Abstract
Aims/Introduction
It is suggested that a positive psychosocial condition has a good effect on health and glycemic control. However, there has been no research to evaluate the association between positive psychosocial factors and diabetic nephropathy (DN). The aim of the present study was to evaluate the association between psychosocial factors and DN in patients with type 2 diabetes.
Material and Methods
To assess psychosocial condition, six indicators (happiness score, Life Orientation Test‐revised score as an indicator of dispositional optimism, laughter frequency, self‐awareness of stress, social network and social support) were assessed by a self‐administered questionnaire, and associations between these psychosocial indicators and the presence of DN were examined.
Results
A cross‐sectional analysis of patients with (n = 123) and without DN (n = 220) showed that a high score for happiness (odds ratio [OR] per 1 standard deviation 0.71, 95% confidence interval [CI] 0.57–0.89, P = 0.003), high Life Orientation Test‐revised score (OR per 1 standard deviation 0.77, 95% CI: 0.61–0.98, P = 0.035), less self‐awareness of stress (OR 0.56, 95% CI: 0.34–0.90, P = 0.017), high connection of social network (OR 0.55, 95% CI: 0.35–0.87, P = 0.010) and high social support (OR 0.61, 95% CI: 0.38–0.96, P = 0.035) were associated with a reduced risk of prevalence of DN. Similar results were observed even after adjustment for the following conventional risk factors of DN: age, sex, duration of diabetes, hemoglobin A1c, hypertension, dyslipidemia and current smoking.
Conclusions
The present study showed that five out of six prespecified indicators of psychosocial condition were significantly associated with the presence of DN in Japanese patients with type 2 diabetes.
Keywords: Diabetic nephropathy, Psychosocial factors, Social support
Introduction
Diabetic nephropathy (DN), a major complication of diabetes, occurs in 20–40% of patients with diabetes, and is the leading cause of end‐stage renal disease. Among patients with type 2 diabetes mellitus, DN is one of the most common causes of hospitalization associated with cardiovascular events, cardiovascular mortality and also total mortality1. As large clinical studies, such as the United Kingdom Prospective Diabetes Study and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation, have shown that good glycemic control and blood pressure control suppress progression of DN, patients with diabetes are instructed to maintain a healthy diet and to exercise, and are prescribed various antidiabetic and antihypertension drugs in a clinical situation. However, the incidence of dialysis with DN has not decreased, despite improvements in the methods of diabetes treatment during the past decade2. Therefore, other renoprotective strategies aiming at the decrease of DN incidence and progression need to be considered.
Recently, the relationship between health and psychosocial factors, including psychological aspects and social support level, has been discussed. Among patients with diabetes, although convincing mechanisms still remain unclear, it was reported that elevated stress and depressive symptoms were risk factors of ischemic heart disease and stroke3. In contrast, it was reported that positive psychological factors, such as self‐esteem, active coping behavior or resilience, influenced glycemic control4, 5, 6, and that a good structure of social support helped patients to maintain recommended diets and exercise for weight loss7. Furthermore, laughing loudly reduced postprandial glucose levels8, and a laughter and exercise program had good effects on glucose control9. These previous reports suggest that a positive psychosocial condition would have a good effect on health and glycemic control, even among diabetes patients.
However, no research has evaluated the association between positive psychosocial factors and DN. Therefore, in the present study, we aimed to evaluate the association between psychosocial factors and DN, as assessed by a self‐administered questionnaire in patients with type 2 diabetes.
Methods
Study design and study population
In the present study, baseline data obtained from a prospective observational study were used to evaluate the association between positive psychosocial factors and diabetic complications in type 2 diabetes patients. Screening of the study participants was carried out consecutively during a 13‐month period (from August 2014 to August 2015) at Osaka University Hospital in Osaka, Japan. The eligibility criteria were as follows: (i) diagnosed as type 2 diabetes based on the criteria of the Japan Diabetes Society; and (ii) aged ≥30 years, but <80 years (regardless of sex). No special exclusion criteria were set. Patients that met the eligibility criteria were asked if they could participate in the present study, and all the patients who agreed to participate were registered. A total of 349 patients (56.2% men; age 65.1 ± 9.6 years) were enrolled. The study protocol was approved by the ethics committee of Osaka University Hospital. Written informed consent was obtained from all the participants after they received a full explanation of the study.
Assessment of psychosocial conditions
To assess positive psychological conditions, six indicators were used, each based on an individual questionnaire. The participants responded to all of these questionnaires at one time, and cases in which the response to a questionnaire was absent or incomplete were excluded from the statistical analyses. The relationships between DN and the results of each questionnaire, and correlations between the indicators were assessed. In some cases, participants were divided into two groups, each consisting of approximately the same number of participants, based on median or mean values of each indicator (Figure S1). The indicators used were as follows:
Happiness score
Evaluated based on the self‐scoring item, ‘How do you rate your happiness status, with 10 as the top end?’ This single‐item score was evaluated from 1 to 10, with 10 as the highest level of happiness.
Dispositional optimism
Defined as the tendency to expect good outcomes in the future. To evaluate dispositional optimism, the Life Orientation Test‐revised (LOT‐R) was used10. LOT‐R consists of a set of statements to which people indicate their agreement or disagreement on a multipoint scale. The points on each scale are summed up to a total score (range 0–24), and a higher score means a higher level of dispositional optimism.
Laughter frequency
The following self‐anchoring single question was used: ‘How often do you laugh loudly in daily life?’ The laughter frequency was scaled as follows: (i) rarely; (ii) one to two times a month; (iii) one to five times a week; and (iv) almost every day. We divided the participants into two groups, the ‘not‐every day laugher’ group (i – iii) and the ‘almost‐every day laugher’ group (iv).
Self‐awareness of stress
The following self‐anchoring single question was used: ‘How often do you suffer stress from your work or daily life?’ The response was scaled as follows: (i) always; (ii) often; (iii) sometimes; and (iv) rarely. The participants who chose (i) or (ii) were categorized to a ‘more‐stress’ group, and those who chose (iii) or (iv) were categorized to a ‘less‐stress’ group.
