Abstract
Our objective was to evaluate the efficacy of a standardised work-up in the diagnosis of pleural tuberculosis (TB) that included fibreoptic bronchoscopy and medical thoracoscopy.
A consecutive series of 52 pleural TB patients observed during the period 2001–2015 was evaluated retrospectively. 20 females, mean (range) age 39.7 (18–74) years, and 32 males, mean (range) age 45.75 (21–83) years, were included (28 non-EU citizens (53.8%)). The diagnosis of TB infections was established by identification (using stains, culture or molecular tests) of Mycobacterium tuberculosis in the pleura, sputum and/or bronchial specimens, or by evidence of caseous granulomas on pleural biopsies. Patients with and without lung lesions were considered separately.
The diagnostic yield of the microbiological tests on pleural fluid was 17.3% (nine out of 52 patients). Among the 18 patients with lung lesions, bronchial samples (washing, lavage or biopsy) were positive in 50% of cases (nine patients). Cultures of pleural biopsies were positive in 63% of cases (29 out of 46 patients); pleural histology was relevant in all patients. Without pleural biopsy, a diagnosis would have been reached in 15 out of 52 patients (28.6%) and in four of them only following culture at 30–40 days.
An integrated diagnostic work-up that includes all the diagnostic methods of interventional pulmonology is required for a diagnosis of pleural TB. In the majority of patients, a diagnosis can be reached only with pleural biopsy.
Short abstract
Diagnosis of tuberculous pleural effusion can be a challenge; medical thoracoscopy greatly increases accuracy http://ow.ly/EnY430gubm9
Introduction
The World Health Organization (WHO) Global Tuberculosis Report 2016 gives an estimated 10.4 million incident cases of tuberculosis (TB) worldwide in 2015 with 1.8 million deaths, an estimated 250 000 deaths being from multidrug-resistant (MDR)-TB (a high proportion compared with the 480 000 incident cases of MDR-TB) [1].
Pleural TB frequency depends on the prevalence of TB and HIV infections in the population; it can affect 4–5% of infected patients in countries with a high prevalence of TB and up to 30% of HIV-positive patients [2–5]. The prevalence of pleural TB in western countries is not well known.
Pleural TB may be unsuspected altogether or its diagnosis may be missed if based exclusively on a positive pleural fluid culture [6, 7]. Pleural TB is typically paucibacillary, being mostly the expression of a post-primary infection that follows the rupture of a subpleural caseous focus into the pleura. Delayed hypersensitivity rather than bacillary proliferation is the greatest contributor to the pathogenesis of the effusion [8].
In the present study, we reviewed our experience in the diagnosis of pleural TB using a standardised work-up protocol that employs fibreoptic bronchoscopy and medical thoracoscopy, and we were able to show that pleural biopsy greatly increases the diagnostic accuracy of TB infection.
Materials and methods
Study base
We evaluated retrospectively all patients with pleural effusion who received a final diagnosis of pleural TB observed over the period January 1, 2001 to December 31, 2015 at the University Hospital of Parma (Parma, Italy).
The inclusion criteria were a definitive diagnosis of TB pleuritis obtained by Mycobacterium tuberculosis identification in pleural fluid, sputum, bronchoscopic samples, pleural fibrin and pleural biopsies by Kinyoun and auramine stain, culture and/or TB nucleic acid amplification test (NAAT), or by evidence of granulomatous inflammation compatible with TB at pathological examination of pleural biopsies. The protocol of the study was approved by the Ethics Committee of the University Hospital of Parma (3448; October 6, 2016).
