Abstract
Aims/Introduction
High fluctuations in blood glucose are associated with various complications. The correlation between glycated hemoglobin (HbA1c) level and fluctuations in blood glucose level has not been studied in Japanese patients with type 2 diabetes. In the present study, blood glucose profile stratified by HbA1c level was evaluated by continuous glucose monitoring (CGM) in Japanese type 2 diabetes patients.
Materials and Methods
Our retrospective study included 294 patients with type 2 diabetes who were divided by HbA1c level into five groups (≥6.0 to <7.0%, ≥7.0 to <8.0%, ≥8.0 to <9.0%, ≥9.0 to <10.0% and ≥10%). The correlation between HbA1c level and CGM data was analyzed. The primary end‐point was the difference in blood glucose fluctuations among the HbA1c groups.
Results
The mean blood glucose level increased significantly with increasing HbA1c (P trend < 0.01). The standard deviation increased with increases in HbA1c (P trend < 0.01). The mean amplitude of glycemic excursions did not vary significantly with HbA1c. The levels of maximum blood glucose, minimum blood glucose, each preprandial blood glucose, each postprandial maximum blood glucose, range of increase in postprandial glucose from pre‐meal to after breakfast, the area under the blood concentration–time curve >180 mg/dL and percentage of the area under the blood concentration–time curve >180 mg/dL were higher with higher HbA1c. Mean glucose level and pre‐breakfast blood glucose level were significant and independent determinants of HbA1c.
Conclusions
In Japanese patients treated for type 2 diabetes, the mean amplitude of glycemic excursions did not correlate with HbA1c, making it difficult to assess blood glucose fluctuations using HbA1c. Parameters other than HbA1c are required to evaluate fluctuations in blood glucose level in patients receiving treatment for type 2 diabetes.
Keywords: Continuous glucose monitoring, Glycated hemoglobin, Mean amplitude of glycemic excursions
Introduction
Large swings in blood glucose levels are known to induce oxidative stress and inflammation1. This phenomenon is a risk factor for diabetic neuropathy2, vascular endothelial dysfunction3, 4 and cognitive dysfunction5, 6, as well as progression of retinopathy7. With the popularization of continuous glucose monitoring (CGM), it is no doubt important to reduce fluctuations in blood glucose concentrations in addition to lowering the glycated hemoglobin (HbA1c) level. However, routine clinical practice emphasizes HbA1c as a treatment guide, as blood glucose fluctuations are difficult to monitor. Analysis of the correlation between HbA1c level and blood glucose fluctuations in drug‐naïve Japanese patients with type 2 diabetes showed a trend towards larger swings in blood glucose levels with high HbA1c levels.8 Diabetes patients seen in clinical practice represent a wide range of patients and include drug‐naïve patients. The relationship between HbA1c levels and blood glucose fluctuation in these patients is not fully understood. In the present study, we used CGM to evaluate blood glucose profile, stratified by HbA1c level, in Japanese inpatients with type 2 diabetes.
Methods
Patients
The study enrolled inpatients with type 2 diabetes mellitus at the Hospital of The University of Occupational and Environmental Health, Kitakyushu, Japan and its affiliated hospitals between April 2010 and April 2015, from whom blood glucose data were collected by a CGM system (CGMS System Gold; Medtronic Inc., Fridley, MN, USA and iPro™2; Medtronic, Northridge, CA, USA) within 5 days of admission while taking medications that remained unchanged throughout the study. The study population included patients of any age who were or were not taking glucose‐lowering agents. In the present study, we defined patients with type 2 diabetes mellitus as those with a family history of diabetes and obesity, those without autoimmune diabetes, and those without hyperglycemia as a result of pancreatic failure or medication. The study excluded patients with type 1 diabetes, pancreatic diabetes, steroid diabetes, severe infection, pre‐ or postoperative status and serious trauma. The study protocol was approved by the review board of The University of Occupational and Environmental Health. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Study protocol
In the present retrospective study, 24‐h CGM data were extracted from the second or third day of glucose monitoring. Patients had hospital meals at 25–30 kcal/kg standard bodyweight according to the dietary therapy recommended by the Japan Diabetes Society9, and underwent blood testing under fasting conditions on the second day of glucose monitoring. For statistical analysis, the patients were stratified by HbA1c level on admission into five subgroups (≥6.0 to <7.0%, ≥7.0 to <8.0%, ≥8.0 to <9.0%, ≥9.0 to <10.0% and ≥10%).
