Abstract
Background
The clinical spectrum of community-acquired pneumonia ranges from infections that can be treated on an outpatient basis, with 1% mortality, to those that present as medical emergencies, with a mortality above 40%.
Methods
This article is based on pertinent publications and current guidelines retrieved by a selective search of the literature.
Results
The radiological demonstration of an infiltrate is required for the differentiation of pneumonia from acute bronchitis regardless of whether the patient is seen in the outpatient setting or in the emergency room. For risk prediction, it is recommended that the CRB-65 criteria, unstable comorbidities, and oxygenation should be taken into account. Amoxicillin is the drug of choice for mild pneumonia; it should be given in combination with clavulanic acid if there are any comorbid illnesses. The main clinical concerns in the emergency room are the identification of acute organ dysfunction and the management of sepsis. Intravenous beta-lactam antibiotics should be given initially, in combination with a macrolide if acute organ dysfunction is present. The treatment should be continued for 5–7 days. Cardiovascular complications worsen the patient’s prognosis and should be meticulously watched for. Structured follow-up care includes the follow-up of comorbid conditions and the initiation of recommended preventive measures such as antipneumococcal and anti-influenza vaccination, the avoidance of drugs that increase the risk, smoking cessation, and treatment of dysphagia, if present.
Conclusion
Major considerations include appropriate risk stratification and the implementation of a management strategy adapted to the degree of severity of the disease, along with the establishment of structured follow-up care and secondary prevention, especially for patients with comorbidities.
Community-acquired pneumonia (CAP) is, in a global perspective, the infectious disease that most commonly causes death (1). Current figures from health services research in Germany, based on data from the legally mandated health-insurance carriers, reveal an incidence of 9.7 cases per 1000 person-years, corresponding to more than 660 000 patients per year. Both the incidence and the mortality of CAP are age- and comorbidity-dependent (2, 3). 46.5% of patients with CAP, and more than half of those aged 60 or above, are admitted to hospital for treatment (2). Hospitalized patients with CAP have a high in-hospital mortality of circa 13% (4). Even after bedridden persons and persons living in nursing homes are excluded from the statistics, 2.4% of the admitted patients die within 72 hours of admission. These patients account for 33% of all in-hospital deaths of patients with CAP, or ca. 3700 patients in Germany each year (5).
Acute respiratory insufficiency and acute extrapulmonary organ dysfunction due to sepsis or comorbidities are the most important early prognostic parameters (6– 8). Even after discharge from the hospital, there is still a high mortality related to comorbidities, particularly in elderly patients: according to a recent German study, in a group of patients whose median age was over 80, most of whom had chronic neurological or cardiac disease, the post-discharge mortality within 30 days of hospitalization was 4.7% (2, 9). These figures have led to major conceptual changes in the characterization and treatment of CAP. Aside from early establishment of the goal of treatment in the emergency room, an important initial consideration is to rapidly identify high-risk patients who need to be treated on an emergency basis so that they can have the best possible outcome. Moreover, aspects of post-hospital treatment, prevention, and palliative care for elderly and multimorbid patients need greater attention. These aspects were already incorporated into the revised guideline of 2016 and, in part, implemented in corresponding recommendations (10).
Mortality.
2.4% of patients admitted to the hospital with community-acquired pneumonia die within 72 hours of admission.
Learning goals
This review should enable the reader to
know the salient epidemiological features of CAP, including its risk factors and short-term and long-term prognosis;
initiate appropriate risk stratification leading to valid recommendations for treatment, both in the outpatient setting and in the hospital;
know the diagnostic and therapeutic steps that should be taken.
Methods
The PubMed database was selectively searched for publications from April 2015 onward containing the term “community-acquired pneumonia” together with “risk stratification,” “diagnosis,” “treatment,” or “prevention.” The current update (2016) of the German guideline on the treatment and prevention of community-acquired pneumonia in adults (10) and its systematic literature search up to March 2015 were also consulted.