Standardised work-up for TB pleuritis diagnosis
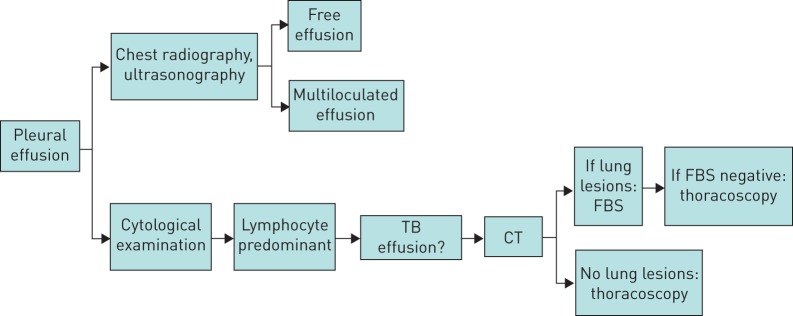
All patients with a pleural effusion associated with fever or with clinically suspected infectious aetiology underwent a standardised work-up (figure 1) that included: peripheral blood tests, standard radiographic imaging (computed tomography (CT) scans were performed only in patients with suspected parenchymal lesions), thoracocentesis (for total proteins, lactate dehydrogenase, cholesterol, glucose, microbiology and cytology with a differential cell count; pleural fluid was considered an exudate if the fluid met Light's criteria [9]) and tuberculin skin tests with 5 tuberculin units of purified protein derivative (the QuantiFERON test was performed in selected patients). If parenchymal involvement was detected, a fibreoptic bronchoscopy was performed and bronchial washings or bronchoalveolar lavage (BAL) were taken for microbiology; in cases with enlarged hilo-mediastinal nodes, transbronchial needle aspiration was performed. Sputum was collected in all patients able to produce sputum spontaneously. HIV infection was tested whenever clinically indicated. The patients underwent medical thoracoscopy under local anaesthesia or sedation in accordance with the technique described by various authors and parietal pleura biopsies were obtained [10, 11]. We performed seven to 10 pleural biopsies in each patient; two or three of them were sent for culture and those remaining were sent for histopathology.
FIGURE 1.
Diagnostic work-up of patients with suspected pleural tuberculosis effusion. CT: computed tomography; FBS: fibreoptic bronchoscopy.
Microbiological investigations
All the specimens for microbiological study were stained with Kinyoun and auramine stain, and cultured on both solid (Löwenstein–Jensen; bioMérieux, Marcy l'Etoile, France) and liquid (Bactec MGIT semiautomated system; Becton Dickinson, Sparks, MD, USA) media. In some cases, a TB NAAT was also performed (Probe Tec ET M. tuberculosis complex; Becton Dickinson).
The culture-positive specimens were tested by molecular methods to distinguish M. tuberculosis complex from other nontuberculous mycobacteria and to verify their genetic resistance to rifampicin and isoniazid, and also by in vitro antimicrobial susceptibility testing.
Histological investigations
Pleural biopsies for histology were fixed in 10% buffered formalin, paraffin embedded, and routinely stained with haematoxylin/eosin, Giemsa, periodic acid–Schiff, Grocott and Ziehl–Neelsen stains.
In order to minimise the risk of exposure to M. tuberculosis of healthcare workers and other patients, various precautions are adopted in our thoracic endoscopy service in accordance with international guidelines [12].
Statistical analysis
Comparisons between groups were calculated using the Chi-squared test or Fisher's exact test as required. All tests were two-sided; significance was for p-values <0.05.
Results
Patients and symptoms
52 patients met the inclusion criteria: 20 females (nine non-EU citizens), mean (range) age 39.7 (18–74) years, and 32 males (19 non-EU citizens), mean (range) age 45.75 (21–83) years. Figure 2 shows the distribution of patients according to age and country of origin. The majority of patients (28 out of 52 (53.8%)) were non-EU citizens coming from areas with a high prevalence of TB such as India, Africa, Eastern Europe or South America; three were pregnant (one Italian, one Indian and one from Eastern Europe), two were HIV-infected and three were alcoholic patients.
FIGURE 2.
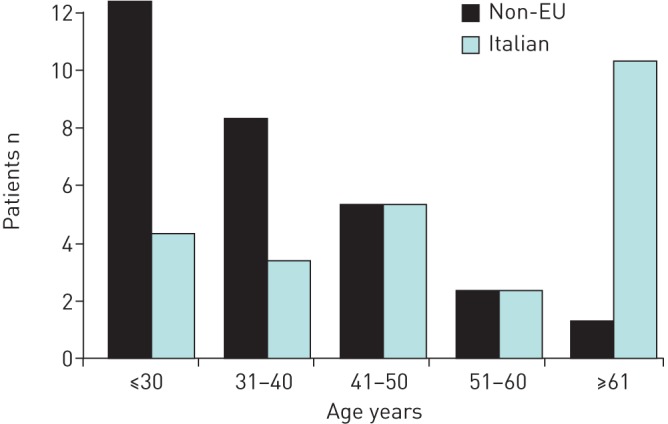
Distribution of patients according to age and nationality.
Patients presented with an acute (n=45) or subacute (n=7) clinical picture characterised by fever (n=48), chest pain (n=45), cough (n=41) or dyspnoea (n=24). The tuberculin skin test was performed in 38 patients (positive in 31 patients (81.6%)) and the QuantiFERON test was performed in nine patients (positive in five patients (55%)).