Measurements of biochemical variables
The CGM devices used in the present study included the Gold™ (Medtronic Inc.) and iPro™2 (Medtronic). A temporary subcutaneous electrode measured glucose concentration in the tissue interstitial fluid within a range of 40–400 mg/dL at a frequency of 288 times/day.10 The sensor readings were calibrated against blood glucose levels measured in the morning, noon and evening, and before bed (4 times/day). The glucose concentration measured by CGM is reported to correlate with the venous blood glucose level. Although CGM actually measures the glucose concentration in the interstitial fluid, it is corrected by self‐monitoring of blood glucose (hereafter, blood glucose level). We excluded patients with microcirculatory impairment, which might affect the sensor performance. Data over a period of 24 h extracted from the second or third day of glucose monitoring were used to calculate the mean blood glucose level ± standard deviation (SD), mean amplitude of glycemic excursions (MAGE), maximum blood glucose level, minimum blood glucose level, area under the blood concentration‐time curve (AUC) >180 mg/dL, percentage of AUC >180 mg/dL, area over the blood concentration–time curve (AOC) <70 mg/dL, percentage of AOC <70 mg/dL, each preprandial blood glucose level, each postprandial maximum blood glucose level, time to each postprandial maximum blood glucose level and magnitude of postprandial blood glucose elevation. The preprandial blood glucose levels were measured at 08.00 hours, 12.00 hours and 18.00 hours. The peak postprandial glucose level was defined as the highest blood glucose value recorded after a meal by the CGM system.
MAGE was calculated using Glycemic Variability Analyzer Program 1.1 (MATLABR 2010b; MathWorks, Inc., Natick, MA, USA).11 HbA1c (%) was determined using National Glycohemoglobin Standardization Program (NGSP) calculated by the equation: HbA1c (NGSP) (%) = HbA1c (Japan relationship of HbA1c [Japan Diabetes Society] × 1.02 + 0.25 [%]).12 Serum lipids were tested in a sample collected under fasting conditions for at least 12 h using a Hitachi 7350 autoanalyzer (Hitachi Co., Tokyo, Japan). Low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol and triglyceride concentrations were determined by enzyme assays; low‐density lipoprotein cholesterol was determined by a direct method. The equation used to calculate the estimated glomerular filtration rate was 194 × serum creatinine − 1.094 × age − 0.287 for men, and 194 × serum creatinine − 1.094 × age − 0.287 × 0.739 for women. Insulin and C‐peptide levels were measured using chemiluminescence immunoassay. The homeostasis model assessment of insulin resistance was calculated by the equation of fasting plasma glucose (mg/dL) × fasting plasma insulin (μU/mL)/405, and the urinary C‐peptide reactivity was determined in a 24‐h pooled urine sample. Homeostasis model assessment of insulin resistance is not always correct in patients with blood glucose levels >150 mg/dL.
The primary study end‐points included SD representing blood glucose fluctuations in the HbA1c subgroups and the differences in MAGE. The secondary study end‐points were the maximum blood glucose level, minimum blood glucose level, AUC >180 mg/dL, percentage of AUC >180 mg/dL, AOC <70 mg/dL, percentage of AOC <70 mg/dL, each preprandial blood glucose level, each postprandial maximum blood glucose level, time to each postprandial maximum blood glucose level and magnitude of postprandial blood glucose elevation.
Statistical analysis
Data are expressed as mean ± SD. One‐way analysis of variance (anova) was used for comparison between groups. The linear trend of association was assessed by a regression model that assigned scores to the group (scores 1–5). The χ2‐test was used to assess categorical data. For multivariate analysis, a stepwise variable selection procedure was used. Each test was carried out at a significance level of 0.05. All statistical analyses were carried out using JMP 11 (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics
The background characteristics of the study patients are summarized in Table 1. The study population consisted of 294 patients (178 men and 116 women), who were divided into five groups according to the level of HbA1c: Group6–7 with HbA1c of ≥6.0 to <7.0% (n = 54), Group7–8 (HbA1c: ≥7.0 to <8.0%, n = 64), Group8–9 (HbA1c: ≥8.0 to <9.0%, n = 73), Group9–10 (HbA1c: ≥9.0 to <10%, n = 49) and Group≥10 (HbA1c: ≥10.0%, n = 54). Patients of the latter group were significantly younger, and had a shorter duration of diabetes, higher body mass index, higher homeostasis model assessment of insulin resistance and higher urinary C‐peptide reactivity levels than those of the other groups. With regard to the therapeutic regimens, 50% of the patients of Group6–7 were not taking any oral glucose‐lowering agents, whereas approximately half of the patients of Group7–8, Group8–9 and Group9–10 were using dipeptidyl peptidase‐4 inhibitors. The proportion of insulin users was the highest in Group6–7 (24%), and the lowest in Group≥10 (2%).
Table 1.
