Abstract
Introduction
The present phase 3, randomized, open‐label study compared the efficacy and safety of basal insulin peglispro with insulin glargine after 26 weeks of treatment when added to oral antihyperglycemic medications in insulin‐naïve Asian patients with type 2 diabetes.
Materials and Methods
The primary objective was to show non‐inferiority of the change in glycated hemoglobin from baseline to 26 weeks.
Results
At 26 weeks, insulin peglispro was non‐inferior to glargine, meeting the primary objective. Patients receiving insulin peglispro (n = 192) showed a greater reduction in glycated hemoglobin from baseline compared with glargine (n = 196); −1.6 vs −1.4%, P = 0.005) and in fasting serum glucose (−61.2 vs −54.8 mg/dL, P = 0.02). A significantly higher proportion of patients receiving insulin peglispro achieved glycated hemoglobin <7% (57 vs 44%, P = 0.012). Insulin peglispro patients showed significantly less weight gain from baseline (1.1 vs 1.6 kg, P = 0.03). Relative rates (insulin peglispro/glargine) of total and nocturnal hypoglycemia through 26 weeks were 1.06 (P = 0.67) and 0.7 (P = 0.10), respectively. Significantly more insulin peglispro‐treated patients experienced adverse events compared with glargine‐treated patients (P = 0.042). Alanine aminotransferase and aspartate aminotransferase were significantly increased from baseline with insulin peglispro compared with glargine at week 26 (3.5 vs −4.6 IU/L and 2.8 vs −1.5 IU/L, respectively; P < 0.001). The incidence of injection site reactions was low and did not differ between the treatments.
Discussion
Insulin peglispro provided better glycemic control vs glargine with no differences in hypoglycemia and increased aminotransferases in insulin‐naïve Asian patients with type 2 diabetes.
Keywords: Basal insulin peglispro, Insulin glargine, Type 2 diabetes
Introduction
The prevalence of diabetes is rapidly increasing globally, especially in the western Pacific region, possibly as a result of the aging population, urbanization, changing dietary habits and increasing obesity1, 2. The typical characteristics of Asian patients with type 2 diabetes compared with Caucasian patients include lower body mass index with more impaired pancreatic β‐cell function than increased peripheral insulin resistance3. Many patients with type 2 diabetes fail to achieve glycemic control with oral antihyperglycemic medications (OAMs), and initiate insulin therapy4, 5. Basal insulins added to OAMs are widely used to initiate insulin therapy in Asian countries6, 7; however, Asian patients with type 2 diabetes might require lower insulin doses compared with Western patients with type 2 diabetes8, 9.
The novel basal insulin peglispro (BIL) is insulin lispro covalently bound through a urethane bond to a 20‐kDa polyethylene glycol molecule10. The molecular weight of BIL is approximately 25.8 kDa; however, its hydrodynamic size is larger and comparable with albumin (for example). This results in delayed absorption and clearance, both contributing to a longer time–action profile, with a half‐life of 2–3 days.
BIL is hypothesized to have restricted passage through the vascular endothelium to peripheral tissues, but ready access to the liver through the fenestrations in the hepatic sinusoidal endothelium11.
The restricted peripheral action of BIL might explain the consistent findings of the phase 3 program in type 2 diabetes, with superior glycemic control (glycated hemoglobin [HbA1c]), reduction in nocturnal hypoglycemia and less insulin‐induced weight gain (or weight loss in type 1 diabetes) compared with insulin glargine (GL), but also higher triglycerides, liver fat content (LFC) and alanine aminotransferase (ALT) compared with GL.
In a 78‐week, phase 3, double‐blind, multinational study in insulin‐naïve patients with type 2 diabetes (IMAGINE‐2), BIL resulted in a greater reduction in HbA1c and a greater proportion of patients achieving HbA1c <7% compared with GL, and the nocturnal hypoglycemia rate was statistically significantly lower with BIL compared with GL12. The variability of glucose levels was lower, the dose of basal insulin was higher and weight gain was less with BIL compared with GL. Greater proportions of BIL‐treated patients than GL‐treated patients experienced ALT ≥3‐fold the upper limit of normal (ULN). At week 26, triglycerides were unchanged with BIL, but ALT was higher than at baseline and compared with GL. LFC measured by magnetic resonance imaging was unchanged through 52 weeks with BIL, but decreased with GL.
Because of the known ethnic differences in pathophysiology of type 2 diabetes between Asian and Caucasian patients3, to determine whether BIL has effects similar to those discussed above in an Asian population, the present phase 3, open‐label study compared BIL with GL in insulin‐naïve Asian patients with type 2 diabetes inadequately controlled with OAM therapy.
Methods
Study design
The present study was a 26‐week, multinational, randomized, parallel‐arm trial that compared BIL with GL. The study protocol was approved by the local ethical review boards, and the study was carried out in accordance with the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, the International Conference on Harmonization Good Clinical Practices Guideline, and applicable laws and regulations. All patients provided written informed consent. Study safety was monitored by an unblinded, independent Data Monitoring Committee.
