Abstract
Aims/Introduction
Glucagon‐like peptide‐1 (GLP‐1) secreted from enteroendocrine L cells is an incretin that potentiates insulin secretion and is already applied in therapies for type 2 diabetes. However, detailed examination of L cells throughout the gastrointestinal tract remains unclear, because of difficulties in purifying scattered L cells from other cells. In the present study, we identified characteristics of L cells of the upper small intestine (UI), the lower small intestine (LI) and the colon using glucagon‐green fluorescent protein‐expressing mice that express GFP driven by the proglucagon promoter.
Materials and Methods
The localization and density of primary L cells were evaluated by anti‐green fluorescent protein antibody reactivity. GLP‐1 content, messenger ribonucleic acid (mRNA) expression levels and secretion in purified L cells were measured.
Results
The number of L cells significantly increased toward the colon. In contrast, the GLP‐1 content and secretion from L cells were higher in the UI than in the LI and colon. L cells from the UI and LI expressed notably high mRNA levels of the transcription factor, islet 1. The mRNA expression levels of peptide YY in L cells were higher in the LI than in the UI and colon. The mRNA expression levels of gastric inhibitory polypeptide in L cells from the UI were significantly higher compared with those from the LI and colon.
Conclusions
L cells show different numbers and characteristics throughout the gut, and they express different mRNA levels of transcription factors and gastrointestinal hormones. These results contribute to the therapeutic application of promoting GLP‐1 release from L cells for the treatment of type 2 diabetes.
Keywords: Enteroendocrine cell, Glucagon‐like peptide‐1, Intestinal L cell
Introduction
Gut–brain communication, the so‐called enteric nervous system, maintains complex homeostatic body states including energy homeostasis1, 2. Enteroendocrine cells (EECs) in the gastrointestinal (GI) tract secrete a large number of peptide hormones that form a homologous family; these cells are traditionally classified by immunocytochemistry into at least 10 different cell types3. More than 100 kinds of gut hormones secreted from EECs have been identified; therefore, the gut is recognized as the largest endocrine organ in the body4. However, analysis of EECs has been difficult, because they comprise <1% of all gut epithelial cells, and are not concentrated like other endocrine organs.
Glucagon‐like peptide‐1 (GLP‐1) is a 31‐amino‐acid polypeptide hormone produced in enteroendocrine L cells located mainly in the distal part of the GI tract5. GLP‐1 is one of the major ‘incretins’ that potentiates a greater and more persistent plasma insulin increase with an oral glucose load than it does with intravenous glucose infusion6. GLP‐1 also shows extrapancreatic effects in the cardiovascular system, GI tract and central nervous system5, 6, 7. Therefore, GLP‐1 has been targeted in clinical applications in patients with type 2 diabetes by dipeptidyl peptidase‐4 inhibitors, which delay degradation of endogenous GLP‐1, and GLP‐1 receptor mimetics6, 7. Furthermore, the increase in plasma GLP‐1 levels is beneficial in patients with type 2 diabetes. Another strategy for GLP‐1 treatment stimulates the release of GLP‐1 from L cells8.
Different EECs are developed from close cell lineages, thus, coexistence of several peptide hormones have been distinguished9. Proglucagon protein is produced from the proglucagon (Gcg) gene in L cells. Prohormone convertase (PC) 1/3 cleaves proglucagon into glicentin‐specific peptide, GLP‐1 and GLP‐2 in an L‐cell‐specific manner5, 10. Furthermore, L cells have been shown to co‐express several related intestinal hormones, such as peptide YY (PYY) and gastric inhibitory polypeptide (GIP; also called glucose‐dependent insulinotropic polypeptide)11, 12, 13. Using mice lacking transcription factor genes, such as paired‐box domain transcription factor (Pax) 4, Pax6 and pancreatic duodenal homeobox 1 (Pdx1), several groups of transcription factors have been suggested to have key roles in L cells14, 15, 16.
L cells are located broadly from the upper intestine (UI) to the colon; however, little is known about the L cell distribution throughout the GI tract. Recently, transgenic mouse models expressing green fluorescent protein (GFP) variants under the control of promoters of gut peptide precursors have been generated, enabling EEC analysis17, 18. However, these modified mice are altered through the use of transgenic engineering techniques that represent the gain‐of‐function approach. In the present study, we used previously established glucagon‐GFP knock‐in (Gcg‐GFP) mice that express GFP driven by the native proglucagon promoter19, to analyze and purify L cells as GFP‐positive (GFP[+]) cells by flow cytometry. The purpose of the present study was to elucidate the differences between primary murine L cells in the UI, the lower small intestine (LI) and the colon, with the aim of helping to establish a new therapeutic approach for increasing GLP‐1 secretion from L cells in patients with type 2 diabetes.