Clinical characteristics according to glycated hemoglobin level
| Group6–7 | Group7–8 | Group8–9 | Group9–10 | Group≥10 | P‐value* | |
|---|---|---|---|---|---|---|
| Patients (n) | 54 | 64 | 73 | 49 | 54 | |
| Age (years) | 64.5 ± 14.0 | 65.9 ± 12.9 | 61.2 ± 13.6 | 63.3 ± 11.5 | 55.0 ± 13.5 | <0.01 |
| Sex (male/female) | 29/25 | 33/31 | 47/26 | 30/19 | 39/15 | 0.15 |
| Duration of diabetes (years) | 12.1 ± 13.5 | 11.7 ± 11.1 | 10.4 ± 8.7 | 12.7 ± 10.1 | 6.2 ± 7.1 | <0.01 |
| BMI (kg/m2) | 25.2 ± 4.8 | 25.6 ± 5.0 | 26.5 ± 4.7 | 24.7 ± 4.3 | 26.9 ± 4.3 | 0.03 |
| eGFR (mL/min/1.73 m2) | 62.6 ± 26.2 | 66.1 ± 22.1 | 78.4 ± 25.2 | 74.7 ± 28.0 | 90.9 ± 28.3 | <0.01 |
| SBP (mmHg) | 133.7 ± 19.1 | 137.5 ± 21.5 | 134.1 ± 18.1 | 134.1 ± 22.7 | 135.5 ± 19.1 | 0.74 |
| DBP (mmHg) | 76.8 ± 12.4 | 80.4 ± 13.3 | 79.2 ± 11.5 | 80.6 ± 16.0 | 85.1 ± 13.3 | 0.03 |
| TC (mg/dL) | 175.6 ± 54.0 | 185.0 ± 29.1 | 180.2 ± 44.4 | 176.1 ± 51.9 | 188.2 ± 31.3 | 0.49 |
| TG (mg/dL) | 123.3 ± 57.7 | 135.5 ± 103.3 | 161.1 ± 83.8 | 169.2 ± 116.4 | 170.4 ± 115.8 | 0.01 |
| LDL‐C (mg/dL) | 109.9 ± 38.5 | 114.2 ± 32.9 | 117.6 ± 38.6 | 120.6 ± 40.7 | 118.9 ± 27.1 | 0.57 |
| HDL‐C (mg/dL) | 52.7 ± 16.2 | 54.3 ± 13.2 | 50.9 ± 16.3 | 48.3 ± 12.1 | 47.7 ± 13.7 | 0.02 |
| HbA1c (%) | 6.6 ± 0.3 | 7.4 ± 0.3 | 8.5 ± 0.3 | 9.5 ± 0.3 | 11.3 ± 1.2 | <0.01 |
| FPG (mg/dL) | 120.8 ± 21.2 | 134.4 ± 34.7 | 150.8 ± 39.1 | 164.2 ± 36.9 | 188.6 ± 42.8 | <0.01 |
| HOMA‐IR | 2.3 ± 1.7 (n = 31) | 2.6 ± 1.9 (n = 50) | 3.1 ± 2.6 (n = 50) | 2.4 ± 1.4 (n = 35) | 3.4 ± 2.7 (n = 49) | 0.04 |
| Urinary CPR (μg/day) | 65.4 ± 53.4 | 67.8 ± 45.8 | 81.5 ± 57.8 | 61.5 ± 44.9 | 107.0 ± 67.0 | <0.01 |
| Treatment of diabetes | ||||||
| Without glucose‐lowering agents, n (%) | 27 (50) | 18 (28) | 14 (19) | 8 (16) | 18 (33) | <0.01 |
| Sulfonylureas, n (%) | 5 (9) | 22 (34) | 23 (32) | 18 (37) | 21 (39) | 0.01 |
| Biguanides, n (%) | 4 (7) | 16 (25) | 16 (22) | 15 (31) | 11 (20) | 0.05 |
| DPP4i, n (%) | 13 (24) | 27 (42) | 38 (52) | 27 (55) | 17 (31) | <0.01 |
| Thiazolidinedione, n (%) | 6 (11) | 8 (13) | 7 (10) | 6 (12) | 7 (13) | 0.98 |
| Glinide, n (%) | 1 (2) | 0 (0) | 0 (0) | 2 (5) | 0 (0) | 0.15 |
| αGI, n (%) | 4 (7) | 5 (8) | 10 (14) | 3 (6) | 3 (6) | 0.46 |
| Insulin, n (%) | 13 (24) | 13 (20) | 14 (19) | 9 (18) | 1 (2) | 0.02 |
| GLP‐1, n (%) | 1 (2) | 1 (2) | 2 (3) | 1 (2) | 3 (6) | 0.70 |
| Combination therapy | ||||||
| Insulin only, n (%) | 7 (13) | 5 (8) | 8 (11) | 0 (0) | 0 (0) | <0.01 |
| Without insulin | ||||||
| Oral hypoglycemic drugs only 1, n (%) | 8 (15) | 15 (24) | 18 (25) | 14 (29) | 17 (32) | 0.29 |
| Oral hypoglycemic drugs ≥2, n (%) | 0 (0) | 22 (35) | 29 (40) | 21 (43) | 18 (34) | 0.05 |
| With insulin | ||||||
| Oral hypoglycemic drugs only 1, n (%) | 4 (7) | 6 (9) | 3 (4) | 8 (16) | 0 (0) | 0.01 |
| Oral hypoglycemic drugs ≥2, n (%) | 2 (4) | 2 (3) | 3 (4) | 1 (2) | 1 (2) | 0.94 |
Data are mean ± standard deviation, unless otherwise indicated. *anova for comparisons between each group, χ2‐test for sex differences. Group6–7, glycated hemoglobin (HbA1c): ≥6.0 to <7.0%; Group7–8, HbA1c: ≥7.0 to <8.0%; Group8–9, HbA1c: ≥8.0 to <9.0%; Group9–10, HbA1c: ≥9.0 to <10%; Group≥10, HbA1c: ≥10.0%. αGI, α‐glucosidase inhibitor; BMI, body mass index; CPR, C‐peptide immunoreactivity; DBP, diastolic blood pressure; DPP4i, dipeptidyl peptidase‐4 inhibitor; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GLP‐1, glucagon‐like peptide‐1; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
CGM data
CGM data are presented according to HbA1c groups in Table 2. The mean 24‐h blood glucose levels ± 1 × SD for the individual HbA1c groups and all patients are shown in Figures 1 and 2, respectively.