Definition
CAP is defined as pneumonia acquired outside the hospital by an immune-competent individual. It is to be distinguished, on the basis of a wider spectrum of pathogens, from nosocomial pneumonia (which arises more than 48 hours after hospital admission or within 3 months of discharge) and from pneumonia in an immunocompromised host (e.g., in the setting of neutropenia, iatrogenic immunosuppression with drugs, status post organ or stem-cell transplantation, HIV infection, or a congenital immune deficiency) (10, 11).
Diagnostic evaluation
The manifestations of community-acquired pneumonia include respiratory symptoms (cough, sputum, dyspnea, chest pain) and general symptoms of infection (fever, hypothermia, malaise, flu-like symptoms, circulatory symptoms, impaired consciousness), along with the corresponding physical findings (tachypnea, tachycardia, arterial hypotension, focal auscultatory abnormality). As these manifestations are not sensitive or specific enough for definitive diagnosis (e1), a confirmatory chest x-ray is recommended. Infiltrates can also be detected by chest ultrasonography. The following clinical findings elevate the pretest probability that an infiltrate will be present and should prompt a chest x-ray, also in the outpatient setting:
Differentiation from bronchitis.
The demonstration of an infiltrate generally suffices to distinguish CAP from acute bronchitis, which does not need to be treated with antibiotics.
Absence of rhinorrhea
Dyspnea and/or elevated respiratory frequency
focal auscultatory abnormality
Abnormal vital signs (fever, tachycardia >100/min)
Elevated biomarkers (e.g., C-reactive protein [CRP] >20–30 mg/L).
If more than two of these criteria are met, the pretest probability that an infiltrate will be present in a patient with acute lower airway infection rises from <5% to >18% (12).
The demonstration of an infiltrate generally suffices to distinguish CAP from acute bronchitis; the latter does not need to be treated with antibiotics because the undesired effects of antibiotic treatment in such cases outweigh their beneficial effect on symptoms (number needed to treat = 22, number needed to harm = 5, according to a pertinent meta-analysis) (13). Procalcitonin is another biomarker that can be used to help avoid the unnecessary prescribing of antibiotics to outpatients with lower airway infections (14); if point-of-care tests are not available, “delayed prescribing” can be used to avoid unnecessary antibiotics.
Evaluation of the goal of treatment
Predominantly elderly patients.
In Germany, the median age of hospitalized patients with CAP is 76. More than half of all patients have at least one chronic comorbid illness.
In Germany, the median age of hospitalized patients with CAP is 76. More than half of all patients have at least one chronic comorbid illness, and 27% were already chronically bedridden before being admitted with CAP (4). Therefore, a continual re-evaluation and documentation of the goal of treatment for the individual patient, the measures to be taken in pursuit of this goal, and (where applicable) the limitations of treatment are central tasks for the treating physician. The current German guideline deals with these matters in a separate chapter on palliative-care aspects of CAP (10).
The spectrum of pathogens
By far the most important bacterial pathogen causing CAP in Germany is Streptococcus pneumoniae, which was present in 40% of patients in the CAPNETZ cohort in whom a pathogen was found (15). Mycoplasma pneumoniae should be considered as well, mainly in patients under age 60 and without comorbid illnesses (16). Further pathogens include Haemophilus influenzae and, in the winter season, influenza viruses. Patients who have chronic comorbid conditions, who live in nursing homes, or who have severe pneumonia may (rarely) be infected with enterobacteria (Escherichia coli, Klebsiella spp.) or Staphylococcus aureus. Legionella spp., too, should always be suspected in patients with severe pneumonia.
Outpatient treatment
Risk stratification
Risk stratification.
The CRB-65 score, which is easy to calculate without requiring any laboratory tests, is recommended for risk stratification in the outpatient setting.