Methods
Animals
Gcg‐GFP heterozygous (Gcggfp/+) mice generated as reported previously19 were backcrossed with the C57BL/6J strain for more than 10 generations. The blood glucose levels, serum insulin levels, plasma GLP‐1 levels and L‐cell population in the Gcggfp/+ mice were not altered compared with those in wild‐type mice, as previously described (data not shown)19. We used 7–16‐week‐old heteromutant male mice to analyze GFP(+) and GFP‐negative (GFP[−]) cells as L cells and non‐L cells, respectively. The mice were housed in an air‐controlled (25°C) room with a dark–light cycle of 10:14 h. This study was carried out in strict accordance with Directive 2010/63/EU for animal experiments. Animal care and procedures were approved by the Kyoto University Animal Care Committee. All efforts were made to minimize the suffering of the animals.
Histological and quantitative analyses of the gut
Mice were killed by cervical dislocation and the GI tract was quickly removed. The small intestine was divided into two portions at the middle position, and the oral and rectal portions were defined as the UI and LI, respectively. Samples were fixed in Bouin's solution and embedded in paraffin as previously described20, 21. In brief, paraffin sections were blocked for 15 min in 3% bovine serum albumin at room temperature, and then incubated with a mouse monoclonal anti‐GFP antibody (sc‐9996, 1:100; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) as the primary antibody overnight at 4°C. According to the manufacturer's information, this antibody was raised against amino acids 1–238 representing the full length GFP of Aequorea victoria. The sections were then incubated with a goat anti‐mouse secondary antibody (A‐11001, 1:200; Life Technologies, Tokyo, Japan) for 1 h at room temperature. Images were taken by a fluorescent microscope with a BZ‐X700 system (KEYENCE Corporation, Osaka, Japan). A total of 50 representative crypt–villus units from each slide were randomly selected only when there was a complete longitudinal section of a villus and its associated crypt. The length (μm) of the crypt–villus units was measured from the tip to the base. The number of GFP(+) cells was counted in the 50 representative crypt–villus units. The densities were expressed as the number of GFP(+) cells per micrometer length of a crypt–villus unit (cell/μm; n = 4–5).
Isolation and collection of L cells from mouse intestinal epithelium
The isolation of L cells from mouse intestinal epithelium has been described previously21. In brief, the intestine was injected with Hank's balanced salt solution containing 0.5 mg/mL collagenase P (Roche Diagnostics, Manheim, Germany), and incubated at 37°C for 10 min in Krebs–Ringer bicarbonate buffer. The digested intestinal epithelium was collected and rinsed. Next, samples were filtered with a cell strainer (352340, BD Falcon cell strainer; Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA). GFP(+) and GFP(−) cells from the intestinal epithelium were analyzed and sorted as L cells and non‐L cells, respectively, using the BD FACS Aria™ flow cytometer (Becton Dickinson and Company). Sorted cells were collected into the buffer.
Measurement of GLP‐1 and GIP content in the sorted L cells
GFP(+) and GFP(−) cells (5,000 per segment) were sorted in 0.1 N HCl lysis buffer. The GLP‐1 and GIP content in the cell extracts were assayed using a total GLP‐1 enzyme‐linked immunosorbent assay kit (Meso Scale Discovery, Gaithersburg, Maryland, USA) and a total GIP enzyme‐linked immunosorbent assay kit (Merck Millipore, Darmstadt, Germany), respectively. Protein concentration was determined using the Bio‐Rad Protein Assay Kit (Bio‐Rad Laboratories, Inc., Hercules, California, USA).
Assay of GLP‐1 secretion by the sorted L cells
A total of 5,000 GFP(+) cells were sorted in 5.5 mmol/L glucose Dulbecco's modified Eagle's medium from the UI and the LI of Gcggfp/+ mice after an overnight fast (16 h). After 15 min of incubation with 25 mmol/L glucose Dulbecco's modified Eagle's medium at 37°C, all samples were centrifuged for 3 min. The supernatants were collected and GLP‐1 levels were assayed using a total GLP‐1 enzyme‐linked immunosorbent assay kit.