Chest imaging
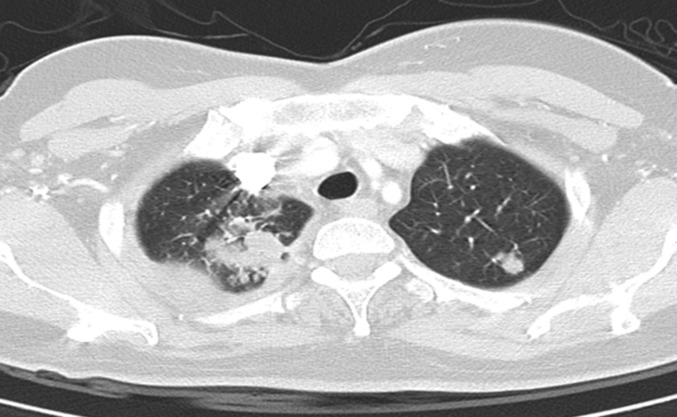
On chest radiography, 27 patients had left pleural effusion, 23 patients had right pleural effusion and two patients had bilateral pleural effusions. 18 patients with parenchymal lesions were further studied with CT. They showed nodules (11 patients) of different size that were bilateral in three patients (figure 3), and cavities (four patients) and infiltrates (two patients). Two patients had mediastinal lymphadenopathy, one associated with lung nodules.
FIGURE 3.
Computed tomography scan of a 50-year-old female with right pleural effusion and bilateral nodules in the upper lobes. Bronchoscopic samples were negative and the diagnosis was obtained only by thoracoscopy.
Pleural fluid, bronchoscopic and thoracoscopic specimens
Pleural fluid was always an exudate. Seven patients had a bloody effusion, the exudate was turbid in four patients, purulent in two patients and yellow in the remaining patients. The effusion was considered lymphocytic when lymphocytes represented >50% of the cells [13]. All patients except one (n=51) had a lymphocytic effusion and in 44 out of 51 patients (86%) the lymphocyte fraction was >85%.
Fibreoptic bronchoscopy was performed in all patients with lung lesions (18 patients) to obtain bronchial washings or BAL at the site of lesions. It was also carried out in nine out of 34 patients without lung lesions because of the presence of secretions.
Medical thoracoscopy was performed on 46 out of 52 patients (88%) without complications. Six patients did not undergo thoracoscopy because a TB diagnosis was obtained on bronchoscopic samples or pleural fluid: four patients with lung lesions (n=1 acid-fast bacilli (AFB) stain, n=2 TB NAAT, and n=1 AFB stain and TB NAAT) and two patients without lung lesions (n=1 AFB stain and n=1 TB NAAT). Two additional patients without lung lesions had TB NAAT-positive pleural fluid; both were non-EU citizens, and therefore a thoracoscopy was performed to obtain pleural samples for histology and culture before the microbiological tests on the fluid were completed.
The most frequent endoscopic picture at thoracoscopy, seen in 26 out of 46 patients (56.5%), was the presence of small nodules on the parietal pleura (figure 4). Nonspecific features such as hyperaemia, thickening of the parietal pleura and adhesions were also frequently observed. The histology was compatible with TB in all patients with pleural biopsy.
FIGURE 4.
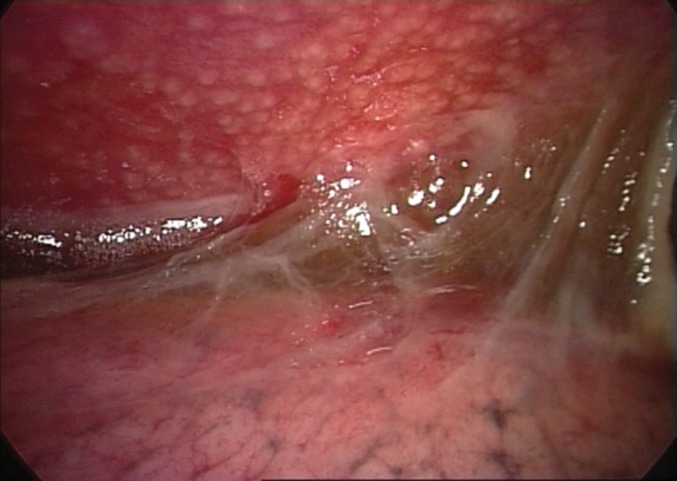
Endoscopic imaging shows a typical diffuse dissemination of micronodules on the parietal pleura and fibrin adhesions between visceral and parietal pleura.
Microbiological investigations
Patients without lung lesions
The results obtained by microbiological investigations for the 34 patients without lung lesions are shown in table 1.
TABLE 1.