Table 2.
Patient profile and summary of glycemic variations according to glycated hemoglobin level
| Group6–7 | Group7–8 | Group8–9 | Group9–10 | Group≥10 | P‐trenda | |
|---|---|---|---|---|---|---|
| Mean glucose level (mg/dL) | 144.2 ± 31.9 | 156.8 ± 26.3 | 164.3 ± 32.9 | 181.3 ± 39.2 | 210.0 ± 59.6 | <0.01 |
| SD (mg/dL) | 31.4 ± 13.8 | 33.7 ± 14.3 | 36.5 ± 12.1 | 39.6 ± 15.3 | 39.7 ± 11.9 | <0.01 |
| MAGE (mg/dL) | 90.1 ± 25.0 | 97.3 ± 32.3 | 98.2 ± 25.0 | 105.6 ± 26.2 | 97.9 ± 23.5 | 0.05 |
| Maximum glucose level (mg/dL) | 222.5 ± 54.8 | 236.0 ± 53.5 | 250.4 ± 48.5 | 267.2 ± 67.9 | 298.1 ± 66.8 | <0.01 |
| Minimum glucose level (mg/dL) | 95.8 ± 23.1 | 102.4 ± 23.8 | 103.5 ± 29.7 | 120.6 ± 37.9 | 142.5 ± 47.4 | <0.01 |
| AUC >180 (mg/dL) | 7.3 ± 17.4 | 9.7 ± 11.6 | 13.1 ± 17.5 | 23.2 ± 27.5 | 44.3 ± 45.7 | <0.01 |
| Percentage of AUC >180 (%) | 16.9 ± 19.9 | 25.6 ± 21.0 | 32.0 ± 24.8 | 39.8 ± 27.6 | 59.8 ± 33.1 | <0.01 |
| AOC <70 (mg/dL) | 0.03 ± 0.14 | 0.01 ± 0.04 | 0.07 ± 0.31 | 0 | 0 | 0.37 |
| Percentage of AOC<70 (%) | 0.35 ± 1.40 | 0.26 ± 1.18 | 1.06 ± 4.11 | 0 | 0 | 0.35 |
| Pre‐meal glucose level (mg/dL) | ||||||
| Breakfast | 124.5 ± 23.5 | 138.7 ± 27.2 | 139.2 ± 34.8 | 160.0 ± 36.7 | 180.0 ± 50.8 | <0.01 |
| Lunch | 130.5 ± 40.4 | 151.3 ± 37.5 | 162.2 ± 43.6 | 174.3 ± 47.7 | 204.2 ± 75.4 | <0.01 |
| Dinner | 126.3 ± 44.2 | 134.4 ± 26.8 | 144.3 ± 34.7 | 157.9 ± 45.4 | 168.5 ± 60.0 | <0.01 |
| Postprandial peak glucose (mg/dL) | ||||||
| Breakfast | 202.9 ± 44.5 | 224.5 ± 47.0 | 227.9 ± 51.2 | 250.6 ± 62.1 | 275.6 ± 70.7 | <0.01 |
| Lunch | 188.5 ± 58.9 | 203.5 ± 44.8 | 215.8 ± 47.1 | 234.2 ± 64.4 | 263.1 ± 75.2 | <0.01 |
| Dinner | 202.9 ± 48.6 | 216.8 ± 39.8 | 227.1 ± 49.6 | 241.5 ± 64.8 | 262.0 ± 65.7 | <0.01 |
| Range of increase in postprandial glucose from pre‐meal (mg/dL) | ||||||
| Breakfast | 78.4 ± 36.7 | 85.8 ± 42.5 | 88.7 ± 41.7 | 90.7 ± 51.2 | 96.0 ± 48.0 | 0.03 |
| Lunch | 58.0 ± 40.0 | 52.1 ± 38.3 | 53.7 ± 29.2 | 59.9 ± 44.0 | 58.9 ± 31.9 | 0.53 |
| Dinner | 76.7 ± 36.7 | 82.4 ± 39.6 | 82.8 ± 41.4 | 83.6 ± 49.5 | 93.4 ± 39.8 | 0.05 |
| Time to glucose peaks (min) | ||||||
| Breakfast | 83.6 ± 33.2 | 109.9 ± 53.0 | 117.0 ± 56.2 | 111.8 ± 47.3 | 100.4 ± 41.2 | 0.11 |
| Lunch | 95.1 ± 56.9 | 86.9 ± 67.1 | 104.2 ± 65.0 | 99.5 ± 68.5 | 78.2 ± 37.8 | 0.42 |
| Dinner | 98.3 ± 51.7 | 105.2 ± 44.7 | 107.7 ± 54.8 | 95.1 ± 54.2 | 88.5 ± 34.6 | 0.10 |
Trend analysis for comparisons between each group. Group6–7, glycated hemoglobin (HbA1c): ≥6.0 to <7.0%; Group7–8, HbA1c: ≥7.0 to <8.0%; Group8–9, HbA1c: ≥8.0 to <9.0%; Group9–10, HbA1c: ≥9.0 to <10%; Group≥10, HbA1c: ≥10.0%. AOC, area over the blood concentration–time curve; AUC, area under the blood concentration–time curve; MAGE, mean amplitude of glycemic excursions; SD, standard deviation.