Ribonucleic acid extraction and quantitative reverse transcription polymerase chain reaction
Total ribonucleic acid (RNA) was extracted with the PicoPure RNA isolation kit (Applied Biosystems, Inc., Alameda, California, USA) from 2,000 sorted cells and treated with deoxyribonuclease (Qiagen Inc., Valencia, California, USA). Complement deoxyribonucleic acid was prepared by reverse transcriptase (Invitrogen, Carlsbad, California, USA) with an oligo(dT) primer (Invitrogen). The messenger RNA (mRNA) levels were measured in a total volume of 20 μL by SYBR Green‐based reverse transcription polymerase chain reaction using the StepOnePlus™ Real‐Time PCR System (Applied Biosystems Inc., Foster City, California, USA). Polymerase chain reaction analysis was carried out with 0.2 μmol/L of the primers listed in Table S1. Thermal cycling conditions were denatured at 95°C for 10 min followed by 50 cycles of 95°C for 15 s and 60°C for 1 min. Melting curve dynamics and the absence of primer dimers were confirmed. Peptidylprolyl isomerase A was used as the internal control for normalization. All data are shown using the Δcycle threshold (ΔCT) method (CT [internal control] − CT [target gene]).
Statistical analysis
The results are given as mean ± standard error of the mean (n = number of mice). Statistical significance was determined using paired and unpaired Student's t‐test and analysis of variance with the Games–Howell test. P < 0.05 was considered statistically significant.
Results
Density and quality of L cells in Gcggfp/+ mice
Gcggfp/+ mice enabled the identification of L cells and non‐L cells as GFP(+) cells and GFP(−) cells, respectively (Figure 1a). L cells were observed in the UI, LI and colon of Gcggfp/+ mice. The length of the crypt–villus units of the LI (319.0 ±17.5 μm), and the colon (194.3 ± 5.7 μm) was significantly shorter than that of the UI (583.6 ± 19.6 μm; Figure 1b). The number of L cells per crypt–villus unit was larger in the LI (0.46 ± 0.02) and the colon (0.44 ± 0.04) than that in the UI (0.12 ± 0.36; Figure 1c). The density of L cells in the length of the crypt–villus was the highest in the colon (0.114 ±0.012 cells/μm), followed by the LI (0.073 ± 0.004 cells/μm) and then the UI (0.010 ± 0.002 cells/μm; Figure 1d).
Figure 1.
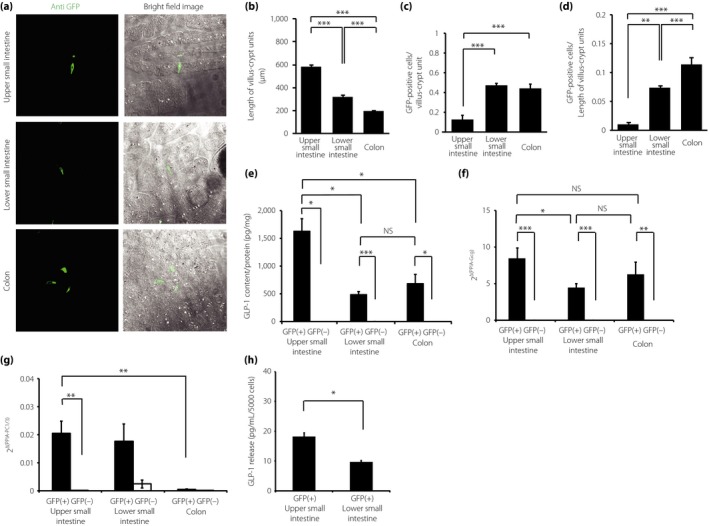
The density and quality of L cells in the gastrointestinal (GI) tract of glucagon (Gcg)‐green fluorescent protein (GFP) knock‐in (Gcg‐GFP) heterozygous (Gcggfp/+) mice. (a) Immunohistochemical images of the upper small intestine (UI), lower small intestine (LI) and colon of Gcggfp/+ mice (bright field image and fluorescence image). (b) The length of the crypt–villus units in the UI, LI and colon was measured by immunohistochemistry (n = 5). (c) The number of GFP‐positive (+) cells per crypt–villus unit (n = 5). (d) The density of GFP(+) cells was normalized against the length of the crypt–villus unit (n = 5). (e) Glucagon‐like peptide‐1 (GLP‐1) content in GFP(+) cells and GFP(–) cells from the UI, LI and colon (n = 5). (f, g) The messenger ribonucleic acid expression levels of Gcg and prohormone convertase (PC) 1/3 in GFP(+) and GFP(–) cells from the UI, LI, and colon (n = 5). Expression levels were normalized against the internal control, peptidylprolyl isomerase A (PPIA). (h) GLP‐1 secretion from GFP(+) cells from the UI and LI (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. NS, not significant.