Microbiological results obtained for 34 patients with pleural tuberculosis (TB) without parenchymal lesions
| Patients | Positive AFB smear | Positive TB NAAT | Positive culture | |
| Pleural fluid | 34 | 1/34 (2.9) | 5/30 (16.6) | 7/34 (20.5) |
| Sputum | 3 | 0 | 0 | 0 |
| Fibreoptic bronchoscopy | 9 | 0 | 0 | 0 |
| Thoracoscopy | 32 | |||
| Biopsy | 32 | 2/32 (6.2) | 19/25 (76) | 22/32 (68.7) |
| Fibrin# | 19 | 2/19 (10.5) | 5/19 (26) | 9/19 (47) |
Data are presented as n or n/N (%). AFB: acid-fast bacilli; NAAT: nucleic acid amplification test. #: in one patient, only the culture of the fibrin was positive.
Pleural fluids were tested in all 34 patients; sputum was also tested in three patients and in bronchoscopic specimens in nine patients. Thoracoscopy was performed in 32 out of 34 patients: one 18-year-old girl, whose pleural fluid was TB NAAT-positive, and another patient with AFB-positive smear and a positive NAAT, refused the procedure. Thoracoscopy was performed in two patients with NAAT-positive pleural fluid because they were non-EU citizens and a MDR mycobacterial infection was suspected, requiring M. tuberculosis isolation.
Cultures yielded higher diagnostic rates than stains and/or TB NAATs in all types of sample. The most informative specimens for culture were pleural biopsies (22 out of 32 (68.7%)) and pleural fibrin (nine out of 19 patients (47%)) (p<0.001); one patient was positive only for fibrin, hence the overall diagnostic yield of thoracoscopy was 71.8% (23 out of 32 patients). Pleural fluid culture was positive in seven out of 34 patients (20.5%). No positive result was obtained in spontaneously collected sputum (three patients) and in bronchoscopic specimens (nine patients).
Patients with lung lesions
Table 2 shows the results of microbiological investigation for the 18 patients with lung lesions. There were three out of 13 TB NAAT-positive pleural fluid samples, no AFB-positive smears and two out of 18 patients had positive cultures (11.1%). Sputum culture was positive for two out of 11 patients (18.1%) who also had positive culture in bronchoscopic samples. All patients underwent fibreoptic bronchoscopy; AFB-positive smears were obtained in two patients, positive TB NAATs in five patients and culture-positive bronchoscopic specimens in nine patients (50%). The nine positive cultures were obtained in four patients with cavities and in five patients with nodules.
TABLE 2.
Results of microbiological investigations in tuberculosis (TB) patients with pleural TB and parenchymal lesions
| Patients | Positive AFB smear | Positive TB NAAT | Positive culture | |
| Pleural fluid | 18 | 0 | 3/13 (23) | 2/18 (11.1) |
| Sputum | 11 | 0 | 0 | 2/11 (18)# |
| Fibreoptic bronchoscopy | 18 | 2/18 (11) | 5/18 (27) | 9/18 (50) |
| Thoracoscopy | 14 | |||
| Biopsy | 14 | 1/14 (7) | 4/13 (30) | 7/14 (50) |
| Fibrin | 8 | 0 | 1/8 (12.5) | 2/8 (25) |
Data are presented as n or n/N (%). AFB: acid-fast bacilli; NAAT: nucleic acid amplification test. #: the two patients with positive sputum culture also had positive fibreoptic bronchoscopy culture.
Thoracoscopy was performed in 14 out of 18 patients; four patients were omitted because of AFB-positive smear at bronchoscopy (two patients) and positive TB NAATs in bronchial specimens (two patients), followed by positive cultures on bronchial specimens. Three additional non-EU patients were TB NAAT-positive on fluid, but thoracoscopy was performed for the reasons specified earlier. Cultures on pleural biopsy were positive in seven out of 14 (50%) patients; there were two positive cultures on pleural fibrin. In the 14 patients tested by thoracoscopy specimens and other procedures, the diagnosis was achieved by culture of bronchial washings (three patients), of thoracoscopic specimens (five patients), culture of both (two patients), TB NAAT in pleural fluid (one patient) and histology (three patients).
Considering all patients, cultures were positive in nine out of 52 (17.3%) pleural fluids and in 30 out of 46 (65.2%) thoracoscopic specimens (pleural biopsy and fibrin) (p<0.0001). Drug susceptibility testing identified four resistant M. tuberculosis strains among the 28 non-EU patients (14.2%). There were two isoniazid-resistant strains, one rifampicin-resistant strain and one MDR-TB strain.