Patients
Asian male or female patients aged ≥20 years with type 2 diabetes (per World Health Organization criteria13) who were insulin‐naïve were eligible if duration of diabetes was ≥1 year, they were receiving stable doses of ≥2 OAMs, had HbA1c of 7.0–11.0%, inclusive, and body mass index ≤35.0 kg/m2. Patients who had signs or symptoms of liver disease, acute or chronic hepatitis, non‐alcoholic steatohepatitis, or elevated liver enzyme measurements (total bilirubin 2‐fold ULN, ALT and/or aspartate aminotransferase [AST] >2.5‐fold ULN) were excluded. In addition, patients who had fasting triglycerides >400 mg/dL were excluded.
Treatments and outcome measures
Patients were randomized in a 1:1 ratio to BIL or GL treatment. Randomization was stratified according to baseline HbA1c (≤8.5 and >8.5%), low‐density lipoprotein cholesterol (<100 and ≥100 mg/dL), country and sulfonylurea/meglitinide use.
The initial study insulin dose was 8 U, administered once daily at bedtime at approximately the same time every night. The insulin dose of 8 U was chosen due to a lower physiological insulin demand in the Asian population compared with Caucasians9, 14, 15. For both BIL and GL, the dose was adjusted for target fasting blood glucose (FBG) of 71–100 mg/dL without hypoglycemia using a dosing algorithm based on the patient's blood glucose (BG; Table S1). Glycemic goals were attained solely by insulin adjustment; OAM doses were altered only for non‐glycemic side‐effects and safety.
Patients carried out self‐monitored BG (SMBG) testing at least once daily (pre‐morning meal [fasting]). Nine‐point SMBG profiles (pre‐ and 2 h post‐morning meal, pre‐ and 2 h post‐midday meal, pre‐ and 2 h post‐evening meal, bedtime, 03.00 hours, and fasting the subsequent morning [next day]) were carried out on two non‐consecutive days in the week before certain predetermined study visits.
A hypoglycemic event was defined based on a measured SMBG ≤70 mg/dL with or without signs/symptoms of hypoglycemia, or with signs/symptoms of hypoglycemia in the absence of glucose measurements. Nocturnal hypoglycemia was defined as any hypoglycemia occurring between bedtime and waking. Severe hypoglycemia was defined as an episode requiring the assistance of another person to actively administer carbohydrates, glucagon or other resuscitative actions.
An independent committee adjudicated prespecified cardiovascular events of interest to determine the incidence of major adverse cardiac events (MACE+: adjudicated positive non‐fatal myocardial infarction, stroke, cardiovascular death and unstable angina with hospitalization).
Continuous glucose monitoring (CGM) was carried out in a subset of Japanese patients using the Medtronic iPro™2 Professional Continuous Glucose Monitoring System (iPro2 CGM; Medtronic MiniMed, Inc., Northridge, California, USA) over 24‐h periods. The primary CGM end‐point was to compare BIL vs GL for the duration of nocturnal hypoglycemia, measured by total minutes with a BG level ≤70 mg/dL from midnight to 06.00 hours, at 26 weeks of treatment. Secondary CGM end‐points included comparing BIL vs GL for the duration of time with a BG level ≤70 mg/dL over a 24‐h period, and the mean duration of individual hypoglycemic episodes with a BG ≤70 mg/dL over a 24‐h period. The durations per day (in minutes) were derived from the percentages of time during the period where glucose values were within a hypoglycemic range (defined as ≤70 mg/dL [3.9 mmol/L]), and standardized to account for times when gaps occurred.
Statistical analysis
The primary objective of the present study was to show non‐inferiority between BIL and GL with respect to HbA1c change from baseline at week 26. It was determined that a total of 320 completers (160 completers per arm) would provide 90% statistical power to show non‐inferiority between the treatments using the upper limit of a 95% two‐sided confidence interval (CI; BIL − GL) with assumptions of a randomization ratio of 1:1, a non‐inferiority margin of 0.4%, no true difference between the two arms and a common standard deviation of 1.1%. A total of 380 patients were to be randomized (assuming a 15% dropout rate).
Efficacy data were collected at screening, baseline, and weeks 4, 8, 12, 16 and 26. The primary efficacy measure was the change in HbA1c from baseline to 26 weeks in the full analysis set population. Secondary efficacy measures included HbA1c changes from baseline to each postbaseline visit, proportions of patients with HbA1c <7.0% or HbA1c <7.0% with no nocturnal hypoglycemia, hypoglycemia (total and nocturnal, incidence and rates), fasting serum glucose (FSG) from central laboratory, SMBG nine‐point profiles, FBG from SMBG, within‐day and between‐day glycemic variability measured by FBG, insulin doses, and bodyweight.
All analyses were carried out with SAS® 9.2 (SAS Institute, Cary, North Carolina, USA). Changes in HbA1c from baseline were analyzed with a mixed model for repeated measures, as were changes in FSG, SMBG and weight. The proportions of patients achieving HbA1c <7.0% or HbA1c <7.0% with no nocturnal hypoglycemia were analyzed with longitudinal logistic regression. Hypoglycemia rates were analyzed with negative binomial regression. The three key CGM end‐points were analyzed using a constrained longitudinal data analysis model.