Figure 1.
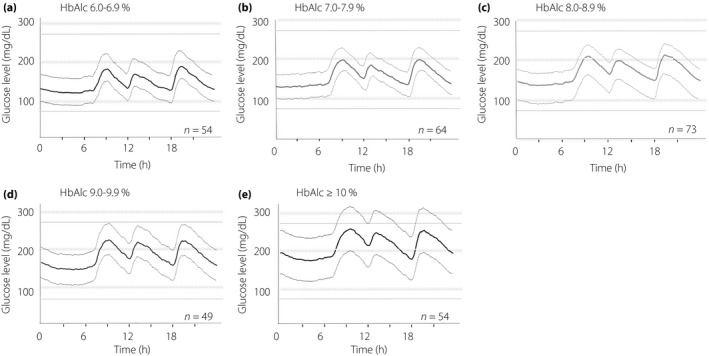
The 24‐h glycemic variations ± 1 standard deviation in type 2 diabetes patients receiving treatment according to glycated hemoglobin (HbA1c) level. Continuous glucose monitoring (CGM) was applied for 2 or 3 days.
Figure 2.
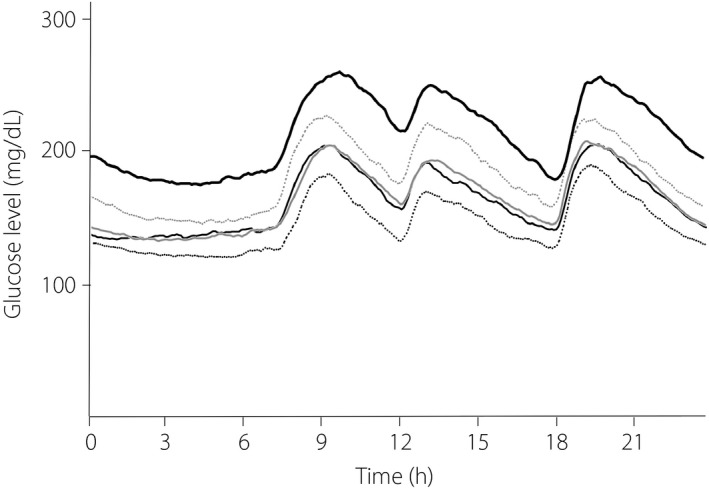
The 24‐h changes in blood glucose level in type 2 diabetes patients receiving treatment. Black dotted line, glycated hemoglobin (HbA1c): ≥6.0 to <7.0%; black thin line, HbA1c: ≥7.0 to <8.0%; gray line, HbA1c: ≥8.0 to <9.0%; gray dotted line, HbA1c: ≥9.0 to <10.0%; black thick line, HbA1c: ≥10.0%.
Mean glucose level
The mean fasting blood glucose levels were 144.2, 156.8, 164.3, 181.3, and 210.0 mg/dL for Group6–7, Group7–8, Group8–9, Group9–10 and Group≥10, respectively, showing a significant increase with higher HbA1c (P trend < 0.01).
SD and MAGE
The SD was already high at 31.4 mg/dL in Group6–7, and even higher at 39.6 mg/dL in Group9–10 and 39.7 mg/dL in Group≥10; SD increased with increases in HbA1c (P trend < 0.01). In contrast, MAGE was already high at 90.1 mg/dL in Group6–7 and similarly high at 97.3, 98.2, 105.6, and 97.9 mg/dL in Groups7–8, 8–9, 9–10 and ≥10, respectively, with no significant differences based on HbA1c (P trend = 0.05).
Other CGM parameters
The maximum blood glucose level, minimum blood glucose level and AUC >180 mg/dL significantly increased with increasing HbA1c (P trend < 0.01). In contrast, none of the patients with HbA1c of ≥9% had AOC <70 mg/dL, and the percentage of AOC <70 mg/dL differed significantly among the five groups. Morning, midday and evening preprandial blood glucose levels increased significantly with higher HbA1c (P trend < 0.01), whereas the midday preprandial blood glucose level was the highest in all five groups (P < 0.01).