If the results of the microbiological tests were considered excluding those performed on thoracoscopy specimens (table 3), a diagnosis of pleural TB would have been reached in 11 patients (21.2%) within the first 3–5 days after admission (two patients with positive AFB stains in fluid and/or fibreoptic bronchoscopy and nine patients positive by TB NAAT-positive in fluid and/or fibreoptic bronchoscopy). Approximately 30 days after admission, 20 positive cultures of 15 patients were obtained (nine on fluid, two on sputum and nine on fibreoptic bronchoscopy), confirming the results for the 11 patients positive at admission and identifying four additional positive patients. Overall, a final diagnosis of pleural TB would have been achieved without thoracoscopy in 15 out of 52 (28.8%) patients, whereas 37 out of 52 (71.2%) patients would have been classified as “aspecific pleuritis”.
TABLE 3.
Results of diagnostic tests excluding those performed on thoracoscopy specimens
| Samples | Positive AFB smear | Positive TB NAAT | Positive culture from pleural fluid | Positive culture from sputum | Positive culture from fibreoptic bronchoscopy |
| Pleural fluid | 1/52# | 8/43+ | 9/52ƒ,## | 0 | 0 |
| Sputum | 0 | 0 | 0 | 2/14¶¶,ƒ | 0 |
| Fibreoptic bronchoscopy | 2/27#,¶ | 5/27+,¶ | 0 | 0 | 9/27¶¶,##,ƒ |
| Total | 2 | 13 | 9 | 2 | 9 |
Data are presented as n/N (%). AFB: acid-fast bacilli; NAAT: nucleic acid amplification test. #: one patient with positive AFB on pleural fluid and fibreoptic bronchoscopy; ¶: one patient with AFB and positive TB NAAT on fibreoptic bronchoscopy; +: three patients with positive TB NAAT on pleural fluid and fibreoptic bronchoscopy; ƒ: one patient with positive culture on sputum, pleural fluid and fibreoptic bronchoscopy; ##: two patients with positive culture on pleural fluid and fibreoptic bronchoscopy; ¶¶: two patients with positive culture on sputum and fibreoptic bronchoscopy.
Discussion
Italy is a country with a low prevalence of TB, but in recent decades it has faced significant migratory pressure [1]. Accordingly, the majority of patients with pleural TB observed in the present study (28 out of 52 patients (53.8%)) were non-EU citizens coming from countries with a high TB prevalence. MDR-TB and extensively drug-resistant TB strains are prevalent in these geographical areas, and they represent a challenge for TB control. Thus, there is a specific need to identify MDR-TB by a definitive diagnosis of the infection that includes microbial culture and testing of drug susceptibility.
The gold standard for a diagnosis of pleural TB is the isolation of M. tuberculosis from biological samples; in its absence, a diagnosis can be achieved by histology of pleural tissue [6, 14].
Our results clearly show that microbiological investigations on pleural fluid yield a diagnosis of TB in a limited number of cases. Only one out of 52 patients had an AFB-positive smear and positive cultures were obtained in nine out of 52 patients (17.3%). The diagnostic yield of pleural fluid has been reported to be highly variable, ranging from 7% to 58% [2, 6, 7, 14, 15]. A positive TB NAAT was obtained in only nine patients on pleural fluid, bronchial specimens or both and it was not considered conclusive for a definitive diagnosis. The test had a high positive predictive value (100%) but a low sensitivity: 18.6% (eight out of 43 patients) in pleural fluid and 27.7% (five out of 18 patients) in bronchial specimens. These results are not unexpected as the value of the NAAT on extrapulmonary specimens is controversial [16]. All four resistant M. tuberculosis infections in non-EU patients would have been missed by the TB NAAT alone.
The WHO has recently recommended the use in all settings of a next-generation Xpert MTB/RIF assay (Xpert MTB/RIF Ultra) that has a limit of detection of 16 CFU·mL–1 (compared with 114 CFU·mL–1 for Xpert MTB/RIF). The assay showed increased detection of M. tuberculosis in smear-negative culture-positive specimens, paediatric specimens, extrapulmonary specimens and HIV-positive individuals whose infections are frequently paucibacillary. A new generation of molecular assays might impact the accuracy profile of current diagnostic approaches, although this most likely applies to areas of high TB prevalence where therapeutic decisions are taken without invasive diagnostic techniques [17].