Results
Patient population
A total of 388 patients from Japan, South Korea and Taiwan were randomized to receive either BIL or GL; all of these patients received study medication, and were included in the full analysis set population (Table 1). Baseline characteristics were generally balanced between treatment arms. A total of 182 patients (94.8%) randomized to BIL, and 186 patients (94.9%) randomized to GL completed the study (Figure S1).
Table 1.
Demographics and patient characteristics
| GL (n = 196) | BIL (n = 192) | |
|---|---|---|
| Age (years) | 56.9 ± 9.6 | 57.9 ± 10.0 |
| Male, n (%) | 106 (54.1) | 111 (57.8) |
| Weight (kg) | 69.7 ± 12.6 | 70.0 ± 12.7 |
| Body mass index (kg/m2) | 26.0 ± 3.7 | 26.2 ± 3.6 |
| Duration of diabetes (years) | 11.8 ± 6.2 | 12.5 ± 6.5 |
| Region, n (%) | ||
| Japan | 102 (52.0) | 103 (53.6) |
| South Korea | 52 (26.5) | 49 (25.5) |
| Taiwan | 42 (21.4) | 40 (20.8) |
| Oral antihyperglycemic medications, n (%) | ||
| Biguanides | 167 (85.2) | 164 (85.4) |
| Sulfonylurea | 158 (80.6) | 159 (82.8) |
| DPP‐4 inhibitor | 139 (70.9) | 129 (67.2) |
| α‐Glucosidase inhibitor | 32 (16.3) | 27 (14.1) |
| Thiazolidinediones | 22 (11.2) | 28 (14.6) |
| Oral antihyperglycemic medications during treatment, n (%) | ||
| 2 | 75 (38.3) | 73 (38.0) |
| 3 | 108 (55.1) | 108 (56.3) |
| ≥4 | 13 (6.6) | 11 (5.7) |
| Lipid‐lowering medications, n (%) | 118 (60.2) | 114 (59.4) |
| Hypertension, n (%) | 125 (63.8) | 120 (62.5) |
All patients were Asian. Data are mean ± standard deviation unless otherwise noted. BIL, basal insulin peglispro; DPP‐4, dipeptidyl peptidase‐4; GL, glargine; SD, standard deviation.
Efficacy
The least squares mean (LS mean) treatment difference (BIL − GL) in the change in HbA1c from baseline at week 26 was −0.24% (95% CI: −0.41, −0.07; P = 0.005); BIL was considered non‐inferior to GL, meeting the primary objective of the study. Table 2 summarizes treatment outcomes at baseline and week 26. The BIL group had significantly lower LS mean HbA1c at week 26 compared with the GL group (P = 0.005). LS mean (SE) HbA1c values (%) by treatment over time are plotted in Figure 1.
Table 2.
Treatment outcomes at baseline and after 26 weeks of treatment
| Baseline | Week 26 | P‐value BIL vs GL | |||
|---|---|---|---|---|---|
| GL (n = 196) | BIL (n = 192) | GL (n = 196) | BIL (n = 192) | ||
| HbA1c (%) | 8.48 ± 0.07 | 8.57 ± 0.07 | 7.17 ± 0.06 | 6.92 ± 0.06 | 0.005 |
| Change from baseline | – | – | −1.36 ± 0.06 | −1.61 ± 0.06 | |
| Fasting serum glucose (mg/dL) | 167 ± 3 | 164 ± 3 | 110 ± 2 | 104 ± 2 | 0.018 |
| Change from baseline | – | – | −54.8 ± 1.9 | −61.2 ± 1.9 | |
| HbA1c <7%, n (%) | 0 (0.0) | 2 (1.0) | 81 (44.0) | 102 (57.0) | 0.012 |
| HbA1c <7.0% and no nocturnal hypoglycemia (BG ≤70 mg/dL), n (%) | 0 (0.0) | 2 (1.0) | 59 (32.1) | 74 (41.3) | 0.035 |
| Bodyweight (kg) | 69.9 ± 0.90 | 69.9 ± 0.91 | 71.4 ± 0.16 | 70.9 ± 0.16 | 0.026 |
| Change from baseline | – | – | 1.6 ± 0.16 | 1.1 ± 0.16 | |
| Insulin dose | |||||
| U | – | – | 19.2 ± 0.80 | 19.0 ± 0.81 | 0.872 |
| U/kg | – | – | 0.26 ± 0.01 | 0.26 ± 0.01 | 0.969 |
| Lipids (mg/dL) | |||||
| TG | 125.9 ± 6.1 | 131.9 ± 6.2 | 122.8 ± 4.7 | 132.4 ± 4.7 | 0.148 |
| Change from baseline | – | – | −2.3 ± 4.7 | 7.3 ± 4.7 | |
| LDL‐C | 97.9 ± 2.4 | 101.6 ± 2.4 | 101.1 ± 1.5 | 98.0 ± 1.5 | 0.135 |
| Change from baseline | – | – | 1.4 ± 1.5 | −1.8 ± 1.5 | |
| HDL‐C | 50.9 ± 1.0 | 51.8 ± 1.0 | 53.0 ± 0.5 | 51.6 ± 0.5 | 0.051 |