Social network and social support
Social network and support are broadly defined as the existence or connection of people on whom one can rely. In the present study, two kinds of instruments were used to evaluate this factor. One was the Social Network Index (SNI), which was designed for the Alameda study, containing a question about the number of relatives or friends one feels close to, and the frequency of the contacts with informal and formal association in informal or formal group associations11. The SNI comprises the numbers of social ties. The SNI score ranges from 0 to 4, and in this study the score was grouped into two categories: score 0 and 1 as the category of low‐connection of social network, and score 2–4 as that of high‐connection of social network. The other instrument used was the Enhancing Recovery in Coronary Heart Disease Patients Social Support Instrument (ESSI), which was used in the Enhancing Recovery in Coronary Heart Disease Patients trial12, 13. The ESSI is a seven‐item self‐report measure, and summed up for a total score, with higher scores indicating greater social support. The participants were grouped into two categories based on a total score of 26, the median of the present study; a score of 5–26 as the category of less‐social support and a score of 27–31 as that of more‐social support.
Assessment of clinical and biochemical characteristics
Clinical and biochemical data were collected at baseline. A structured questionnaire was used to determine medical history, including duration of diabetes, current medication use, cigarette smoking and alcohol consumption status. Current smoker was defined as one who currently smokes any amount of cigarettes, and a person who drinks alcohol habitually as one who drinks once or more a week. Blood pressure was measured at rest with a mercury and/or automated sphygmomanometer. Fasting blood samples were collected, and hemoglobin A1c, glycoalbumin, serum total, low‐density lipoprotein and high‐density lipoprotein cholesterol, serum triglyceride, and creatinine levels were measured using standard laboratory protocols. The presence of hypertension and dyslipidemia was diagnosed by the primary doctors based on the criteria of the Japanese Society of Hypertension14 and the Japan Atherosclerosis Society15.
Assessment of diabetic microvascular complications
Estimated glomerular filtration rate (eGFR; mL/min/1.73 m2) was calculated using the equation proposed by the Japanese Society of Nephrology16. Urinary albumin and creatinine (Cr) concentrations were measured using early morning spot urine, and urinary albumin‐to‐creatinine ratio was calculated. According to the criteria of the Japan Diabetes Society17, the presence and the stage of DN were determined based on the eGFR and the presence of albuminuria or proteinuria as follows: normoalbuminuria, urinary albumin‐to‐creatinine ratio <30 mg/gCr; microalbuminuria, 30–300 mg/gCr; or macroalbuminuria, >300 mg/gCr. Proteinuria of >0.5 g/gCr was defined as overt proteinuria. The participants with a albumin‐to‐creatinine ratio in the normal range and eGFR >30 mL/min/1.73 m2 were categorized as the non‐DN group, and the participants with micro‐ or macroalbuminuria, overt proteinuria and/or eGFR <30 mL/min/1.73 m2 and under dialysis therapy were categorized as the DN group.
Diabetic retinopathy was defined by the presence of characteristic changes, such as microaneurysms, hemorrhages, cotton wool spots, new vessel formation and so on. The participants with past treatment of retinopathy, such as laser photocoagulation and vitreous surgery, were also categorized to the group with diabetic retinopathy. The participants were diagnosed by ophthalmologists based on the criteria of the Japan Diabetes Society.
To evaluate the presence of diabetic neuropathy, the abbreviated diagnostic criteria proposed by the Diabetic Neuropathy Study Group in Japan were used18. The criteria consist of three items: (i) symptom such as tingling pain, numbness and cramping; (ii) absence of the Achilles tendon reflex; and (iii) reduced sense of vibration. When more than two items were positive, the participant was diagnosed as having diabetic neuropathy.
Statistical analysis
Data were presented as mean ± standard deviation (SD) or median and interquartile range for continuous variables, or as percentages for dichotomous variables. Between‐group differences of the average were compared using the unpaired t‐test for parametric data, and the Mann–Whitney U‐test for non‐parametric data. Between‐group differences of numbers and percentages were compared using the χ2‐test, and relationships among six indicators of psychosocial factors were assessed using Spearman's rank correlation coefficient. Multiple logistic regression models were used to estimate the odds ratio and 95% confidence interval of each psychosocial factor as a risk factor for DN. A P‐value of <0.05 was considered to show significance. The statistical analyses were carried out using Spss version 22 (SPSS, Chicago, Illinois, USA).
Results
Participants
A total of 349 participants were enrolled in the present study, and the evaluation of DN was successful in 343 participants. Among them, 123 participants were diagnosed as having DN at baseline. The clinical characteristics of the participants with and without DN are described in Table 1. The duration of diabetes, systolic blood pressure and serum triglyceride levels were significantly higher in participants with DN as compared with those without DN (P < 0.05). Frequencies of male sex, current smoking, hypertension, dyslipidemia, insulin and antihypertensive medication use were also higher in participants with DN (P < 0.05). There was no significant difference between the two groups regarding the other parameters, including hemoglobin A1c levels. These findings were almost consistent with previous reports.
Table 1.