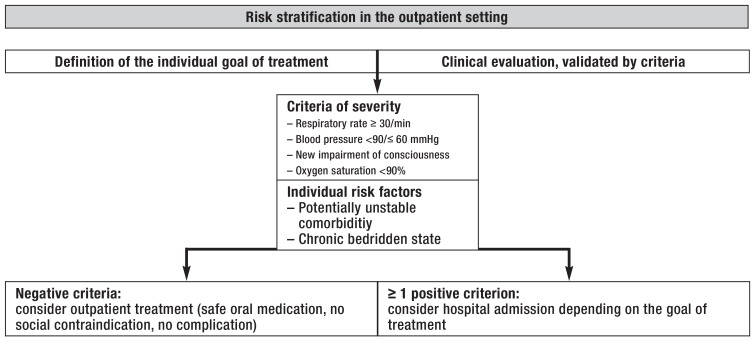
The main goal of risk stratification in the outpatient setting is to accurately identify the patients who are at low risk of dying from pneumonia and are amenable to outpatient treatment. The physician’s impression of clinical severity should be objectified with properly validated criteria. The CRB-65 score, which is easy to calculate without requiring any laboratory tests, is recommended in Germany for this purpose (table 1) (17). Nonetheless, multimorbid patients may have a poor prognosis despite a low score (18), and acute cardiac complications in patients with CAP substantially worsen the prognosis as well (6). Patients must often be hospitalized because of hypoxemia despite a low CRB-65 score (19). The negative predictive value of the CRB-65 criteria can be markedly improved by the additional consideration of three further parameters—oxygen saturation, potentially decompensated comorbidity, and chronic bedridden state (table 1) (18, 20), as recommended in the current German S3 guideline, before any decision is taken to treat CAP on an outpatient basis (10). A corresponding risk stratification process for outpatient practice is depicted in Figure 1.
Table 1. CRB-65 criteria and additional parameters for risk prediction before outpatient treatment (17, 18, 20).
| Parameter | CRB-65 criteria | Additional parameters |
| Impaired consciousness |
newly arisen | |
| Respiratory rate | ≥ 30/min | |
| Blood pressure | <90 mmhg / ≤ 60 mmhg | |
| Age | ≥ 65 years | |
| Comorbidities* | (potentially) decompensated | |
| Oxygen saturation | <90% | |
| Functional status | chronically bedridden (>50% of the day) |
* chronic cardriac, neurological, hepatic, renal, or malignant accompanying disease
Figure 1.
Management-based risk stratification for community-acquired pneumonia in the outpatient setting (from [21])
Outpatient treatment of community-acquired pneumonia
Diagnostic evaluation.
In patients with mild CAP that is amenable to outpatient treatment, microbiological determination of the pathogen is generally not needed.
In patients with mild CAP that is amenable to outpatient treatment according to the criteria outlined above, microbiological determination of the pathogen is generally not needed (10). The recommended antimicrobial treatment of choice for patients without comorbid conditions is monotherapy with high-dose amoxicillin. Patients with mild CAP who have chronic accompanying illnesses should be treated with a combination including a beta-lactamase inhibitor (amoxicillin/clavulanic acid), which widens the spectrum of efficacy to cover S. aureus, enterobacteria, and beta-lactamase-producing H. influenzae. Patients who are allergic to or cannot tolerate penicillin can be given a fluoroquinolone with efficacy against pneumococci (moxifloxacin, levofloxacin). Randomized trials have shown that the use of these antibiotics on an outpatient basis results in cure rates from 76% to 89%, without any relevant differences between the individual substance classes (e2). Oral cephalosporins should not be given because of their inadequate oral bioavailability, the elevated risk of selecting extended-spectrum beta-lactamase (ESBL) bacteria, and the risk of Clostridium difficile colitis, along with an elevated risk of treatment failure in the outpatient setting, as shown in the CAPNETZ cohort (odds ratio [OR]: 2.9) (22). The corresponding treatment recommendation is summarized in Table 2. Patients undergoing outpatient treatment should be clinically re-evaluated within 48 to 72 hours, as this is the time frame in which clinical worsening can occur despite ongoing treatment. If the treatment yields no improvement, admission to hospital is necessary in the vast majority of cases. If there is clinical improvement, including defervescence, then the antimicrobial treatment should be continued for 5–7 days at most (10).