| Change from baseline | – | – | 1.7 ± 0.5 | 0.4 ± 0.5 | |
| TC | 173. 8 ± 2.8 | 179.1 ± 2.8 | 177.9 ± 1.6 | 175.8 ± 1.7 | 0.357 |
| Change from baseline | – | – | 2.1 ± 1.6 | −0.0 ± 1.7 | |
| ALT (IU/L) | 30.3 ± 1.3 | 32.1 ± 1.3 | 26.6 ± 0.9 | 34.7 ± 1.0 | <0.001 |
| Change from baseline | – | – | −4.6 ± 0.9 | 3.5 ± 1.0 | |
| AST (IU/L) | 24.6 ± 0.8 | 26.5 ± 0.8 | 24.0 ± 0.6 | 28.3 ± 0.6 | <0.001 |
| Change from baseline | – | – | −1.5 ± 0.6 | 2.8 ± 0.6 | |
| Patients with any CGM data (n) | 51 | 49 | 49 | 46 | – |
| Duration of BG ≤70 mg/dL, min (nocturnal period) | 5.70 ± 1.64 | 1.80 ± 1.67 | 24.86 ± 5.57 | 20.37 ± 5.80 | 0.578 |
| Duration of BG ≤70 mg/dL, min (24‐h period) | 8.38 ± 2.54 | 3.01 ± 2.59 | 46.64 ± 9.67 | 34.58 ± 10.23 | 0.396 |
| Duration of individual episodes with BG ≤70 mg/dL, min (24‐h period) | 93.06 ± 1.44 (n = 10)† | 53.23 ± 19.57 (n = 4)† | 117.87 ± 14.77 (n = 22)† | 86.67 ± 15.11 (n = 20)† | 0.148 |
Data are least squares mean ± SE unless otherwise noted. †The number of patients with hypoglycemic episodes. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BG, blood glucose; BIL, basal insulin peglispro; CGM, continuous glucose monitoring; GL, glargine; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; LS mean, least‐squares mean; n, number of patients randomized and treated; SE, standard error; TC, total cholesterol; TG, triglycerides.
Figure 1.
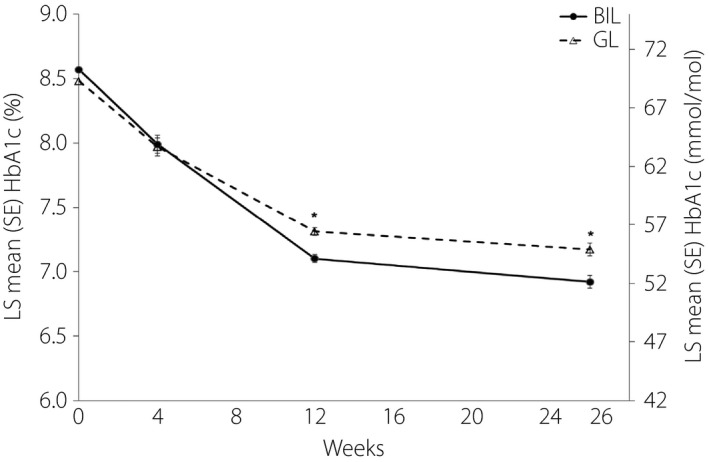
Least squares (LS) mean (standard error [SE]) glycated hemoglobin (HbA1c [%]) over time by treatment. *P < 0.005. BIL, basal insulin peglispro; GL, glargine; HbA1c, glycated hemoglobin; LS, least‐squares; SE, standard error.
At week 26, significantly greater proportions of patients in the BIL group compared with the GL group achieved the HbA1c target <7.0% (P = 0.012), and achieved the HbA1c target <7.0% with no nocturnal hypoglycemia (P = 0.035; Table 2).
At week 26, patients treated with BIL had a greater reduction in FSG (central laboratory) from baseline compared with patients treated with GL (P = 0.018;Figure S2). At week 26, both treatment groups had significant decreases from baseline for all time‐points of the nine‐point SMBG profile (P < 0.001); the treatment differences were not statistically significant (data not shown). There were no statistically significant treatment differences in FBG from SMBG at any time (data not shown). There were no statistically significant treatment differences for between‐day or within‐day glucose variability at week 26 (data not shown).
There were no statistically significant treatment differences in insulin dose (in U/kg/day or in U/day) at any time during treatment (data not shown). Insulin doses at week 26 are shown in Table 2. Patients in the BIL group had significantly smaller LS mean increases in bodyweight at week 26 compared with the GL group (P = 0.026; Table 2).
The total and nocturnal hypoglycemia rates from baseline to week 26 (events/30 days) did not differ significantly between the treatments (1.28 vs 1.21 events/30 days and 0.19 vs 0.27 events/30 days, respectively, for BIL vs GL); relative rates (BIL/GL) of total and nocturnal hypoglycemia through 26 weeks were 1.06 (P = 0.67) and 0.7 (P = 0.10), respectively. Figure 2a,b plot the cumulative numbers of events for total and nocturnal hypoglycemia, respectively, by treatment from baseline through week 26. No severe hypoglycemia occurred during the treatment period.
Figure 2.