Baseline characteristics of participants with or without diabetic nephropathy
| Total n = 343 | Non‐diabetic nephropathy n = 220 | Diabetic nephropathy n = 123 | P‐value | |
|---|---|---|---|---|
| Sex (male) | 194 (56.6%) | 115 (52.3%) | 79 (64.2%) | 0.032 |
| Age | 65.1 ± 9.5 | 64.9 ± 9.0 | 65.4 ± 10.4 | 0.654 |
| Duration of diabetes (years) | 15.1 ± 9.9 | 13.9 ± 9.5 | 17.2 ± 10.3 | 0.003 |
| Body mass Index (kg/m2) | 25.9 ± 5.3 | 25.6 ± 5.3 | 26.5 ± 5.3 | 0.154 |
| Systolic blood pressure (mmHg) | 131.6 ± 16.4 | 130.1 ± 15.4 | 134.4 ± 17.9 | 0.020 |
| Diastolic blood pressure (mmHg) | 75.0 ± 12.2 | 74.9 ± 11.3 | 75.1 ± 13.8 | 0.845 |
| Alcohol (once a week or more) | 115 (33.9%) | 76 (34.7%) | 39 (32.5%) | 0.682 |
| Current smoker | 43 (12.6%) | 19 (8.7%) | 24 (19.7%) | 0.003 |
| HbA1c (%) | 7.8 ± 1.5 | 7.8 ± 1.4 | 7.9 ± 1.7 | 0.632 |
| Glycated albumin (%) | 21.0 ± 5.1 | 21.3 ± 5.1 | 20.6 ± 5.1 | 0.243 |
| Creatinine (mg/dL) | 0.99 ± 1.1 | 0.78 ± 0.20 | 1.35 ± 1.71 | <0.001 |
| eGFR (mL/min/1.73 m2) | 66.7 ± 20.6 | 70.5 ± 16.1 | 59.9 ± 25.4 | <0.001 |
| Total‐cholesterol (mg/dL) | 188.5 ± 37.3 | 187.3 ± 37.4 | 190.8 ± 37.2 | 0.429 |
| Triglyceride (mg/dL) | 117 (84–169) | 105 (76–155) | 139 (104–185) | <0.001 |
| LDL cholesterol (mg/dL) | 106.7 ± 28.0 | 106.9 ± 26.7 | 106.3 ± 30.2 | 0.834 |
| HDL cholesterol (mg/dL) | 54.4 ± 16.5 | 55.9 ± 16.2 | 51.9 ± 16.9 | 0.036 |
| Hypertension | 240 (70.0%) | 141 (64.1%) | 99 (80.5%) | 0.001 |
| Dyslipidemia | 226 (65.9%) | 135 (61.4%) | 91 (74.0%) | 0.018 |
| Malignancy | 75 (21.9%) | 55 (25.0%) | 20 (16.3%) | 0.060 |
| Macroangiopathy | 68 (19.8%) | 27 (12.3%) | 41 (33.3%) | <0.001 |
| Nephropathy | – | – | ||
| Microalbuminuria | 75 (61.0%) | |||
| Macroalbuminuria and eGFR ≥30 (mL/min/1.73 m2) | 33 (26.8%) | |||
| Macroalbuminuria and eGFR <30 (mL/min/1.73 m2) | 11 (8.9%) | |||
| Under dialysis | 4 (3.3%) | |||
| Retinopathy (n = 312) | 104 (33.3%) | 43 (21.3%) | 61 (55.5%) | <0.001 |
| Neuropathy (n = 328) | 153 (46.6%) | 91 (42.7%) | 62 (53.9%) | 0.053 |
| Medications | ||||
| Antidiabetics | 314 (91.5%) | 197 (89.5%) | 117 (95.1%) | 0.075 |
| Insulin | 115 (33.5%) | 59 (26.8%) | 56 (45.5%) | <0.001 |
| Antihypertensives | 223 (65.0%) | 130 (59.1%) | 93 (75.6%) | 0.002 |
| Renin–angiotensin system inhibitor | 182 (53.1%) | 104 (47.3%) | 78 (63.4%) | 0.004 |
| Lipid‐lowering drug | 174 (50.7%) | 107 (48.6%) | 67 (54.5%) | 0.300 |
| Psychosocial factors | ||||
| Happiness score (range 1–10, higher = happier) | 7.3 ± 1.7 | 7.5 ± 1.6 | 6.9 ± 1.9 | 0.003 |
| Optimism: LOT‐R (n = 336) (range 0–24, higher = greater optimism) | 13.5 ± 3.1 | 13.7 ± 3.1 | 13.0 ± 3.0 | 0.033 |
| Laughter frequency (n = 340) | ||||
| Non‐every day laughers | 205 (60.3%) | 129 (58.6%) | 76 (63.3%) | 0.398 |
| Almost every day laughers | 135 (39.7%) | 91 (41.4%) | 44 (36.7%) | |
| Self‐awareness of stress (n = 342) | ||||
| More stress (‘always’ and ‘often’) | 113 (33.0%) | 63 (28.8%) | 50 (40.7%) | 0.025 |
| Less stress (‘sometimes’ and ‘rarely’) | 229 (67.0%) | 156 (71.2%) | 73 (59.3%) | |
| Social network: SNI (n = 337) | ||||
| Low connection (SNI score; 0 and 1) | 153 (45.4%) | 87 (40.3%) | 66 (54.5%) | 0.012 |
| High connection (SNI score; 2–4) | 184 (54.6%) | 129 (59.7%) | 55 (45.5%) | |
| Social support: ESSI (n = 336) | ||||
| Less support (ESSI score: 5–26) | 176 (52.4%) | 102 (47.2%) | 74 (61.7%) | 0.011 |
| More support (ESSI score: 27–31) | 160 (47.6%) | 114 (52.8%) | 46 (38.3%) | |
Values are shown as mean ± standard deviation, median (interquartile) or number (percentage). Quantitative data between the groups of non‐diabetic and diabetic nephropathy were compared by two‐tailed unpaired t‐test or Mann–Whitney test, depending on the distribution pattern of the data. Categorical data were analyzed using the χ2‐test or Fisher's exact test. The threshold of statistical significance was defined as P < 0.05. Macrovascular disease includes ischemic heart disease, cerebrovascular disease and peripheral artery disease. Microalbuminuria is urinary albumin and creatinine ≥30 and <300 mg/gCr, and macroalbuminuria is urinary albumin and creatinine ≥300 mg/gCr. eGFR, estimated glomerular filtration rate; ESSI, the Enhancing Recovery in Coronary Heart Disease Patients Social Support Instrument; HbA1c, hemoglobin A1c; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low density lipoprotein cholesterol; LOT‐R, Life Orientation Test‐revised; SNI, the Social Network Index; UACR, urinary albumin‐to‐creatinine ratio.
Association between psychosocial condition and clinical and biochemical characteristics
There were significant associations among the six indicators of positive psychosocial factors: happiness score, optimism, laughter frequency, self‐awareness of stress, and social network and support. In particular, a higher happiness score was associated with a higher level of optimism, and with more social support with greater relative strength (P < 0.001 for all; Figure 1). We also carried out factor analysis on these six indicators with the method of maximum likelihood, and found one factor with an Eigenvalue >1 (2.77). This one‐factor structure explained 46% of variance, and all six indicators had absolute loading values >0.40. This result of factor analysis supported our assumption that these six indicators reflect one factor: ‘positive psychosocial factor. ’ (Table S3)
Figure 1.