The GLP‐1 content in the L cells from the UI (1630 ± 224 pg/mL/protein), LI (487 ± 54 pg/mL/protein) and colon (684 ± 165 pg/mL/protein) was significantly higher than that in the non‐L cells of each part (Figure 1e). Furthermore, the L cells from the UI contained approximately threefold more GLP‐1 compared with the L cells from the LI and colon (Figure 1e). Because GLP‐1 is processed from the proglucagon gene5, GLP‐1 expression was assessed by the expression levels of Gcg mRNA. A set of primers for the Gcg mRNA was designed to span an exon–intron junction from exon 3 to exon 4. Neither GLP‐1 content nor Gcg mRNA was detected in non‐L cells of each part. The mRNA expression levels of Gcg were significantly higher in L cells than in non‐L cells of all parts of the GI tract (Figure 1f). The mRNA expression levels of Gcg in L cells from the UI were 1.8‐fold higher than those in cells from the LI (Figure 1f). GLP‐1 is produced in L cells through cleavage of proglucagon by PC1/3. The mRNA expression levels of PC1/3 were significantly higher in L cells than those in non‐L cells in the UI, and tended to be higher in L cells than those in non‐L cells in the LI (Figure 1g). The expression levels of PC1/3 mRNA were very low in L cells from the colon. PC2 mRNA was not detected in either L cells or non‐L cells from the UI, LI and colon (data not shown). GLP‐1 secretion in response to glucose stimulation was significantly higher in the L cells from the UI, compared with that in cells from the LI (Figure 1h).
Expression levels of transcription factors in L cells
We measured the mRNA expression levels of representative transcription factors in sorted L cells (Figure 2). These transcription factors have been suggested to be expressed in L cells and other EECs. The expression levels of islet 1 (Isl1) mRNA were significantly higher in L cells compared with those in non‐L cells from the UI and LI (Figure 2b). Furthermore, L cells from the UI and LI showed significantly higher Isl1 expression than L cells from the colon. L cells from the LI showed significantly higher expression of Pax6 mRNA than L cells from the colon (Figure 2c). The expression levels of regulatory factor X 6 (Rfx6) mRNA in L cells from the UI were significantly higher than those in non‐L cells from the UI (Figure 2e). Finally, there were no differences in the mRNA expression of Pdx1 and Pax4 between the L cells of each part (Figure 2a,d).
Figure 2.
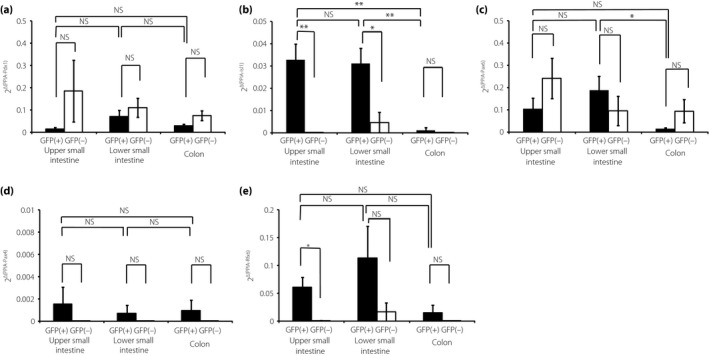
Expression of transcription factors in L cells. (a–e) The messenger ribonucleic acid expression levels of transcription factors in green fluorescent protein (GFP)‐positive (GFP[+]) cells and GFP‐negative (GFP[–]) cells from the upper small intestine (UI), lower small intestine (LI) and colon (n = 5). Expression levels were normalized against the internal control, peptidylprolyl isomerase A (PPIA). *P < 0.05, **P < 0.01, ***P < 0.001. Isl1, islet 1; NS, not significant; Pax4, paired box gene 4; Pax6, paired box gene 6; Pdx1, pancreatic duodenal homeobox 1; Rfx6, regulatory factor X 6.