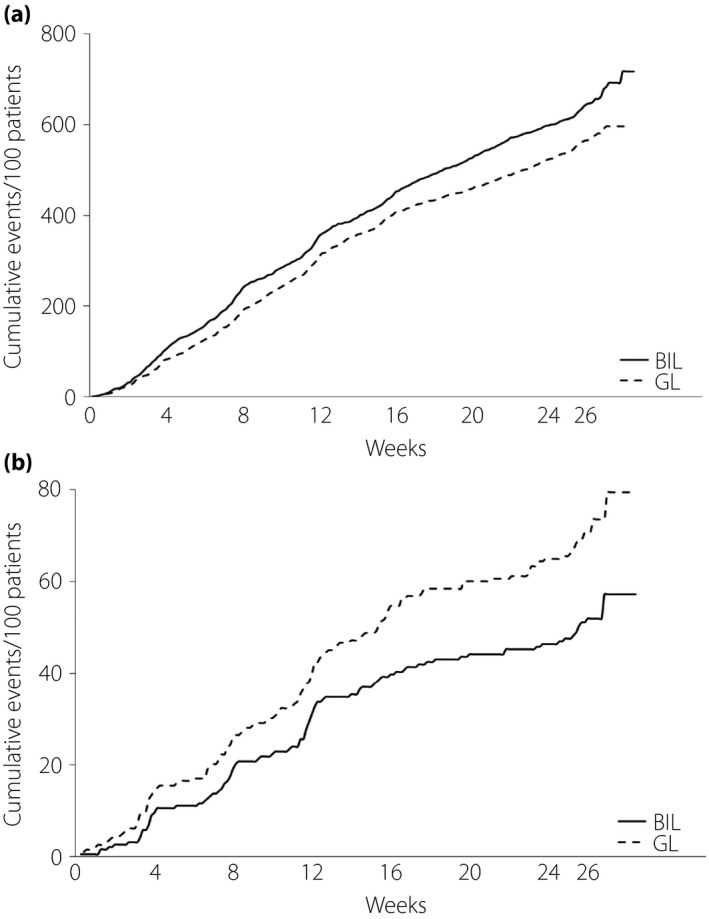
Hypoglycemia. (a) Cumulative numbers of total hypoglycemia events by treatment over time. (b) Cumulative numbers of nocturnal hypoglycemia by treatment over time. BIL, basal insulin peglispro; GL, glargine.
The use and doses of concomitant OAMs were mostly unchanged from baseline to week 26 (Table S2), and, therefore, would have little impact on insulin dosage, bodyweight and hypoglycemic events.
The primary CGM end‐point, duration of nocturnal hypoglycemia (total minutes with a BG ≤70 mg/dL from midnight to 06.00 hours) at week 26, was not significantly different between BIL (n = 49) and GL (n = 51; Table 2). The LS mean difference (BIL − GL) in duration of hypoglycemia during the nocturnal period was −4.49 min/day (95% CI: −20.47, 11.49; P = 0.578). The other key CGM end‐points at week 26 were also not significantly different between treatments (Table 2). At week 26, the LS mean difference in duration of hypoglycemia measured with CGM during the 24‐h period was −12.06 min/day (95% CI: −40.46, 16.03; P = 0.396), and the LS mean difference in duration of individual hypoglycemic episodes over a 24‐h period was −31.19 min (95% CI: −73.98, 11.59; P = 0.148).
Safety
During the 26‐week treatment period, 14 patients (3.6%) experienced at least one serious adverse event (BIL, 9 [4.7%]; GL, 5 [2.6%]), with no clinically important treatment group imbalances (Table S3). A significantly greater percentage of patients in the BIL group (n = 114 [59.4%]) reported at least one treatment‐emergent adverse event compared with the GL group (n = 96 [49.0%]; P = 0.042). In the BIL group, 62 patients (32.3%) had treatment‐emergent adverse events in the system organ class of infections and infestations, compared with 45 patients (23.0%) in the GL group (P = 0.042); there were no statistically significant treatment group differences for any other system organ class (Table S3). Overall, the most frequently reported treatment‐emergent adverse events included nasopharyngitis (16.5%), increased weight (3.1%) and pharyngitis (2.8%), with no statistically significant difference between treatment groups. There were no events adjudicated as MACE+.
In the BIL group, at week 4 LS mean triglycerides increased from baseline, whereas in the GL group, LS mean triglycerides decreased; at weeks 4 and 12, the treatment differences in changes from baseline were significant (LS mean changes were week 4: BIL, 2.99 mg/dL; GL, −11.10 mg/dL [P = 0.002]; week 12: BIL, 13.65 mg/dL; GL, −9.94 mg/dL [P = 0.003]). At week 26, the within‐BIL group change was not statistically significant, and the treatment difference was not significant (LS mean changes were BIL, 7.29 mg/dL; GL, −2.31 mg/dL; P = 0.148; Table 2). No statistically significant treatment differences were observed in changes from baseline in high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol or total cholesterol at week 26 (Table 2).