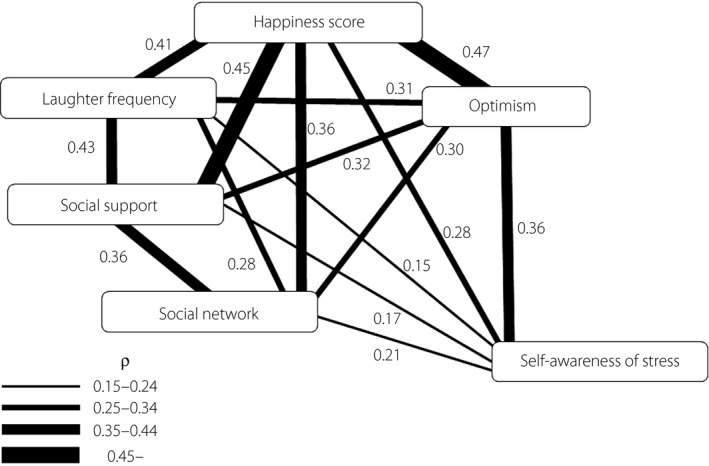
The relationships among indicators of psychosocial factors. Relationships among six indicators of psychosocial factors were assessed using Spearman's rank correlation coefficient. The strength of the relationship is shown by ρ‐values and the width of the connecting line.
Older age was associated with a higher score of happiness and optimism, and less stress‐awareness, but was not associated with social support and network or laughter frequency. Women showed a higher happiness score and more social support status, and were more likely to laugh every day as compared with men. Regarding the other indicators, there was no sex difference (data not shown).
Age‐ and sex‐adjusted associations between the aforementioned indicators of psychosocial conditions and clinical and biochemical characteristics were evaluated (Tables 2 and 3). The happiness score was inversely associated with body mass index (β = −0.13, P = 0.012) and serum low‐density lipoprotein cholesterol (β = −0.12, P = 0.012). The LOT‐R score was positively associated with drinking frequency (OR per 1 SD 1.58, 95% CI: 1.22–2.07, P = 0.001). The participants who laughed every day and those who felt less stress in daily life showed the tendency to consume alcohol more frequently (laugher every day OR 1.81 95% CI: 1.09–3.03, P = 0.023; less stress‐awareness OR 1.80 95% CI: 1.05–3.11, P = 0.033). Laughter frequency and self‐awareness of stress were also inversely associated with serum low‐density lipoprotein cholesterol levels (β = −0.12, P = 0.025 and β = −0.13, P = 0.017, respectively). Social network was inversely associated with the presence of hypertension: high connection was associated with a reduced risk of the presence of hypertension (OR 0.58, 95% CI: 0.36–0.95, P = 0.031). There were no relationships between psychosocial conditions and medication for diabetes or use of insulin.
Table 2.
Associations between psychosocial factors and clinical characteristics (health behaviors and complicated disease)
| Current smoker | Alcohol consumption frequency | Hypertension | Dyslipidemia | Medication for diabetes | Use of insulin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Happiness score (range 1–10, higher = happier) | 0.97 (0.70–1.36) | 0.893 | 1.25 (0.98–1.61) | 0.076 | 0.83 (0.65–1.05) | 0.123 | 0.91 (0.72–1.15) | 0.422 | 1.06 (0.73–1.53) | 0.772 | 0.97 (0.77–1.21) | 0.783 |
| Optimism (LOT‐R) (range 0–24, higher = greater optimism) | 1.09 (0.78–1.53) | 0.620 | 1.58 (1.22–2.07) | 0.001 | 0.84 (0.66–1.07) | 0.159 | 0.90 (0.71–1.14) | 0.366 | 1.14 (0.78–1.67) | 0.505 | 1.03 (0.82–1.31) | 0.776 |
| Laughter frequency (non‐every day/almost every day) | 1.41 (0.71–2.82) | 0.327 | 1.81 (1.09–3.03) | 0.023 | 0.84 (0.51–1.37) | 0.482 | 1.02 (0.64–1.64) | 0.939 | 1.96 (0.83–4.66) | 0.127 | 1.28 (0.80–2.04) | 0.307 |
| Self‐awareness of stress (more‐stress/less‐stress) | 1.31 (0.63–2.75) | 0.465 | 1.80 (1.05–3.11) | 0.033 | 1.14 (0.69–1.89) | 0.608 | 0.71 (0.43–1.17) | 0.181 | 0.63 (0.26–1.53) | 0.312 | 1.11 (0.68–1.81) | 0.688 |
| Social network (low‐connection/high‐connection) | 1.33 (0.68–2.62) | 0.402 | 1.47 (0.90–2.40) | 0.125 | 0.58 (0.36–0.95) | 0.031 | 1.55 (0.98–2.44) | 0.062 | 1.11 (0.52–2.38) | 0.792 | 0.73 (0.46–1.15) | 0.172 |
| Social support (less‐support/more‐support) | 1.34 (0.67–2.68) | 0.408 | 1.04 (0.63–1.70) | 0.884 | 0.70 (0.43–1.14) | 0.152 | 1.57 (0.98–2.52) | 0.060 | 2.04 (0.90–4.62) | 0.088 | 0.94 (0.59–1.49) | 0.780 |
Logistic regression analyses adjusted for sex and age were carried out to select the psychosocial factors significantly associated with clinical characteristics (current smoking, alcohol consumption, presence of hypertension, presence of dyslipidemia, medication for diabetes and use of Insulin.). Odds ratios (OR) (95% confidence interval [CI]) per 1 standard deviation are shown for the happiness score and Life Orientation Test‐revised (LOT‐R) score. The threshold of statistical significance was defined as P < 0.05. Alcohol consumption frequency was defined as drinking alcohol more than once per week.
Table 3.