Expression of other intestinal hormones in L cells
We next measured the content of another incretin peptide, GIP, and the mRNA expression levels of other enteroendocrine hormones in L cells (Figure 3). In the UI, the GIP content in L cells was significantly higher than that in non‐L cells (Figure 3a). Figure 3a and b shows that expression levels of GIP protein parallel mRNA expression levels of GIP in L cells. The mRNA expression levels of GIP in L cells from the UI were significantly higher compared with those in cells from the LI and colon (Figure 3b). The mRNA expression levels of PYY, secretin and cholecystokinin (CCK) were significantly higher in L cells than those in non‐L cells in all parts of the GI tract (Figure 3c–e). The mRNA expression of PYY in L cells from the LI was higher than that in cells from the UI and colon (Figure 3c). The expression of secretin mRNA in the L cells from the UI and LI was higher than that in cells from the colon (Figure 3d). The expression levels of CCK mRNA in L cells were the highest in the UI, second highest in the LI and the lowest in the colon (Figure 3e). Somatostatin mRNA was barely expressed in the L cells of each segment, similar to the case of non‐L cells (Figure 3f).
Figure 3.
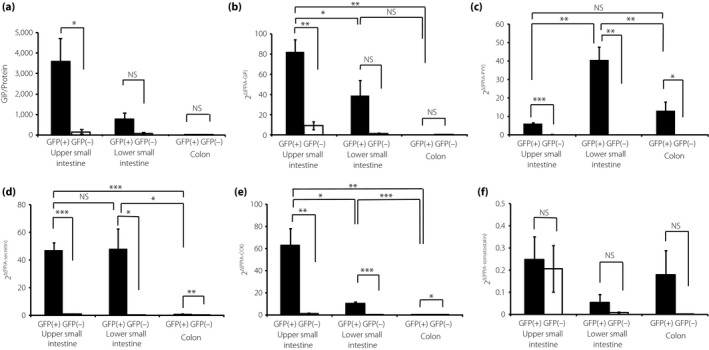
Expression of enteroendocrine hormones in L cells. (a) Gastric inhibitory polypeptide (GIP) content in GFP‐positive (GFP[+]) cells and GFP‐negative (GFP[–]) cells from the upper small intestine (UI), lower small intestine (LI), and colon (n = 5). (b–f) GIP, peptide YY (PYY), secretin, cholecystokinin (CCK) and somatostatin messenger ribonucleic acid levels in GFP(+) and GFP(–) cells from the UI, LI, and colon (n = 5). Expression levels were normalized against the internal control, peptidylprolyl isomerase A (PPIA). *P < 0.05, **P < 0.01, ***P < 0.001. NS, not significant.
Discussion
In the present study, Gcg‐GFP mice expressing GFP under the control of the native proglucagon promoter enabled us to directly assess L cells, revealing quantitative and qualitative differences between L cells in the UI, LI, and colon. The present findings showed that the number of L cells increases from the UI to the colon, and that they have different expression patterns of transcription factors and hormones.
The classifications of EECs and hormones have historically relied on granule morphology and immunochemical studies3, 4. The immunoreactivity technique has shown that L‐cells contain several hormones4, 13. Although the specificity of antibodies has always been discussed, L cells have been shown to exist broadly in the GI tract and occur most often in the LI5. A recent report using fluorescently labeled L cells showed an increase in density from the duodenum to the colon17. Our results of the localization and the number of L cells using anti‐GFP antibody reactivity matched the findings of previous studies showing an increase in the number of L cells toward the colon5. Additionally, the function of the GI tract has been evaluated by the surface area, which differs in each part of the tract. Therefore, we measured the density of L cells as L cells/length of the crypt–villus unit to compensate for the different surface area of each part. Our study showed that the highest density of L cells throughout the GI was in the colon. In the present study, we used only adult male mice to evaluate the characteristics and distribution of L cells. The effect of aging and sex on the localization and density of primary L cells is yet unknown.
We also characterized L cells from the UI, LI and colon. The GLP‐1 content and expression levels of Gcg mRNA were higher in the UI compared with the LI and colon. Previous reports have shown lower expression levels of Gcg mRNA in L cells from the UI compared with the colon11, 17. Conversely, recent reports have shown that L cells in the UI exhibit GLP‐1 secretion12. This discrepancy might stem from the differences between transgenic mice and our knock‐in mice. Thus, these data indicate that L cells exhibit differences not only in their numbers throughout the GI tract, but also in terms of molecular characteristics.