LS mean (±SE) ALT and AST were significantly increased from baseline at week 26 in the BIL group (within‐group P < 0.001 for both), and significantly decreased from baseline in the GL group (within‐group P < 0.001 and P < 0.009, respectively); the treatment differences were significant (LS mean differences: ALT, 8.12 IU/L; AST, 4.29 IU/L [P < 0.001, both]; Table 2). A small number of patients experienced study‐emergent ALT and/or AST values >3‐fold ULN, with no significant differences between the treatments: seven patients had ALT ≥3‐fold ULN (BIL, 4 [2.1%]; GL, 3 [1.5%]; P = 0.723), and three patients had AST ≥3‐fold ULN (BIL, 2 [1.0%]; GL, 1 [0.5%]; P = 0.622). No patients met the criteria for Hy's law (ALT or AST ≥3‐fold ULN, and total bilirubin ≥2‐fold ULN without findings of cholestasis and without any other apparent cause), showing that there was no evidence of acute, severe or drug‐induced liver injury16. The incidence of injection site reaction was low and did not differ between the treatments (Table S3). A total of 10 patients had potential systemic hypersensitivity reactions (Table S3); no patients had any potential systemic hypersensitivity reaction judged by the investigator to be possibly related to the study drug.
Discussion
This is the first trial in insulin‐naïve Asian patients with type 2 diabetes assessing the efficacy and safety of the hepato‐preferential insulin, BIL. BIL was non‐inferior for change in HbA1c from baseline compared with GL at 26 weeks, with a significantly greater reduction in HbA1c than GL (−1.6 vs −1.4%; P = 0.005). BIL treatment also resulted in a statistically significantly greater proportion of patients achieving HbA1c <7% compared with GL at week 26. These data are consistent with results from five multinational, phase 3 studies of BIL comparing its effects with GL in patients with type 2 diabetes or type 1 diabetes11, 17, and they show that BIL also results in better glycemic control compared with GL in insulin‐naïve Asian patients with type 2 diabetes.
Hypoglycemia and weight gain are two side‐effects patients fear when trying to attain glycemic control with insulin18. Although BIL resulted in greater reduction in HbA1c compared with GL after 26 weeks of treatment, the rates of total and nocturnal hypoglycemia were not statistically significantly different between the treatments. It is noteworthy that the numerically 30% lower rates of nocturnal hypoglycemia with BIL were consistent with the statistically significant results of IMAGINE‐2 (25 and 46% lower with BIL vs GL at 52 and 78 weeks of treatment, respectively)12. The absolute rate of nocturnal hypoglycemia with BIL in the present study was 0.19 events/30 days, which is numerically lower than observed with BIL in other studies in insulin‐naïve patients with type 2 diabetes: IMAGINE‐212 (0.30 events/30 days) and IMAGINE‐6, a multinational, phase 3 study of BIL compared with human insulin isophane suspension (0.31 events/30 days)19. The smaller sample size, the shorter study duration and relatively low rates of hypoglycemia in the present study are possible reasons why the difference in nocturnal hypoglycemia rates between BIL and GL did not reach statistical significance. Prolonged hypoglycemia is one of the concerns of longer‐acting basal insulins; however, no statistically significant differences were observed between the treatment groups in time spent with BG <70 mg/dL (3.9 mmol/L) or in the duration of a single hypoglycemic event in the nocturnal period or over 24 h measured by CGM in a subset of 100 patients. The similar durations of hypoglycemic episodes suggests that BIL did not prolong hypoglycemia compared with GL.
The insulin dose at week 26 was 0.26 U/kg/day with BIL, lower than those in the IMAGINE‐212 and IMAGINE‐619 studies (0.45 [week 52] and 0.40 U/kg/day [week 26], respectively), probably as a result of the ethnic difference in insulin sensitivity between East Asian and Caucasian patients with type 2 diabetes2, 3. Similar ethnic differences in basal insulin doses have been seen in other Asian, Japanese and global studies of long‐acting insulin analogs6, 8, 20, 21, 22, 23. Despite the lower basal insulin dose in the present study, glycemic control as measured by HbA1c and FSG was similar to that observed in the IMAGINE‐212 and IMAGINE‐619 studies. The basal insulin doses were consistently higher with BIL compared with GL at the study end‐point in other IMAGINE studies; however, the insulin doses were similar for both treatments in the present study at week 26. In this study, patients treated with BIL had less bodyweight gain throughout the 26‐week treatment period compared with GL, possibly as a result of less peripheral activity with BIL.
During the first 12 weeks of treatment in the study, serum triglycerides increased with BIL and decreased with GL; however, the treatment differences were not statistically significant at week 26, and were similar to those observed in insulin‐naïve patients with type 2 diabetes in IMAGINE‐212 and IMAGINE‐619 studies, and in a pooled analysis of six phase 3 trials of BIL24. This contrasts with the findings observed in the IMAGINE‐5 study, in which BIL‐ and GL‐treated patients had increased triglycerides from baseline, and the treatment differences at weeks 26 and 52 were statistically significant25. Treatment with GL and other conventional insulins has resulted in reduced triglycerides26, 27, possibly as a result of relatively more peripheral and less hepatic action of exogenously‐administered insulins. Therefore, the suppressive effect on triglycerides from the pre‐study basal insulin was attenuated when patients switched to BIL, resulting in increased triglycerides with BIL in the IMAGINE‐5 study. There were no statistically significant differences in total cholesterol, high‐density lipoprotein cholesterol or low‐density lipoprotein cholesterol between the treatment groups at 26 weeks in the present study.