Relationships between psychosocial factors and clinical parameters
| Duration of diabetes (years) | BMI (kg/m2) | HbA1c (%) | GA (%) | SBP (mmHg) | T‐chol (mg/dL) | TG (mg/dL) | LDL‐C (mg/dL) | HDL‐C (mg/dL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | β | P | β | P | β | P | β | P | |
| Happiness score (range 1–10, higher = happier) | −0.02 | 0.687 | −0.13 | 0.012 | −0.08 | 0.159 | −0.09 | 0.122 | 0.04 | 0.424 | −0.10 | 0.073 | −0.08 | 0.117 | −0.12 | 0.026 | −0.05 | 0.377 |
| Optimism (LOT‐R) (range 0–24, higher =greater optimism) | 0.04 | 0.429 | −0.02 | 0.644 | −0.01 | 0.883 | −0.01 | 0.834 | −0.00 | 0.953 | 0.02 | 0.751 | −0.01 | 0.896 | −0.01 | 0.791 | 0.02 | 0.710 |
| Laughter frequency (non‐every day/almost every day) | −0.05 | 0.375 | 0.06 | 0.225 | 0.01 | 0.899 | 0.01 | 0.935 | −0.00 | 0.950 | −0.08 | 0.157 | 0.02 | 0.743 | −0.12 | 0.025 | −0.04 | 0.439 |
| Self‐awareness of stress (more stress/less stress) | −0.00 | 0.999 | 0.01 | 0.871 | 0.01 | 0.878 | −0.05 | 0.431 | −0.06 | 0.254 | −0.12 | 0.035 | −0.02 | 0.682 | −0.13 | 0.017 | 0.02 | 0.784 |
| Social network (low connection/high connection) | 0.00 | 0.979 | −0.06 | 0.269 | 0.04 | 0.445 | 0.03 | 0.565 | −0.03 | 0.571 | −0.04 | 0.509 | −0.06 | 0.297 | 0.01 | 0.839 | −0.07 | 0.221 |
| Social support (less support/more support) | −0.04 | 0.414 | 0.01 | 0.846 | 0.00 | 0.981 | −0.09 | 0.121 | −0.02 | 0.707 | −0.09 | 0.137 | 0.09 | 0.116 | −0.11 | 0.051 | −0.06 | 0.290 |
Multivariate linear regression analyses adjusted for sex and age were carried out to select the psychosocial factors significantly associated with clinical characteristics. The threshold of statistical significance was defined as P < 0.05. BMI, body mass index; HbA1c, hemoglobin A1c; HDL‐C, high density lipoprotein cholesterol; GA, glycoalbumin; LDL‐C, low density lipoprotein cholesterol; LOT‐R, Life Orientation Test‐revised; SBP, systolic blood pressure; T‐chol, total cholesterol; TG, triglyceride.
Association between psychosocial conditions and DN
Participants with DN showed a lower happiness score, lower LOT‐R score, lower level of social support and network, and felt more stress in daily life and work than the participants without DN. Although laugher every day showed a tendency to be more frequent in the non‐DN group as compared with the DN group, it did not reach statistical significance (Tables 1 and S1). In the logistic regression analysis adjusted for age and sex, a high happiness score (OR per 1 SD 0.71, 95% CI: 0.57–0.89, P = 0.003), high LOT‐R score (OR per 1 SD 0.77, 95% CI: 0.61–0.98, P = 0.035), less self‐awareness of stress (OR 0.56, 95% CI: 0.34–0.90, P = 0.017), high connection of social network (OR 0.55, 95% CI: 0.35–0.87, P = 0.010) and more social support (OR 0.61, 95% CI: 0.38–0.96, P = 0.035) were associated with a reduced risk of the presence of DN (Figure 2).
Figure 2.
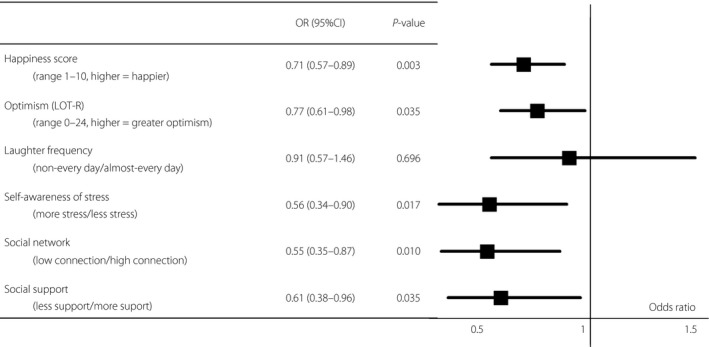
Odds ratios (OR) for diabetic nephropathy of six indicators of psychosocial factors adjusted for age and sex. Logistic regression analysis was carried out to evaluate whether psychosocial factors were significantly associated with an increase in the risk of diabetic nephropathy. The threshold of statistical significance was defined as P < 0.05. The OR of ‘happiness score’ and ‘optimism’ are shown as per 1 standard deviation. In the logistic regression analysis adjusted for age and sex, a high happiness score (OR per 1 standard deviation 0.72, 95% CI: 0.57–0.90, P = 0.004), high LOT‐R score (OR per 1 standard deviation 0.79, 95% CI: 0.62–0.99, P = 0.044), less self‐awareness of stress (OR 0.59, 95% CI: 0.36–0.94, P = 0.028), high connection of social network (OR 0.57, 95% CI: 0.37–0.90, P = 0.015) and more social support (OR 0.61, 95% CI: 0.38–0.96, P = 0.042) were associated with reduced risk of presence of diabetic nephropathy. CI, confidence interval; LOT‐R, Life Orientation Test‐revised; OR, odds ratio.
Similar results were observed even after adjustment for other conventional risk factors of DN (age, sex, duration of diabetes, prevalence of hypertension, prevalence of dyslipidemia, hemoglobin A1c, current smoking behavior and the presence of macroangiopathy; Table 4).
Table 4.
