Figure 24.
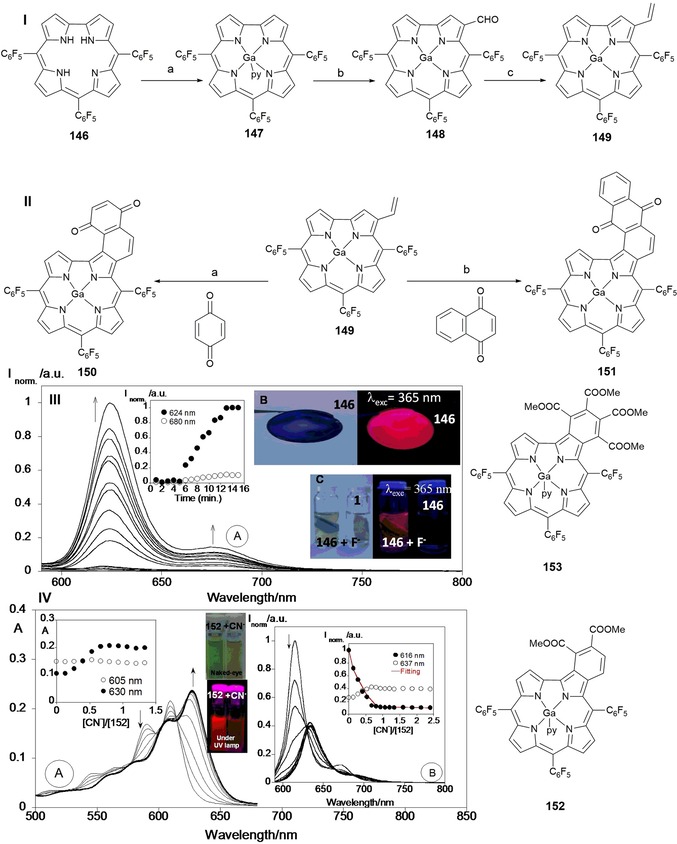
Reagents and conditions for Scheme I: a) i) GaCl3, pyridine, reflux, ii) 1. 36 % HCl, 2. aq NaHCO3; b) Vilsmeier–Haack reagent; c) CH3PPh3Br, NaH in THF, RT, N2. Reagents and conditions for Scheme II: a) reflux, toluene, N2; b) reflux, toluene, N2. Chemical structures of compounds 146–153. Panel III) a) Emission spectra of acrylamide gel doped with compound 146 in the presence of fluoride as a function of time (T=298 K, λ ex=570 nm). b) Polymethylmethacrylate film with 146 and c) polyacrylamide gel of 146 in the presence of fluoride (F−). Reproduced from Ref. 210 with permission from The Royal Society of Chemistry. Panel IV) a) Spectrophotometric and b) spectrofluorimetric titration of compound 152 with the addition of CN− in toluene. The inset represents a) the absorption at λ=605 and 630 nm and b) the emission intensity at λ=616 and 637 nm ([152]=1×10−5 m, λ ex=590 nm, T=298 K). Reproduced with permission from Ref. 211. Copyright 2015 Elsevier B. V.