We found that the exudative effusions were lymphocytic predominant (≥50%) in 51 out of 52 patients [13, 18], but this finding is not specific as lymphocyte-predominant effusions can be seen in malignancy, congestive heart failure, chylothorax, lymphoma, yellow nail syndrome, chronic rheumatoid pleurisy, sarcoidosis and post-coronary artery bypass graft effusions [19–21]. One patient had a neutrophilic effusion early in the clinical course with a shift after 2 weeks towards lymphocytosis [22–24]; interestingly, the patient had no lung lesions and had positive cultures on pleural fluid. It has been reported that it is easier to find a positive culture in neutrophilic than in lymphocytic effusions (50% versus 10%) [25].
It is clinically useful to separate patients with pleural TB into those with (18 out of 52 patients (34.6%)) and without (34 out of 52 patients (65.4%)) parenchymal involvement. The reported prevalence of pulmonary parenchymal TB on CT is variable and differs among studies, ranging from 18.9% to 86% [3, 13, 26–29]. An unexpected pulmonary involvement can sometimes be found in extrapulmonary TB patients [30] and CT imaging is an important complement to the clinical work-up.
The involvement of the lung warrants the search for M. tuberculosis in sputum, preferably with sputum induction [7]. In patients with lung lesions, we collected sputum for bacteriology when present spontaneously (11 out of 18 patients) and we also performed fibreoptic bronchoscopy (18 out of 18 patients). However, there were only two out of 11 (18%) positive cultures on sputum. Fibreoptic bronchoscopy can also be useful in patients with lung lesions; a bronchial washing or a BAL can be obtained at the site of lesions when secretions are present. In these specimens, two AFB-positive smears, five positive TB NAATs and nine out of 18 (50%) positive cultures were obtained. Conversely, in patients without lung lesions, sputum and fibreoptic bronchoscopy were always negative. This supports the American Thoracic Society statement [31] that in the absence of concurrent pulmonary lesions, a diagnosis of pleural TB requires thoracocentesis and, usually, pleural biopsy.
Overall, a medical thoracoscopy was performed in 46 patients and all procedures were diagnostic. All biopsies had a histology compatible with TB and cultures of biopsies were positive in 29 out of 46 cases (63%), in agreement with the results obtained by other authors [6]. Percutaneous needle biopsy with a Cope or Abrams needle still has a role because of its high diagnostic yield; however, since 1986, we have adopted medical thoracoscopy as the best diagnostic procedure. With thoracoscopy within 2 days, a diagnosis could be obtained by histology (23 out of 38 patients (60.5%)) and the TB NAAT (19 out of 25 patients (76%)), and subsequently with culture (29 out of 46 patients (63%)), with a higher yield compared with other procedures. Waiting for the result of the pleural fluid culture is impractical as the sensitivity is low and the results are obtained with delay.
The main message of this study is that if thoracoscopy had not been performed, a diagnosis of pleural TB would have been achieved in 15 out of 52 patients (28.8%) and in four of them only with the culture after 30–40 days (table 3). Spontaneous resolution of pleural TB is a well-known phenomenon [32], but 65% of patients may progress to pulmonary or extrapulmonary TB within 5 years [33, 34]. The usefulness of medical thoracoscopy for the diagnosis and treatment of pleural TB has been previously reported [35]. It is important to emphasise that medical thoracoscopy is minimally invasive and can be done under local anaesthesia [36].
Other diagnostic tests such as adenosine deaminase (ADA), interferon (IFN)-γ, interleukin-2, tumour necrosis factor-α, NAATs and IFN-γ release assays are used to diagnose TB pleural effusion [37], but they are only suggestive of the diagnosis, do not identify resistant mycobacteria and cannot guide the therapy. Recently, new-generation assays have been developed that seem to have improved sensitivity in TB detection in patients with extrapulmonary TB [17]. A lymphocyte-prominent exudative effusion with an ADA level >40 IU in settings with a high TB prevalence is considered diagnostic for pleural TB [6]. However, in areas with a low TB prevalence, the positive predictive value of the test is 50% and this limits its clinical usefulness [13]. Thus, increased ADA levels should not be equated to M. tuberculosis isolation in pleural fluid or biopsy specimens [38]. In the presence of a suspicion of pleural TB, every diagnostic method should be used to achieve the diagnosis and empiric therapy is not justified.
The study is biased by a retrospective design and a long period of accrual due to the rarity of the disease. The lack of a true control group does not allow for a direct assessment of the sensitivity of our approach that is inferred ex-post. These arguments call for caution in the conclusions, which need to be confirmed by prospective observations and in different settings. The study is representative of the epidemiological and healthcare scenario of a tertiary hospital in a developed country where TB is not prevalent and where access to diagnostic procedures is widespread. Its conclusions cannot be easily transferred to other settings such as small health centres in developing countries where invasive procedures, although easily manageable like medical thoracoscopy, are not recommended unless emergency surgical facilities are available. Nonetheless, medical thoracoscopy definitely sets a standard for the diagnosis of pleural TB that will be useful to test the efficacy of new biomarkers on blood or fluid for a rapid, noninvasive diagnosis in developing countries.