Each postprandial maximum blood glucose level increased significantly with increasing HbA1c in the morning, midday and evening. The range of increase in postprandial glucose level from pre‐meal to after breakfast increased significantly with higher HbA1c levels. The magnitude of blood glucose rise was smallest for midday preprandial data in all five groups (P < 0.01). The time to each postprandial maximum blood glucose level was not significantly different among the five groups.
Factors contributing to HbA1c
Univariate analysis showed that mean blood glucose level, SD, maximum blood glucose level, minimum blood glucose level, AUC >180 mg/dL, percentage of AUC >180 mg/dL, each preprandial blood glucose level and each postprandial maximum blood glucose level correlated significantly with HbA1c (Table 3). Multivariate analysis was carried out for the variables used in the univariate analysis after adjusting for age, sex and body mass index. The contributing factors to HbA1c were mean glucose level and preprandial glucose level (breakfast; Table 3).
Table 3.
Results of liner univariate analysis and linear multivariate analysis with glycated hemoglobin as the dependent variable
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | P‐value | β | 95% CI | P‐value | |
| Intercept | 7.0 | 6.1, 8.0 | <0.01 | |||
| Mean glucose level | 0.02 | 0.01, 0.02 | <0.01 | 0.02 | 0.01, 0.03 | <0.01 |
| SD | 0.03 | 0.01, 0.04 | <0.01 | |||
| MAGE | 0.01 | 0, 0.01 | 0.12 | |||
| Maximum glucose levels | 0.02 | 0, 0.01 | <0.01 | |||
| Minimum glucose levels | 0.02 | 0.02, 0.03 | <0.01 | |||
| AUC >180 mg/dL | 0.03 | 0.02, 0.03 | <0.01 | |||
| Percentage of AUC >180 mg/dL | 0.03 | 0.02, 0.04 | <0.01 | |||
| AOC <70 mg/dL | –0.74 | –1.9, 0.4 | 0.19 | |||
| Percentage of AOC <70 mg/dL | –0.07 | –0.15, 0.02 | 0.12 | |||
| Pre‐meal glucose level (breakfast) | 0.02 | 0.01, 0.02 | <0.01 | 0.01 | 0, 0.01 | 0.02 |
| Pre‐meal glucose level (lunch) | 0.01 | 0.01, 0.02 | <0.01 | |||
| Pre‐meal glucose level (dinner) | 0.02 | 0.01, 0.02 | <0.01 | |||
| Postprandial peak glucose (breakfast) | 0.01 | 0, 0.01 | <0.01 | |||
| Postprandial peak glucose (lunch) | 0.01 | 0, 0.01 | <0.01 | |||
| Postprandial peak glucose (dinner) | 0.01 | 0, 0.01 | <0.01 | 0 | –0.01, 0 | 0.14 |
Multivariate stepwise regression analysis with glycated hemoglobin as the dependent variable, and age, sex, body mass index, mean glucose level, standard deviation (SD), mean amplitude of glycemic excursions (MAGE), maximum glucose levels, minimum glucose levels, area under the blood concentration–time curve (AUC) >180 mg/dL, percentage of AUC >180 mg/dL, area over the blood concentration–time curve (AOC) <70 mg/dL, percentage of AOC <70 mg/dL, pre‐meal glucose level (breakfast), pre‐meal glucose level (lunch), pre‐meal glucose level (dinner), postprandial peak glucose (breakfast), postprandial peak glucose (lunch), postprandial peak glucose (dinner) as the independent variables. All variables were measured by the continuous glucose monitoring system.
Factors contributing to MAGE
On univariate analysis, MAGE correlated with mean blood glucose level, SD, maximum blood glucose level, AUC >180 mg/dL, percentage of AUC >180 mg/dL, pre‐lunch blood glucose level and each postprandial maximum blood glucose level (Table 4). Multivariate analysis for MAGE was carried out for the variables used in the univariate analysis after adjusting for age, sex and body mass index. The results showed that HbA1c was not a contributing factor to MAGE (Table 4).
Table 4.