ALT was increased from baseline with BIL treatment, and was statistically significantly higher compared with GL at week 26. The mean changes in aminotransferases were consistent with results reported in other phase 3 BIL studies12, 28. The mechanism(s) and clinical significance of mean increases in aminotransferases with BIL have not been established. LFC was measured in patients with type 2 diabetes in some of the other phase 3 BIL studies. The analysis of LFC in insulin‐naïve patients with type 2 diabetes in the IMAGINE‐2 study showed similar trends to triglycerides: no changes from baseline with BIL, and decreases with GL12. Although LFC was not measured in the present study, based on similar patient populations, results would have been expected to be similar to those observed in the IMAGINE‐2 study. In contrast, in the IMAGINE‐5 study, prior treatment of type 2 diabetes patients with basal insulin was associated with increases in LFC from baseline in patients with BIL treatment, but there was no change in LFC from baseline in patients assigned to GL treatment after treatment up to 52 weeks25, which was consistent with the changes in triglycerides observed in the study. The clinical implications of the ALT findings are still unclear. In the present study, there was no evidence of acute, severe, hepatocellular, drug‐induced liver injury. However, it is unknown whether higher LFC in BIL‐ vs glargine‐treated patients, shown in other BIL phase 3 trials, would alter the future risk of steatohepatitis28.
In December 2015, Eli Lilly and Company formally announced that they would cease development of BIL based on discussions with regulatory authorities and other external experts, particularly around the liver fat changes that were observed in the IMAGINE trials. The company concluded that further studies to address the safety findings would have required a significant amount of time and investment, and stated that it was unclear whether any such studies would produce conclusive answers on the liver data.
Limitations of the present study included the short duration of treatment, the open‐label design and a relatively small number of patients, which resulted in insufficient power to detect clinically meaningful differences in hypoglycemia between the treatments. Strengths included the rigorous execution of a treat‐to‐target treatment algorithm, with good adherence in both treatment groups (data not shown).
In summary, the present study showed that BIL provides greater glycemic‐lowering efficacy than GL in insulin‐naïve Asian patients with type 2 diabetes inadequately controlled by OAMs. This was achieved with less weight gain, but also higher ALT and AST compared with GL. The efficacy and safety findings were similar to those observed in other multinational studies that included a wide variety of ethnicities.
Disclosure
TH has received lecture fees from Sanofi, Eli Lilly, Novo Nordisk, Takeda Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe, MSD, Dainippon Sumitomo, Novartis, Kissei Pharma, Boehringer Ingelheim, Ono Pharmaceutical and AstraZeneca; scholarships from Boehringer Ingelheim and AstraZeneca; and research endowments from Sanofi, Eli Lilly, Novo Nordisk, Mitsubishi Tanabe, Kissei Pharma, Boehringer Ingelheim, Ono Pharmaceutical, AstraZeneca, Taisho‐Toyama. The other authors are employees of Eli Lilly.
Supporting information
Figure S1 ¦ Patient disposition. BIL, basal insulin peglispro; GL, glargine.
Figure S2 ¦ Least squares (LS) mean (standard error [SE]) fasting serum glucose (FSG; mg/dL) over time by treatment.
Table S1 ¦ Treat‐to‐target insulin dosing algorithm.
Table S2 ¦ Mean doses of concomitant oral antihyperglycemic medications at baseline and week 26.
Table S3 ¦ Summary of safety data during the 26‐week treatment period.
Acknowledgments
The authors thank the participants, investigators and contributors. Barbara Nambu PhD, Teri Tucker (inVentiv Health Clinical) and Maki Miyamoto (Eli Lilly Japan K.K) provided writing assistance. This research was supported by Eli Lilly and Company.
J Diabetes Investig 2018; 9: 100–107
Clinical Trial Registry ClinicalTrials.govNCT01894568
References
- 1. Rhee EJ. Diabetes in Asians. Endocrinol Metab (Seoul) 2015; 30: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013; 1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fukushima M, Usami M, Ikeda M, et al Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Standards of medical care in diabetes. Diabetes Care 2016; 39(Suppl 1): S1–S106.26696671 [Google Scholar]
- 5. The Japan Diabetes Society . Evidence‐Based Practice Guideline for the Treatment of Diabetes in Japan 2013. Nankodo Co,. Ltd, Bunkyo‐ku, Tokyo, Japan, 2013. Available from: http://www.jds.or.jp/modules/en/index.php?content_id=44 Accessed January 20, 2017 (in Japanese) [Google Scholar]
- 6. Odawara M, Ohtani T, Kadowaki T. Dosing of insulin glargine to achieve the treatment target in Japanese type 2 diabetes on a basal supported oral therapy regimen in real life: ALOHA study subanalysis. Diabetes Technol Ther 2012; 14: 635–643. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki D, Umezono T, Miyauchi M, et al Effectiveness of basal‐supported oral therapy (BOT) using insulin glargine in patients with poorly controlled type 2 diabetes. Tokai J Exp Clin Med 2012; 37: 41–46. [PubMed] [Google Scholar]
- 8. Tsai S, Pathan F, Ji L, et al First insulinization with basal insulin in patients with Type 2 diabetes in a real‐world setting in Asia. J Diabetes 2011; 3: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The Treat‐to‐Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26: 3080–3086. [DOI] [PubMed] [Google Scholar]
- 10. Beals JM, Cutler GB Jr, Vick A, et al LY2605541: leveraging hydrodynamic size to develop a novel basal insulin. Diabetologia 2012; 55(Suppl 1): S23. [Google Scholar]
- 11. Jacober SJ, Prince MJ, Beals JM, et al Basal insulin peglispro: overview of a novel long‐acting insulin with reduced peripheral effect resulting in a hepato‐preferential action. Diabetes Obes Metab 2016; 18(Suppl 2): 3–16. [DOI] [PubMed] [Google Scholar]
- 12. Davies MJ, Russell‐Jones D, Selam J‐L, et al Basal insulin peglispro versus insulin glargine in insulin‐naïve type 2 diabetes: IMAGINE 2 randomized trial. Diabetes Obes Metab 2016; 18: 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. 14 January 2006. Available from: http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf Accessed January 20, 2017.