L cells secrete GLP‐1 into the circulation in response to various nutrients and neuromodulators. Whether the early postprandial GLP‐1 secretion originates from a few L cells in the UI or from L cells located further down the tract in the LI and colon is not clear. It has been suggested that a neural signal from the UI reaches L cells in the more distal intestine and colon22. The present data showed that GFP(+) L cells from the UI had the highest amount of GLP‐1 content, the highest expression levels of Gcg mRNA. Furthermore, an ex vivo GLP‐1 secretion experiment showed higher GLP‐1 secretion from L cells in the UI. Therefore, the small number of L cells in the UI might explain the capacity to activate early postprandial secretion of GLP‐1.
PC1/3 is an essential enzyme for producing GLP‐1 from proglucagon protein. Expression levels of PC1/3 mRNA were increased in L cells of UI compared with those of LI, suggesting that GLP‐1 synthesis is increased in L cells of the UI. In contrast, PC1/3 mRNA expression levels were extremely low, but GLP‐1 protein levels were detectable in L cells of the colon. It is unclear how much PC1/3 mRNA expression level in L cells is required for PC1/3 enzymatic activity, but there would be a possibility that PC1/3 enzymatic activity in L cells is differently regulated depending on the distribution. Although we could not measure the PC1/3 enzymatic activity in L cells, further study is required to clarify the mechanism.
Studies using transcription factor‐deficient mice have indirectly confirmed the requirement of specific transcription factors in the development of the EEC lineage23, 24, 25. However, little is known about the expression of transcription factors in L cells. Although Pax6 has been shown to activate the proglucagon gene in L cells15, our data showed that Pax6 was expressed in L cells and non‐L cells at the same level. We could not detect any transcription factor specific to L cells, even in L‐cells from the UI, which contained the highest amount of GLP‐1 (Figure 2). Nevertheless, the present results revealed that Isl1 showed significantly higher expression in L cells from the UI and LI. Isl1 is a LIM‐homeodomain transcription factor that promotes islet cell proliferation26. Additionally, mice with intestinal epithelial‐specific deletion of Isl1 showed loss of GLP‐1, GIP, CCK and somatostatin‐expressing cells, and impaired glucose homeostasis27. We found that Isl1 was expressed at higher levels in L cells from the UI and LI. Isl1 might not only be involved in L cell development, but also in GLP‐1 synthesis; however, further experiments are required to clarify the role of Isl1 in GLP‐1 expression. In Rfx6‐deficient mice, none of the endocrine cells, excluding pancreatic polypeptide‐expressing cells, were detected in the islets of these mice28. Here, we confirmed a notable mRNA expression of Rfx6 and GIP in L cells from the UI. GIP is one other incretin secreted from K cells located in the duodenum and UI29. In our previous report21, we showed that Rfx6 plays critical roles in GIP expression, but not in GLP‐1 expression. Another report has shown that L cells in the UI are more similar to K cells in the UI than to L cells in the LI and colon11. Considering our results and these reports, we propose that Rfx6 induces GIP expression in L cells located in the UI, but further study is required.
The developmental relationships between EECs that arise from secretory precursors have been examined by genetically engineered mice9, 18, 29. The ablation of secretin‐producing cells decreased the number of L cells and CCK‐producing cells, suggesting that these cells are developmentally related9. Tissue‐specific post‐translational processing of proglucagon in L cells produces GLP‐1 and GLP‐2. Elimination of the proglucagon promoter resulted in reduction in GLP‐1, PYY, CCK, secretin and GIP18. These results suggest that EECs co‐express several kinds of functionally related hormones. Recent studies have shown that different EECs have more hormones than originally thought, and that the expression patterns of GI hormones depend on their localization11. L cells from Gcg‐GFP mice also expressed several GI hormones: PYY, GIP, secretin and CCK. We observed significant differences in the expression patterns of these GI hormones depending on their distribution. PYY has also been identified for its role in regulating appetite and bodyweight30. We observed a significant high expression of PYY in L cells from the LI. This result is consistent with that by Svendsen et al.12, who showed that PYY is secreted from L cells in the distal part of the intestine. CCK‐GFP mice have shown that CCK‐producing cells in the duodenum express Gcg, PYY, GIP and secretin31. Using specific genetically engineered Gcg‐GFP mice, we confirmed that L cells from the UI also express significantly high CCK and secretin mRNA.