Multivariate associations with diabetic nephropathy and psychosocial factors
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Happiness score (range 1–10, higher = happier) | 0.74 (0.58–0.93) | 0.011 | 0.74 (0.58–0.94) | 0.013 | 0.74 (0.58–0.94) | 0.013 | 0.72 (0.56–0.92) | 0.008 |
| Optimism: LOT‐R (range 0–24, higher = greater optimism) | 0.74 (0.57–0.97) | 0.026 | 0.74 (0.57–0.97) | 0.027 | 0.74 (0.57–0.97) | 0.027 | 0.71 (0.54–0.93) | 0.013 |
| Laughter frequency (non‐every day/almost every day) | 0.90 (0.55–1.48) | 0.678 | 0.92 (0.56–1.52) | 0.747 | 0.91 (0.55–1.51) | 0.725 | 0.79 (0.47–1.34) | 0.388 |
| Self‐awareness of stress (more stress/less stress) | 0.51 (0.31–0.85) | 0.010 | 0.54 (0.32–0.90) | 0.019 | 0.54 (0.32–0.91) | 0.021 | 0.49 (0.29–0.83) | 0.008 |
| Social network (low connection/high connection) | 0.53 (0.33–0.86) | 0.010 | 0.55 (0.34–0.89) | 0.015 | 0.55 (0.34–0.90) | 0.016 | 0.54 (0.33–0.90) | 0.017 |
| Social support (less support/more support) | 0.57 (0.35–0.93) | 0.026 | 0.59 (0.36–0.98) | 0.040 | 0.59 (0.36–0.97) | 0.038 | 0.51 (0.30–0.86) | 0.011 |
Multivariate logistic regression analysis was carried out to evaluate whether positive psychosocial factors were significantly associated with an increase in the risk of diabetic nephropathy. The threshold of statistical significance was defined as P < 0.05. Odds ratios (OR) (95% confidence interval [CI]) per 1 standard deviation are shown for the happiness score and Life Orientation Test‐revised (LOT‐R) score. Model 1: adjusted for age, sex, duration of diabetes mellitus, hypertension, dyslipidemia, current smoker and hemoglobin A1c. Model 2: adjusted for all factors in Model 1, plus body mass index and systolic blood pressure. Model 3: adjusted for all factors in model 1, plus body mass index, systolic blood pressure and treatment with an renin‐angiotensin system (RAS) inhibitor. Model 4: adjusted for all factors in model 1, plus body mass index, systolic blood pressure, treatment with an RAS and the presence of macroangiopathy.
Discussion
Previous studies have suggested that positive psychosocial condition has good effects on health, even among diabetes patients6, 7, 9. However, there has still been no research that has evaluated the association between positive psychosocial factors and diabetic microangiopathies. This is the first study to evaluate the association between psychosocial factors assessed by self‐administered questionnaire and DN, one of the most clinically important diabetic microangiopathies, in Japanese patients with type 2 diabetes.
There have been several reports on the association between psychosocial factors and health disorders, whereas the indicators of psychosocial condition used in each study were different. For example, the incidence of cardiovascular disease, mortality and hospital readmission as a result of coronary artery bypass surgery were reported to be lower among patients with high LOT‐R score, high perceived level of life enjoyment19, 20, 21 and rich social support and network11, 22. To the contrary, the incidence of cerebro‐cardiovascular disease was higher in the patients who were depressive and had low well‐being3, 23. Thus, there are several indicators of psychosocial condition that reflect different aspects of psychosocial condition, such as mental, social support and network, culture, and religion. In the present study, therefore, we selected six major indicators of psychosocial condition: happiness, optimism (LOT‐R), laughter, stress‐awareness, social support (ESSI) and social network (SNI), and then evaluated the association between these and DN. These six indicators were also used in the 100‐thousand‐scale cohort studies in Japan, such as the Japan Public Health center‐based prospective study and the Japan Gerontological Evaluation study24, 25. LOT‐R, laughter, ESSI and SNI were also shown to be applicable to diabetes patients25, 26, 27, 28.
Interestingly, the present study showed that five out of six major indicators were associated with the presence of DN in Japanese patients with type 2 diabetes: higher scores of happiness and optimism, more enriched social support level, and less self‐awareness of stress were associated with less prevalence of DN (Table 4; Figure 2). These findings support the idea that various positive psychosocial factors are associated with DN.
The mechanism of how psychosocial factors influence health remains unknown, despite much clinical evidence regarding their associations. In particular, regarding the association between the psychosocial factors and DN, even whether there is a causal link between them remains to be established. However, we can assume several possible scenarios, as below.
One possible scenario is that lifestyle habit improvement, which is strongly related to positive psychosocial factors as well as the prevention of DN, brings beneficial renoprotective effects. Generally, patients with lifestyle‐related diseases, including diabetes, are urged to engage in health‐promoting behaviors, such as recommended diet, physical activity, no smoking, moderation in drinking and good medication compliance. Unfortunately, we did not collect information about the lifestyle factors, such as dietary habit, physical activity and good medication compliance, and thus, could not assess whether these factors might have confounded the association between positive psychosocial factors and DN found in the present study. However, it has been reported that the patients with positive psychosocial factors understood the importance of good health behavior, and felt that maintaining a healthy lifestyle was less troublesome as compared with the patients without positive psychosocial factors29, 30. Optimists understood their disease more correctly, and had more motivation for a healthy life as compared with pessimists4, 29. Social support and social network also play important roles in the lifestyle habit improvement31. A recent study showed that social support was related to adherence to physical activity aspects of a weight loss program in overweight patients with type 2 diabetes7.
Indeed, in the present study, several psychosocial factors were significantly associated with the prevalence of hypertension and body mass index: overweight participants were likely to show a lower happiness score, and the prevalence of hypertension was related to a lower level of social network (Tables 2 and 3). As obesity and hypertension are established risk factors for DN, these positive psychosocial factors might indirectly influence the incidence of DN through improvement of obesity and/or hypertension. However, the present study also showed that the psychosocial factors remained independent risk factors for DN, even after adjustment for conventional risk factors for DN, such as hypertension, obesity and dyslipidemia (Table 4), suggesting that positive psychosocial factors are associated with DN through other mechanisms, as well.