Conclusions
The present results support a diagnostic work-up of pleural TB that includes all the diagnostic methods of interventional pulmonology: thoracocentesis, bronchoscopy (when lung lesions are present) and medical thoracoscopy. In the majority of patients with tuberculous pleural effusion, the correct diagnosis can be reached only with the pleural biopsy: tissue is the issue.
Acknowledgements
Author contributions: A.G. Casalini was responsible for the conception and design of the study, participated in the acquisition, analysis and interpretation of the data, and writing the article. P.A. Mori, M. Majori and M. Anghinolfi were involved in the acquisition of the data. E.M. Silini was involved in analysis and interpretation of the cyto-histological data, and writing the article. L. Gnetti was involved in analysis and interpretation of the cyto-histological data. A. Calderaro, F. Motta, S. Larini and S. Montecchini were involved in analysis and interpretation of the microbiological data.
Footnotes
Conflict of interest: None declared
References
- 1.World Health Organization. Global Tuberculosis Report 2016 www.who.int/tb/publications/global_report/en Date last accessed: November 11, 2017.
- 2.Seibert AF, Haynes J Jr, Middleton R, et al. Tuberculous pleural effusion: twenty-year experience. Chest 1991; 99: 387–390. [DOI] [PubMed] [Google Scholar]
- 3.Qiu L, Teeter LD, Liu Z, et al. Diagnostic associations between pleural and pulmonary tuberculosis. J Infect 2006; 53: 377–386. [DOI] [PubMed] [Google Scholar]
- 4.Vidal R, de Garcia J, Ruiz J, et al. Estudiocontrolado de 637 pacientes con tuberculosis. Diagnostico y resultadosterapéuticos con esquemas de 9 y 6 meses. [Controlled study of 637 patients with tuberculosis. Diagnosis and therapeutic results with 9- and 6-month regimens.] Med Clin 1986; 87: 368–370. [PubMed] [Google Scholar]
- 5.Saks AM, Posner R. Tuberculosis in HIV positive patients in South Africa: a comparative radiological study with HIV negative patients. Clin Radiol 1992; 46: 387–390. [DOI] [PubMed] [Google Scholar]
- 6.Diacon AH, Van de Wal BW, Wyser C, et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur Respir J 2003; 22: 589–591. [DOI] [PubMed] [Google Scholar]
- 7.Conde MB, Loivos AC, Rezende VM, et al. Yield of sputum induction in the diagnosis of pleural tuberculosis. Am J Respir Crit Care Med 2003; 167: 723–725. [DOI] [PubMed] [Google Scholar]
- 8.Light RW. Update on tuberculous pleural effusion. Respirology 2010; 15: 451–458. [DOI] [PubMed] [Google Scholar]
- 9.Light RW, MacGreggor I, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507–513. [DOI] [PubMed] [Google Scholar]
- 10.Boutin C, Viallat JR, Aelony Y. Practical Thoracoscopy. Berlin, Springer, 1991. [Google Scholar]
- 11.Mori PA, Casalini AG, Melioli A. Toracoscopia medica: metodica e complicanze. [Medical thoracoscopy: method and complications.] In: Casalini AG, ed. Pneumologia Interventistica. [Interventional Pneumology.] Milan, Springer, 2006; pp. 461–472. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, 2005. MMWR Recomm Rep 2005; 54: RR-17, 1–141. [PubMed] [Google Scholar]
- 13.Valdes L, Alvarez D, San José E, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med 1998; 158: 2017–2021. [DOI] [PubMed] [Google Scholar]
- 14.Valdes L, San Jose ME, Pose A, et al. Diagnosing tuberculous pleural effusion using clinical data and pleural fluid analysis. A study of patients less than 40 years-old in an area with a high incidence of tuberculosis. Respir Med 2010; 104: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 15.Sahn SA, Huggins JT, San José ME, et al. Can tuberculous pleural effusions be diagnosed by pleural fluid analysis alone? Int J Tuberc Lung Dis 2013; 17: 787–793. [DOI] [PubMed] [Google Scholar]
- 16.Trajman A, da Silva Santos Kleiz de Oliveira EF, Bastos ML, et al. Accuracy of polimerase chain reaction for the diagnosis of pleural tuberculosis. Respir Med 2014; 108: 918–923. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Meeting report of a Technical Expert Consultation: Non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF 2017. www.who.int/tb/publications/2017/XpertUltra/en Date last accessed: November 11, 2017.