In clinical practice, GLP‐1 secretion in patients with type 2 diabetes is impaired; however, the incretin effect of GLP‐1 is preserved32. Therefore, GLP‐1‐based therapies have been developed for patients with type 2 diabetes5, 6, 7. Recent investigations have shown that the increased plasma GLP‐1 concentration in patients with type 2 diabetes who underwent a gastric bypass or sleeve gastrectomy surgery normalized their blood glucose levels7, 33. It has been reported that the possible mechanisms are that these surgeries lead to gut hypertrophy and an increase in the number of L cells in the LI, resulting in increased plasma GLP‐1 levels34, 35. However, these kinds of surgery are greatly invasive and expensive. As we showed that L cells from the UI contain the highest amount of GLP‐1, a strategy for stimulating GLP‐1 exocytosis might be efficient for L cells in the UI. Additionally, increasing the number of L cells in the UI by promoting differentiation might enable patients to significantly elevate their plasma GLP‐1 levels. The present results suggest the therapeutic potential of promoting GLP‐1 release from L cells for the treatment of type 2 diabetes; however, further research is required.
In conclusion, using Gcg‐GFP mice as an L cell reporter model, we clearly showed that L cells exhibit different numbers and characteristics depending on their distribution in the gut. L cells also express different levels of gut hormones and transcription factors’ mRNA.
Disclosure
Dr Inagaki reports that personal fees were received from the following companies outside the duration of the submitted work: Takeda Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corporation; MSD; Sanofi; Novartis Pharma; Dainippon Sumitomo Pharma; Kyowa Hakko Kirin Co., Ltd.; Eli Lily Japan; Shiratori Pharmaceutical; Roche Diagnostics Japan Tobacco (JT); Nippon Boehringer Ingelheim Co., Ltd.; Astellas Pharma Inc.; Daiichi Sankyo Company, Ltd.; Ono Pharmaceutical Co., Ltd.; and Taisho Toyama Pharmaceutical Co., Ltd.
Supporting information
Table S1 ¦ Oligonucleotide primers used for the quantitative reverse transcription polymerase chain reaction analysis.
Acknowledgment
We gratefully thank the Kyoto University Gender Equality Promotion Center for financial support for employing research assistants. Dr Inagaki reports receiving grants from the following offices during this study: Ministry of Education, Culture, Sports, Science and Technology (MEXT); Japan Society for the Promotion of Science (JSPS); the Ministry of Health, Labor and Welfare; the Ministry of Agriculture, Forestry and Fisheries; the Japan Diabetes Foundation; the Japan Association for Diabetes Education and Care; Merck Sharp & Dohme (MSD); Novo Nordisk Pharma; and Banyu Life Science Foundation International.
J Diabetes Investig 2018; 9: 25–32
References
- 1. Mayer EA. Gut feelings: the emerging biology of gut‐brain communication. Nat Rev Neurosci 2011; 12: 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science (New York, NY) 2005; 307: 1909–1914. [DOI] [PubMed] [Google Scholar]
- 3. Solcia E, Pearse AGE, Grube D, et al Revised Wiesbaden classification of gut endocrine cells. Rendic Gastroenterol 1973; 5: 13–16. [Google Scholar]
- 4. Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev 1998; 78: 1087–1108. [DOI] [PubMed] [Google Scholar]
- 5. Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev 2007; 87: 1409–1439. [DOI] [PubMed] [Google Scholar]
- 6. Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. J Diabetes Investig 2010; 1: 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 8. Wu T, Thazhath SS, Bound MJ, et al Mechanism of increase in plasma intact GLP‐1 by metformin in type 2 diabetes: stimulation of GLP‐1 secretion or reduction in plasma DPP‐4 activity? Diabetes Res Clin Pract 2014; 106: e3–e6. [DOI] [PubMed] [Google Scholar]
- 9. Rindi G, Ratineau C, Ronco A, et al Targeted ablation of secretin‐producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development 1999; 126: 4149–4156. [DOI] [PubMed] [Google Scholar]
- 10. Kieffer TJ, Habener JF. The glucagon‐like peptides. Endocr Rev 1999; 20: 876–913. [DOI] [PubMed] [Google Scholar]
- 11. Habib AM, Richards P, Cairns LS, et al Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 2012; 153: 3054–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Svendsen B, Pedersen J, Albrechtsen NJ, et al An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 2015; 156: 847–857. [DOI] [PubMed] [Google Scholar]
- 13. Mortensen K, Christensen LL, Holst JJ, et al GLP‐1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 2003; 114: 189–196. [DOI] [PubMed] [Google Scholar]