Another possible scenario is that immune system malfunction and/or chronic inflammation induced by chronic stress are linked with the psychosocial factors and development of DN. It is well known that inflammation plays important roles in the pathogenesis of DN32, 33. In contrast, several studies showed that psychosocial factors are closely related with the induction of inflammation and immune system malfunction. Lutgendorf et al.34 showed that older women with depression and distress had higher levels of interleukin‐6 as compared with those without. To the contrary, high LOT‐R score, which means more optimistic, was associated with lower levels of interleukin‐6 and C‐reactive protein35. It is also reported that the score of the social network index is inversely associated with elevated interleukin‐6 and intercellular adhesion molecule‐1 levels, after adjusting for confounders including age, smoking, blood pressure, body mass index, medication for dyslipidemia and hypertension, cardiovascular disease, depression, and socioeconomic status36. In addition, an association has been shown between psychosocial factors and response of the hypothalamic–pituitary–adrenal (HPA) axis. It is reported that optimists showed a lower level of HPA response than pessimists in the face of adversity and illness29, 37. This difference might influence the development of DN, as HPA response affects glycemic control and obesity38. Thus, we could assume that the decrease of inflammation biomarkers and downregulation of HPA are associated with suppressed incidence of DN; however, these biochemical markers were not measured in the present study.
Several limitations of the present study should be discussed. First, the number of study participants was relatively small to justify the multiple testing carried out in the present study. Second, this was a cross‐sectional analysis of baseline data of an ongoing project. Because of the cross‐sectional nature of this study, the associations do not necessarily show causality. In particular, there is a concern that an advanced stage of DN could impair psychosocial condition. However, in the present study, the influence of advanced DN would be small, as the number of patients with advanced DN (stage 4 n = 11 and stage 5 n = 4) was small (4.4% of all the participants). Indeed, similar results were obtained through additional statistical analyses, in which the patients with advanced DN (stages 4 and 5) were excluded (Table S2). Further studies would be necessary to confirm the hypothesis that psychosocial factors affect the development of DN in longitudinal settings. For this purpose, a follow‐up prospective study is now ongoing, and the results are planned to be published in 2021.
Third, although we tried to minimize the possible influence of known major confounders, the results might have been confounded by unknown factors. Also, we cannot deny the possibility that the psychosocial factors of some participants might have been affected by concomitant diseases other than diabetes. Therefore, we carried out the same analysis among the participants without malignancy and CVD, and we found that they showed similar results (data not shown). It would support that the relationship between psychosocial factors and DN was independent of concomitant diseases. Fourth, psychosocial factors consist of various aspects, such as mental, social support and network, culture, and religion, and there are many indicators in regard to psychosocial factors that reflect different aspects of psychosocial conditions. However, we could not determine what kind of factor is the most important, as these indicators are associated with each other to varying degrees (Figure 1). Fifth, to understand the impact of psychosocial conditions on DN, the influence of economic status and working status should be also considered. Unfortunately, however, as we did not collect information on economic status, we could not assess its influence on the association between positive psychosocial factors and DN. Regarding working status, current workers showed high scores for LOT‐R and high self‐awareness of stress scores as compared with non‐workers. However, multiple logistic regression analyses showed that LOT‐R and self‐awareness of stress as well as the other psychosocial factors, except laughter‐frequency, were independent determinant factors for the presence of DN, even after adjustment for working status (data not shown). Finally, it is noted that the participants of the present study were Japanese type 2 diabetes patients, and all the study participants were recruited at a single center, in particular a university hospital. It would thus be premature to generalize our findings to other groups.
Notwithstanding these limitations, the present study showed that five out of six prespecified indicators of psychosocial condition were significantly associated with the prevalence of DN in Japanese type 2 diabetes patients. These findings supported the idea that psychosocial factors affect the development of DN.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Distribution of laughter frequency, self‐awareness of stress, social network (SNI score) and social support (ESSI score). Values are shown as number (percentage). The broken line indicates the separation point of two groups consisting of approximately the same number of participants.
Table S1 | Associations between psychosocial factors and diabetic nephropathy.
Table S2 | Multivariate associations with diabetic nephropathy and psychosocial factors on the group without advanced DN subjects.
Table S3 | Result of factor analysis.
Acknowledgments
The authors thank all the clinical staff who participated in this trial. Financial support for this study was provided by the following funding sources: a Health Labor Sciences Research Grant from the Japanese Ministry of Health, Labor and Welfare.
