and Astellas outside the submitted work. ST reports personal fees from Eli Lilly Japan K.K., Sanofi KK., Merck & Co., Inc., AstraZeneca K.K., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ono Pharmaceutical Co., Ltd. and Daiichi Sankyo Company, Ltd. outside the submitted work.
Acknowledgments
This study was funded by the Waksman Foundation of Japan Inc. We are deeply grateful for the cooperation of the participating patients. Re Co., Ltd. provided data management. Clinical study support, Inc. assisted with statistical analysis and draft editing. This study was supported by following investigators participating in this study:Tenri Hospital, Nakai Clinic, Izumi Clinic, Nara Medical University, Nijoekimae Clinic, Yano Clinic, Hikari Clinic, Kindai University Nara Hospital, Nara City Hospital, Matsumura Clinic, Nishimoto Medical Clinic, Sakagami Medical Clinic, Seiwadai Clinic, Yashima Medical Clinic, Kim Clinic, Sasazuka Inoue Clinic, Shimomura Clinic, Tadaoka Clinic and Wada Clinic.
J Diabetes Investig 2018; 9: 137–145
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000006986
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 2. Bolen S, Feldman L, Vassy J, et al Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007; 147: 386–399. [DOI] [PubMed] [Google Scholar]
- 3. The Japan Diabetes Society . Treatment Guide for Diabetes 2016–2017. Tokyo: Bunkodo, 2016. (in Japanese). [Google Scholar]
- 4. American Diabetes Association . Standards of Medical Care in Diabetes‐2016. Abridged for Primary Care Providers. Clin Diabetes 2016; 34: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biderman A, Noff E, Harris SB, et al Treatment satisfaction of diabetic patients: what are the contributing factors? Fam Pract 2009; 26: 102–108. [DOI] [PubMed] [Google Scholar]
- 6. Barbosa CD, Balp MM, Kulich K, et al A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 2012; 6: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pladevall M, Williams LK, Potts LA, et al Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care 2004; 27: 2800–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rhee MK, Slocum W, Ziemer DC, et al Patient adherence improves glycemic control. Diabetes Educ 2005; 31: 240–250. [DOI] [PubMed] [Google Scholar]
- 9. Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care 2002; 25: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 10. Rozenfeld Y, Hunt JS, Plauschinat C, et al Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care 2008; 14: 71–75. [PubMed] [Google Scholar]
- 11. Ishii H, Oda E. Reproducibility and validity of a satisfaction questionnaire on hypoglycemic agents: the Oral Hypoglycemic Agent Questionnaire (OHA‐Q). Diabetol Int 2012; 3: 152–163. [Google Scholar]
- 12. Ishii H, Oda E. Characteristics of patients with type 2 diabetes mellitus grouped by 5 classes of oral hypoglycemic agents based on assessment of treatment satisfaction. Diabetol Int 2014; 5: 134–143. [Google Scholar]
- 13. Kohro T, Yamazaki T, Sato H, et al Trends in antidiabetic prescription patterns in Japan from 2005 to 2011 Impact of the introduction of dipeptidyl peptidase‐4 inhibitors. Int Heart J 2013; 54: 93–97. [DOI] [PubMed] [Google Scholar]
- 14. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016; 7: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishii H, Anderson JH Jr, Yamamura A, et al Improvement of glycemic control and quality‐of‐life by insulin lispro therapy: assessing benefits by ITR‐QOL questionnaires. Diabetes Res Clin Pract 2008; 81: 169–178. [DOI] [PubMed] [Google Scholar]
- 16. Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 2005; 65: 385–411. [DOI] [PubMed] [Google Scholar]
- 17. Bennett WL, Wilson LM, Bolen S, et al Oral Diabetes Medications for Adults With Type 2 Diabetes: An Update. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. https://www.effectivehealthcare.ahrq.gov/ehc/products/607/2215/diabetes-update-2016-report.pdf. Accessed July 19, 2016. [PubMed] [Google Scholar]
- 18. Okayasu S, Kitaichi K, Hori A, et al The evaluation of risk factors associated with adverse drug reactions by metformin in type 2 diabetes mellitus. Biol Pharm Bull 2012; 35: 933–937. [DOI] [PubMed] [Google Scholar]
- 19. Best JH, Rubin RR, Peyrot M, et al Weight‐related quality of life, health utility, psychological well‐being, and satisfaction with exenatide once weekly compared with sitagliptin or pioglitazone after 26 weeks of treatment. Diabetes Care 2011; 34: 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies M, Pratley R, Hammer M, et al Liraglutide improves treatment satisfaction in people with Type 2 diabetes compared with sitagliptin, each as an add on to metformin. Diabet Med 2011; 28: 333–337. [DOI] [PubMed] [Google Scholar]
- 21. Pratley R, Nauck M, Bailey T, et al One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel‐group, open‐label trial. Int J Clin Pract 2011; 65: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakamoto Y, Oyama J, Ikeda H, et al Effects of sitagliptin beyond glycemic control: focus on quality of life. Cardiovasc Diabetol 2013; 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Institute of Medicine (US) Committee on Quality of Health Care in America . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press (US); 2001. PubMed PMID: 25057539. https://www.ncbi.nlm.nih.gov/pubmed/25057539. Accessed July 19, 2016. [PubMed] [Google Scholar]
- 25. Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well‐being with insulin glargine compared with NPH in patients with Type 1 diabetes. Diabet Med 2001; 18: 619–625. [DOI] [PubMed] [Google Scholar]
- 26. Onishi Y, Koshiyama H, Imaoka T, et al Safety of exenatide once weekly for 52 weeks in Japanese patients with type 2 diabetes mellitus. Journal of Diabetes Investigation 2013; 4: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inoue K, Maeda N, Fujishima Y, et al Long‐term impact of liraglutide, a glucagon‐like peptide‐1 (GLP‐1) analogue, on body weight and glycemic control in Japanese type 2 diabetes: an observational study. Diabetology & Metabolic Syndrome 2014; 6: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marre M, Shaw J, Brändle M, et al Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bays HE, Weinstein R, Law G, et al Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring) 2014; 22: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishii H, Niiya T, Ono Y, et al Improvement of quality of life through glycemic control by liraglutide, a GLP‐1 analog, in insulin‐naive patients with type 2 diabetes mellitus: the PAGE1 study. Diabetol Metab Syndr 2017; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]