Results of liner univariate analysis and linear multivariate analysis with mean amplitude of glycemic excursions as the dependent variable
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | P‐value | β | 95% CI | P‐value | |
| Intercept | 38.7 | 20.2, 57.1 | <0.01 | |||
| HbA1c | 1.5 | –0.41, 3.4 | 0.12 | |||
| FPG | 0.06 | –0.01, 0.13 | 0.10 | |||
| Mean glucose level | 0.14 | 0.08, 0.21 | <0.01 | 0.07 | 0.01, 0.12 | 0.01 |
| SD | 1.4 | 1.2, 1.5 | <0.01 | 1.6 | 1.3, 1.8 | <0.01 |
| Maximum glucose levels | 0.21 | 0.17, 0.25 | <0.01 | 0.16 | 0.08, 0.24 | <0.01 |
| Minimum glucose levels | 0.02 | –0.07, 0.10 | 0.69 | 0.25 | 0.14, 0.37 | <0.01 |
| AUC >180 mg/dL | 0.21 | 0.11, 0.32 | <0.01 | –0.14 | –0.32, 0.05 | 0.16 |
| Percentage of AUC >180 mg/dL | 0.29 | 0.19, 0.39 | <0.01 | |||
| AOC <70 mg/dL | –9.0 | –27.6, 9.6 | 0.34 | –11.0 | –23.3, 1.1 | 0.07 |
| Percentage of AOC <70 mg/dL | 0.80 | –3.5, 5.1 | 0.72 | |||
| Pre‐meal glucose level (breakfast) | 0.05 | –0.03, 0.13 | 0.20 | |||
| Pre‐meal glucose level (lunch) | 0.09 | 0.03, 0.14 | <0.01 | |||
| Pre‐meal glucose level (dinner) | 0.04 | –0.03, 0.11 | 0.21 | –0.11 | –0.19, –0.04 | <0.01 |
| Postprandial peak glucose (breakfast) | 0.19 | 0.14, 0.24 | <0.01 | 0.07 | 0.02, 0.12 | 0.01 |
| Postprandial peak glucose (lunch) | 0.13 | 0.09, 0.18 | <0.01 | |||
| Postprandial peak glucose (dinner) | 0.16 | 0.11, 0.21 | <0.01 | |||
Multivariate stepwise regression analysis with mean amplitude of glycemic excursions as the dependent variable, and age, sex, body mass index, glycated hemoglobin, fasting plasma glucose (FPG), mean glucose level, standard deviation (SD), maximum glucose levels, minimum glucose levels, area under the blood concentration–time curve (AUC) >180 mg/dL, percentage of AUC >180 mg/dL, area over the blood concentration–time curve (AOC) <70 mg/dL, percentage of AOC <70 mg/dL, pre‐meal glucose level (breakfast), pre‐meal glucose level (lunch), pre‐meal glucose level (dinner), postprandial peak glucose (breakfast), postprandial peak glucose (lunch), postprandial peak glucose (dinner) as the independent variables. All variables were measured by the continuous glucose monitoring system.
Discussion
We evaluated blood glucose profiles by CGM in Japanese patients with type 2 diabetes, including 72% of those taking medications. The results showed significant increases in the levels of mean blood glucose, maximum blood glucose, minimum blood glucose, each preprandial blood glucose, each postprandial maximum blood glucose, range of postprandial glucose increases from pre‐meal to after breakfast, AUC >180 mg/dL and percentage of AUC >180 mg/dL, with increases in HbA1c level. These results are similar to those described in a previous study of Japanese patients with diabetes who were not taking medications, which reported increases in mean blood glucose level, preprandial blood glucose level and postprandial blood glucose level with increases in HbA1c8.
In this regard, one study reported that the time to postprandial maximum glucose level was delayed with higher levels of HbA1c8. However, the present results showed the time to each postprandial maximum blood glucose level did not vary significantly with HbA1c. We assumed that this was due to the differences in the proportion of patients not taking glucose‐lowering agents and those using insulin among the HbA1c groups, and the greater influence of drugs on the levels of postprandial glucose.
The SD, which represents fluctuations in blood glucose level, increased with increases in HbA1c; however, MAGE was increased in all groups regardless of HbA1c level. MAGE is an indicator of blood glucose fluctuation at and above 1 × SD13. The presence of both hyperglycemia and hypoglycemia sometimes results in an apparently favorable HbA1c value. This might explain the higher MAGE in some patients with low HbA1c level in the present study.
Multivariate analysis identified mean blood glucose levels and pre‐breakfast blood glucose level, but not changes in blood glucose, as significant and independent determinants of HbA1c. According to Monnier et al.14, patients with type 2 diabetes show worsening blood glucose levels with increasing HbA1c in a stepwise manner, starting with an increase in morning preprandial blood glucose level followed by an increase in daytime preprandial blood glucose level, while the contribution of preprandial blood glucose level increased at higher HbA1c levels15. In addition, other reports showed that HbA1c more closely reflects preprandial blood glucose levels than postprandial blood glucose levels16, 17. The present results also support the notion that HbA1c more closely reflects the preprandial blood glucose level than glucose fluctuations or the postprandial rise in blood glucose level. A previous study reported that the preprandial glucose level increased with an increase in the HbA1c level14. This helps explain the association between average glucose levels and HbA1c levels in the present study.
Patients with a relatively favorable HbA1c of ≥6.0 to <7.0% already had a higher MAGE, approximately 1.5‐fold the range of 22–60 mg/dL reported in individuals with normal glucose tolerance13. MAGE represents the mean of daily glycemic fluctuation at and above 1 × SD, which reflects abrupt and greater fluctuations than SD18, and is closely correlated with oxidative stress1. In this regard, a number of studies of patients with type 2 diabetes showed that higher MAGE levels correlated better with risk of cardiovascular events, compared with high HbA1c19, and that a significant reduction in HbA1c was associated with a higher risk of cardiovascular events.20 Our results showed that HbA1c was not a contributing factor to MAGE. Considered together, we believe that any therapeutic strategy for type 2 diabetes that focuses only on HbA1c is limited.