J Diabetes Investig 2018; 9: 162–172
References
- 1. Park CW. Diabetic kidney disease from epidemiology to clinical perspectives. Diabetes Metab J 2014; 38: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanafusa N, Nakai S, Iseki K, et al Japanese society for dialysis therapy renal data registry‐a window through which we can view the details of Japanese dialysis population. Kidney Int Suppl 2011; 2015: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cummings DM, Kirian K, Howard G, et al Consequences of Comorbidity of Elevated Stress and/or Depressive Symptoms and Incident Cardiovascular Outcomes in Diabetes: results From the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Diabetes Care 2016; 39: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rose M, Fliege H, Hildebrandt M, et al The network of psychological variables in patients with diabetes and their importance for quality of life and metabolic control. Diabetes Care 2002; 25: 35–42. [DOI] [PubMed] [Google Scholar]
- 5. Sousa VD, Zauszniewski JA, Musil CM, et al Relationships among self‐care agency, self‐efficacy, self‐care, and glycemic control. Res Theory Nurs Pract 2005; 19: 217–230. [DOI] [PubMed] [Google Scholar]
- 6. Yi JP, Vitaliano PP, Smith RE, et al The role of resilience on psychological adjustment and physical health in patients with diabetes. Br J Health Psychol 2008; 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marquez B, Anderson A, Wing RR, et al The relationship of social support with treatment adherence and weight loss in Latinos with type 2 diabetes. Obesity (Silver Spring) 2016; 24: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayashi K, Hayashi T, Iwanaga S, et al Laughter lowered the increase in postprandial blood glucose. Diabetes Care 2003; 26: 1651–1652. [DOI] [PubMed] [Google Scholar]
- 9. Hirosaki M, Ohira T, Kajiura M, et al Effects of a laughter and exercise program on physiological and psychological health among community‐dwelling elderly in Japan: randomized controlled trial. Geriatr Gerontol Int 2013; 13: 152–160. [DOI] [PubMed] [Google Scholar]
- 10. Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self‐mastery, and self‐esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994; 67: 1063–1078. [DOI] [PubMed] [Google Scholar]
- 11. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine‐year follow‐up study of Alameda County residents. Am J Epidemiol 1979; 109: 186–204. [DOI] [PubMed] [Google Scholar]
- 12. Enhancing recovery in coronary heart disease patients (ENRICHD): study design and methods. The ENRICHD investigators. Am Heart J. 2000; 139: 1–9. [DOI] [PubMed] [Google Scholar]
- 13. Vaglio J Jr, Conard M, Poston WS, et al Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health Qual Life Outcomes 2004; 2: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shimamoto K, Ando K, Fujita T, et al The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37: 253–390. (In Japanese). [DOI] [PubMed] [Google Scholar]
- 15. Teramoto T, Sasaki J, Ishibashi S, et al Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan ‐2012 version. J Atheroscler Thromb 2013; 20: 517–523. [DOI] [PubMed] [Google Scholar]
- 16. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 17. Haneda M, Utsunomiya K, Koya D, et al A new Classification of Diabetic Nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. J Diabetes Investig 2015; 6: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yasuda H, Sanada M, Kitada K, et al Rationale and usefulness of newly devised abbreviated diagnostic criteria and staging for diabetic polyneuropathy. Diabetes Res Clin Pract 2007; 77(Suppl 1): S178–S183. [DOI] [PubMed] [Google Scholar]
- 19. Tindle HA, Chang YF, Kuller LH, et al Optimism, cynical hostility, and incident coronary heart disease and mortality in the Women's Health Initiative. Circulation 2009; 120: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheier MF, Matthews KA, Owens JF, et al Optimism and rehospitalization after coronary artery bypass graft surgery. Arch Intern Med 1999; 159: 829–835. [DOI] [PubMed] [Google Scholar]
- 21. Shirai K, Iso H, Ohira T, et al Perceived level of life enjoyment and risks of cardiovascular disease incidence and mortality: the Japan public health center‐based study. Circulation 2009; 120: 956–963. [DOI] [PubMed] [Google Scholar]
- 22. Bucholz EM, Strait KM, Dreyer RP, et al Effect of low perceived social support on health outcomes in young patients with acute myocardial infarction: results from the VIRGO (Variation in Recovery: role of Gender on Outcomes of Young AMI Patients) study. J Am Heart Assoc 2014; 3: e001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Araki A, Murotani Y, Kamimiya F, et al Low well‐being is an independent predictor for stroke in elderly patients with diabetes mellitus. J Am Geriatr Soc 2004; 52: 205–210. [DOI] [PubMed] [Google Scholar]
- 24. Tsugane S, Sobue T. Baseline survey of JPHC study–design and participation rate. Japan Public Health Center‐based Prospective Study on Cancer and Cardiovascular Diseases. J Epidemiol 2001; 11: 6. supply: S24‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayashi K, Kawachi I, Ohira T, et al Laughter is the Best Medicine? A Cross‐Sectional Study of Cardiovascular Disease Among Older Japanese Adults. J Epidemiol 2016; 26: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puig‐Perez S, Hackett RA, Salvador A, et al Optimism moderates psychophysiological responses to stress in older people with Type 2 diabetes. Psychophisiology 2016. doi:10.1111/psyp.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Donald M, Dower J, Ware R, et al Living with diabetes: rationale, study design and baseline characteristics for an Australian prospective cohort study. BMC Public Health 2012; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eller M, Holle R, Landgraf R, et al Social network effect on self‐rated health in type 2 diabetic patients–results from a longitudinal population‐based study. Int Public Health 2008; 53: 188–194. [DOI] [PubMed] [Google Scholar]
- 29. Carver CS, Scheier MF. Dispositional optimism. Trends Cogn Sci 2014; 18: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huffman JC, DuBois CM, Millstein RA, et al Positive psychological interventions for patients with type 2 diabetes: Rationale, Theoretical Model, and Intervention Development. J Diabetes Res 2015; 2015: 428349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanson BS. Social network, social support and heavy drinking in elderly men–a population study of men born in 1914, Malmo, Sweden. Addiction 1994; 89: 725–732. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki D, Miyazaki M, Naka R, et al In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes 1995; 44: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 33. Cao Z, Cooper ME. Pathogenesis of diabetic nephropathy. J Diabetes Investig 2011; 2: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lutgendorf SK, Garand L, Buckwalter KC, et al Life stress, mood disturbance, and elevated interleukin‐6 in healthy older women. J Gerontol A Biol Sci Med Sci 1999; 54: M434–M439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roy B, Diez‐Roux AV, Seeman T, et al Association of optimism and pessimism with inflammation and hemostasis in the Multi‐Ethnic Study of Atherosclerosis (MESA). Psychosom Med 2010; 72: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loucks EB, Sullivan LM, D'Agostino RB, et al Social networks and inflammatory markers in the Framingham Heart Study. J Biosoc Sci 2006; 38: 835–842. [DOI] [PubMed] [Google Scholar]
- 37. Brunello N, Blier P, Judd LL, et al Noradrenaline in mood and anxiety disorders: basic and clinical studies. Int Clin Psychopharmacol 2003; 18: 191–202. [DOI] [PubMed] [Google Scholar]
- 38. Rosmond R. Stress induced disturbances of the HPA axis: a pathway to Type 2 diabetes? Med Sci Monit 2003; 9: Ra35–Ra39. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Distribution of laughter frequency, self‐awareness of stress, social network (SNI score) and social support (ESSI score). Values are shown as number (percentage). The broken line indicates the separation point of two groups consisting of approximately the same number of participants.
Table S1 | Associations between psychosocial factors and diabetic nephropathy.
Table S2 | Multivariate associations with diabetic nephropathy and psychosocial factors on the group without advanced DN subjects.
Table S3 | Result of factor analysis.