- 14. Larsson LI, St‐Onge L, Hougaard DM, et al Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev 1998; 79: 153–159. [DOI] [PubMed] [Google Scholar]
- 15. Trinh DK, Zhang K, Hossain M, et al Pax‐6 activates endogenous proglucagon gene expression in the rodent gastrointestinal epithelium. Diabetes 2003; 52: 425–433. [DOI] [PubMed] [Google Scholar]
- 16. Fujita Y, Chui JW, King DS, et al Pax6 and Pdx1 are required for production of glucose‐dependent insulinotropic polypeptide in proglucagon‐expressing L cells. Am J Physiol Endocrinol Metab 2008; 295: E648–E657. [DOI] [PubMed] [Google Scholar]
- 17. Reimann F, Habib AM, Tolhurst G, et al Glucose sensing in L cells: a primary cell study. Cell Metab 2008; 8: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egerod KL, Engelstoft MS, Grunddal KV, et al A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP‐1, PYY, and neurotensin but not somatostatin. Endocrinology 2012; 153: 5782–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi Y, Yamamoto M, Mizoguchi H, et al Mice deficient for glucagon gene‐derived peptides display normoglycemia and hyperplasia of islet {alpha}‐cells but not of intestinal L‐cells. Mol Endocrinol 2009; 23: 1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwasaki K, Harada N, Sasaki K, et al Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology 2015; 156: 837–846. [DOI] [PubMed] [Google Scholar]
- 21. Suzuki K, Harada N, Yamane S, et al Transcriptional regulatory factor X6 (Rfx6) increases gastric inhibitory polypeptide (GIP) expression in enteroendocrine K‐cells and is involved in GIP hypersecretion in high fat diet‐induced obesity. J Biol Chem 2013; 288: 1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lim GE, Brubaker PL. Glucagon‐Like Peptide 1 Secretion by the L‐Cell ‐The View From Within‐. Diabetes 2006; 55: S70–S77. [Google Scholar]
- 23. Jenny M, Uhl C, Roche C, et al Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J 2002; 21: 6338–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang Q, Bermingham NA, Finegold MJ, et al Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science (New York, NY) 2001; 294: 2155–2158. [DOI] [PubMed] [Google Scholar]
- 25. Naya FJ, Huang HP, Qiu Y, et al Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD‐deficient mice. Genes Dev 1997; 11: 2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo T, Wang W, Zhang H, et al ISL1 promotes pancreatic islet cell proliferation. PLoS One 2011; 6: e22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Terry NA, Walp ER, Lee RA, et al Impaired enteroendocrine development in intestinal‐specific Islet1 mouse mutants causes impaired glucose homeostasis. Am J Physiol Gastrointest Liver Physiol 2014; 307: G979–G991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith SB, Qu HQ, Taleb N, et al Rfx6 directs islet formation and insulin production in mice and humans. Nature 2010; 463: 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schonhoff S, Baggio L, Ratineau C, et al Energy homeostasis and gastrointestinal endocrine differentiation do not require the anorectic hormone peptide YY. Mol Cell Biol 2005; 25: 4189–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manning S, Batterham RL. The role of gut hormone peptide YY in energy and glucose homeostasis: twelve years on. Annu Rev Physiol 2014; 76: 585–608. [DOI] [PubMed] [Google Scholar]
- 31. Sykaras AG, Demenis C, Cheng L, et al Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 2014; 155: 3339–3351. [DOI] [PubMed] [Google Scholar]
- 32. Nauck MA, Heimesaat MM, Orskov C, et al Preserved incretin activity of glucagon‐like peptide 1 [7‐36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type‐2 diabetes mellitus. J Clin Investig 1993; 91: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Madsbad S, Holst JJ. GLP‐1 as a mediator in the remission of type 2 diabetes after gastric bypass and sleeve gastrectomy surgery. Diabetes 2014; 63: 3172–3174. [DOI] [PubMed] [Google Scholar]
- 34. Hansen CF, Bueter M, Theis N, et al Hypertrophy dependent doubling of L‐cells in Roux‐en‐Y gastric bypass operated rats. PLoS One 2013; 8: e65696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shuang J, Zhang Y, Ma L, et al Relief of diabetes by duodenal‐jejunal bypass sleeve implantation in the high‐fat diet and streptozotocin‐induced diabetic rat model is associated with an increase in GLP‐1 levels and the number of GLP‐1‐positive cells. Exp Ther Med 2015; 10: 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Oligonucleotide primers used for the quantitative reverse transcription polymerase chain reaction analysis.