Although MAGE is clearly an important and useful parameter, it is not practical, as CGM cannot be used in all patients. The present study found high MAGE in patients with low mean blood glucose levels. In actual clinical practice, it is important to treat patients by monitoring blood glucose fluctuations even when HbA1c is low, and by monitoring postprandial blood glucose levels to identify hypoglycemia.
The present study had the following limitations: (i) small sample size; (ii) possible impact of medications on blood glucose profiles due to the large proportion of patients taking medications; and (iii) possible glycemic improvement by inpatient dietary and exercise therapies probably related to the study design of recording CGM data during hospital stay. Future studies should increase the proportion of patients not taking oral glucose‐lowering medications and further evaluate patients in routine practice.
Disclosure
Y Tanaka has received consulting fees, speaking fees, and/or honoraria from Abbvie, Chugai, Astellas, Takeda, Santen, Mitsubishi‐Tanabe, Pfizer, Janssen, Eisai, Daiichi‐Sankyo, UCB, GlaxoSmithKline and Bristol‐Myers, and has received research grants from Mitsubishi‐Tanabe, Chugai, MSD, Astellas and Novartis. Y Okada has received speaking fees from Astellas Pharma, Daiichi‐Sankyo, Eli Lilly Japan, Novartis, Novo Nordisc Pharma and MSD. All other authors declare no conflict of interest.
Acknowledgments
The authors thank Dr Fujino for the analysis, and Ms N Sakaguchi for excellent technical assistance. This study was supported in part by a Research Grant‐In‐Aid for Scientific Research from the Ministry of Health, Labor and Welfare of Japan; the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Joint Research Association for Japanese Diabetes; and the University of Occupational and Environmental Health, Japan.
J Diabetes Investig 2018; 9: 75–82
References
- 1. Monnier L, Mas E, Ginet C, et al Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681–1689. [DOI] [PubMed] [Google Scholar]
- 2. Xu F, Zhao LH, Su JB, et al The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well‐controlled HbA1c. Diabetol Metab Syndr 2014; 6: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torimoto K, Okada Y, Mori H, et al Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol 2013; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azuma K, Kawamori R, Toyofuku Y, et al Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol 2006; 26: 2275–2280. [DOI] [PubMed] [Google Scholar]
- 5. Abbatecola AM, Rizzo MR, Barbieri M, et al Postprandial plasma glucose excursions and cognitive functioning in aged type 2 diabetics. Neurology 2006; 67: 235–240. [DOI] [PubMed] [Google Scholar]
- 6. Rizzo MR, Marfella R, Barbieri M, et al Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 2010; 33: 2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu CR, Chen YT, Sheu WH, et al Glycemic variability and diabetes retinopathy: a missing link. J Diabetes Complications 2015; 29: 302–306. [DOI] [PubMed] [Google Scholar]
- 8. Ando K, Nishimura R, Tsujino D, et al 24‐hour glycemic variations in drug‐naïve patients with type 2 diabetes: a continuous glucose monitoring (CGM)‐based study. PLoS ONE 2013; 8: e71102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. United Kingdom Prospective Diabetes Study (UKPDS) Group . UK Prospective Diabetes Study 7. Response of fasting plasma glucose to diet therapy in newly presenting type 2 diabetic patients, UKPDS Group. Metabolism 1990; 39: 905–912. [PubMed] [Google Scholar]
- 10. Boyne MS, Silver DM, Kaplan J, et al Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 2003; 52: 2790–2794. [DOI] [PubMed] [Google Scholar]
- 11. Marics G, Lendvai Z, Lodi C, et al Evaluation of an open access software for calculating glucose variability parameters of a continuous glucose monitoring system applied at pediatric intensive care unit. Biomed Eng Online 2015; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seino T, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Service FJ, Molmar GD, Rosevear JW, et al Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970; 19: 644–655. [DOI] [PubMed] [Google Scholar]
- 14. Monnier L, Colette C, Dunseath GJ, et al The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007; 30: 263–269. [DOI] [PubMed] [Google Scholar]
- 15. Monnier L, Lapinski H, Colette C, et al Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003; 26: 881–885. [DOI] [PubMed] [Google Scholar]
- 16. Bonora E, Calcaterra F, Lombardi S, et al Plasma glucose levels throughout the day and HbA1c interrelationships in type 2 diabetes: implications for treatment and monitoring of metabolic control. Diabetes Care 2001; 24: 2023–2029. [DOI] [PubMed] [Google Scholar]
- 17. Borg R, Kuenen JC, Carstensen B, et al Associations between features of glucose exposure and A1C: the A1C‐Derived Average Glucose (ADAG) study. Diabetes 2010; 59: 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol 2008; 2: 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Su G, Mi S, Li Z, et al Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol 2011; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Emerging Risk Factors Collaboration , Di Angelantonio E, Gao P, et al Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA 2014; 311: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]