- 18.Dalbeth N, Gary Lee YC. Lymphocytes in pleural disease. Curr Opin Pulm Med 2005; 11: 334–339. [DOI] [PubMed] [Google Scholar]
- 19.Sahn SA. The value of pleural fluid analysis. Am J Med Sci 2008; 335: 7–15. [DOI] [PubMed] [Google Scholar]
- 20.Antony VB, Codbey SW, Kunkel SL, et al. Recruitment of inflammatory cells to the pleural space. Chemotactic cytokines, IL-8, and monocyte chemotactic peptide-1 in human pleural fluids. J Immunol 1993; 151: 7216–7223. [PubMed] [Google Scholar]
- 21.Sahn SA. Pleural disease In: CHEST Pulmonary Medicine Board Review. 25th Edn Glenview, American College of Chest Physicians, 2009; pp. 513–546. [Google Scholar]
- 22.Levine H, Szanto PB, Cugell DW. Tuberculous pleurisy. an acute illness. Arch Intern Med 1968; 122: 329–332. [PubMed] [Google Scholar]
- 23.Vorster MJ, Allwood BW, Diacon AH, et al. Tuberculous pleural effusions: advances and controversies. J Thorac Dis 2015; 7: 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stead WW, Eichenholtz A, Strauss HK. Operative and pathologic findings in 24 patients with the syndrome of idiopathic pleurisy with effusion presumably tuberculous. Am Rev Tuberc 1955; 71: 473–502. [DOI] [PubMed] [Google Scholar]
- 25.Bielsa S, Palma R, Pardina M, et al. Comparison of polymorphonuclear- and lymphocyte-rich tuberculous pleural effusions. Int J Tuberc Lung Dis 2013; 17: 85–89. [DOI] [PubMed] [Google Scholar]
- 26.Liam C-K, Lim K-H, Wong CM-M. Tuberculous pleurisy as a manifestation of primary and reactivation disease in a region with a high prevalence of tuberculosis. Int J Tuberc Lung Dis 1999; 3: 816–822. [PubMed] [Google Scholar]
- 27.Yilmaz MU, Kumcuoglu Z, Utkaner G, et al. Computed tomography findings of tuberculous pleurisy. Int J Tuberc Lung Dis 1998; 2: 164–167. [PubMed] [Google Scholar]
- 28.Kim HJ, Lee HJ, Kwon SY, et al. The prevalence of pulmonary parenchymal tuberculosis in patients with tuberculous pleuritis. Chest 2006; 129: 1253–1258. [DOI] [PubMed] [Google Scholar]
- 29.Parimon T, Spitters CE, Muangman N, et al. Unexpected pulmonary involvement in extrapulmonary tuberculosis patients. Chest 2008; 134: 589–594. [DOI] [PubMed] [Google Scholar]
- 30.Ko JM, Park HJ, Kim CH. Pulmonary changes of pleural TB. Chest 2014; 146: 1604–1611. [DOI] [PubMed] [Google Scholar]
- 31.American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Crit Care Med 2000; 161: 1376–1395. [DOI] [PubMed] [Google Scholar]
- 32.Cohen M, Sahn SA. Resolution of pleural effusions. Chest 2001; 119: 1547–1562. [DOI] [PubMed] [Google Scholar]
- 33.Roper WH, Waring JJ. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc 1955; 71: 616–634. [DOI] [PubMed] [Google Scholar]
- 34.Casalini AG, Cusmano F, Sverzellati N, et al. An undiagnosed pleural effusion with surprising consequences. Respir Med Case Rep 2017; 22: 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong Y, Gao X, Zhu H, et al. Role of medical thoracoscopy in the treatment of tuberculous pleural effusion. J Thorac Dis 2016; 8: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Astoul P, Tassi GF, Tschopp JM. Thoracoscopy for Pulmonologists. A Didactic Approach. Berlin, Springer, 2014. [Google Scholar]
- 37.Trajman A, Pai M, Dheda K, et al. Novel tests for diagnosing tuberculous pleural effusion: what works and what does not? Eur Respir J 2008; 31: 1098–1106. [DOI] [PubMed] [Google Scholar]
- 38.Laniado-Laborın R. Adenosine deaminase in the diagnosis of tuberculous pleural effusion is it really an ideal test? A word of caution. Chest 2005; 127: 417–418. [DOI] [PubMed] [Google Scholar]