- 14. Pan CY, Sinnassamy P, Chung KD, et al LEAD Study Investigators Group. Insulin glargine versus NPH insulin therapy in Asian type 2 diabetes patients. Diabetes Res Clin Pract 2007; 76: 111–118. [DOI] [PubMed] [Google Scholar]
- 15. Kawamori R, Eliaschewitz FG, Takayama H, et al Efficacy of insulin glargine and glimepiride in controlling blood glucose of ethnic Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2008; 79: 97–102. [DOI] [PubMed] [Google Scholar]
- 16. Center for Drug Evaluation and Research, Food and Drug Administration . Guidance for industry: Drug‐induced liver injury: Premarketing clinical evaluation. 2009. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090.pdf Accessed January 20, 2017.
- 17. Rosenstock J, Marre M, Qu Y, et al Reduced nocturnal hypoglycemia with basal insulin peglispro compared with insulin glargine: pooled analyses of five randomized controlled trials. Diabetes Obes Metab 2016; 18: 1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin 2011; 27(Suppl. 3): 13–20. [DOI] [PubMed] [Google Scholar]
- 19. Grunberger G, Chen A, Rodriguez A, et al A randomised clinical trial of basal insulin peglispro versus NPH in insulin‐naïve patients with type 2 diabetes: the IMAGINE 6 Trial. Diabetes Obes Metab 2016; 18(Suppl 2): 34–42. [DOI] [PubMed] [Google Scholar]
- 20. Onishi Y, Iwamoto Y, Yoo SJ, et al Insulin degludec compared with insulin glargine in insulin‐naïve patients with type 2 diabetes: a 26‐week, randomized, controlled, Pan‐Asian, treat‐to‐target trial. J Diabetes Investig 2013; 4: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zinman B, Philis‐Tsimikas A, Cariou B, et al Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terauchi Y, Koyama M, Cheng X, et al New insulin glargine 300 U/mL versus glargine 100 U/mL in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2). Diabetes Obes Metab 2016; 18: 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yki‐Järvinen H, Bergenstal RM, Zieman M, et al New insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 2). Diabetes Care 2014; 37: 3235–3243. [DOI] [PubMed] [Google Scholar]
- 24. Ginsberg H, Cariou B, Orchard TJ, et al Lipid changes during 26‐wk treatment with the novel basal insulin peglispro (BIL) vs. insulin glargine (GL) or insulin NPH in 6 IMAGINE trials. Diabetes 2015; 64(Suppl. 1, 990–P): A251. [DOI] [PubMed] [Google Scholar]
- 25. Buse JB, Rodbard HW, Trescoli Serrano C, et al Randomized clinical trial comparing basal insulin peglispro and insulin glargine in patients with type 2 diabetes previously treated with basal insulin: IMAGINE 5. Diabetes Care 2016; 39: 92–100. [DOI] [PubMed] [Google Scholar]
- 26. Lindström T, Arnqvist HJ, Olsson AG. Effect of different insulin regimens on plasma lipoprotein and apolipoprotein concentrations in patients with non‐insulin‐dependent diabetes mellitus. Atherosclerosis 1990; 81: 137–144. [DOI] [PubMed] [Google Scholar]
- 27. Hollenbeck CB, Chen YD, Greenfield MS, et al Reduced plasma high density lipoprotein‐cholesterol concentrations need not increase when hyperglycemia is controlled with insulin in noninsulin‐dependent diabetes mellitus. J Clin Endocrinol Metab 1986; 62: 605–608. [DOI] [PubMed] [Google Scholar]
- 28. Cusi K, Sanyal A, Zhang S, et al Different effects of basal insulin peglispro and insulin glargine on liver enzymes and liver fat content in patients with type 1 and type 2 diabetes. Diabetes Obes Metab 2016; 18(Suppl 2): 50–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ Patient disposition. BIL, basal insulin peglispro; GL, glargine.
Figure S2 ¦ Least squares (LS) mean (standard error [SE]) fasting serum glucose (FSG; mg/dL) over time by treatment.
Table S1 ¦ Treat‐to‐target insulin dosing algorithm.
Table S2 ¦ Mean doses of concomitant oral antihyperglycemic medications at baseline and week 26.
Table S3 ¦ Summary of safety data during the 26‐week treatment period.