Table 2. Empirical initial treatment for mild CAP that can be treated on an outpatient basis (10).
| Severity class | Primary treatment (standard dose) | Alternative treatment (standard dose) |
| Mild pneumonia without comorbidity, outpatient treatment | Amoxicillin (750–1000 mg tid) | Moxifloxacin (400 mg qd) Levofloxacin (500 mg qd or bid) Clarithromycin (500 mg bid) Azithromycin (500 mg qd × 3 d) Doxycycline (200 mg qd) |
| Mild pneumonia with comorbidity, outpatient treatment – Congestive heart failure – Neurologic disease with dysphagia – Severe COPD, bronchiectasis – Bedridden state, PEG |
Amoxicillin/clavulanic acid (1 g tid) | Moxifloxacin (400 mg qd) Levofloxacin (500 mg qd or bid) |
CAP,community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; PEG, percutaneous endoscopic gastrostomy
The treatment of mild CAP.
The recommended antimicrobial treatment of choice for patients without comorbid conditions is monotherapy with high-dose amoxicillin.
Inpatient treatment
Risk stratification in the emergency room: CAP as an emergency
Re-evaluation.
Patients undergoing outpatient treatment should be clinically re-evaluated in 48 to 72 hours.
In the hospital emergency room, risk evaluation is needed so that the treatment of patients at risk can be intensified. Patients who are acutely in need of mechanical ventilation or vasopressor therapy must be transferred to an intensive care unit at once. A recent CAPNETZ study revealed, however, that patients whose condition is stable at first, but deteriorates later on in the course of their hospitalization to the extent that organ-replacement therapy becomes necessary, suffer an additional increase in mortality by as much as 48% (23). The main predictors of such an occurrence were the following abnormal vital signs on admission:
Tachycardia and tachypnea
Low blood pressure
Hypothermia and
New impairment of consciousness.
High-risk patients.
The identification of high-risk patients and the establishment of treatment goals are the primary initial concerns in the hospital emergency room.
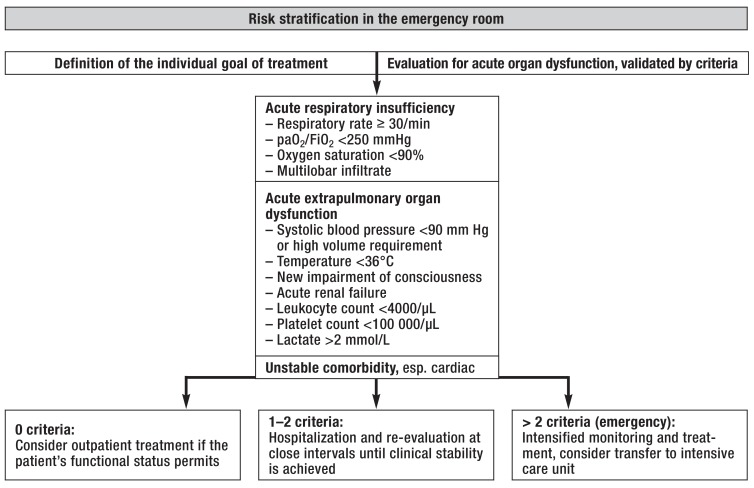
In particular, patients with systemic hypotension, acute respiratory insufficiency, or decompensated cardiac comorbidities are in acute danger of death even if they do not immediately require organ-replacement therapy; they benefit from early intensive treatment of organ dysfunction due to sepsis and comorbid conditions (6, 8, 24, 25). In the current guideline, the use of so-called minor criteria is recommended as a means of objectifying high-risk predictions of this type (box 1) (10). A recent meta-analysis has shown that all nine of these criteria are predictors for the need for organ-replacement therapy; if more than two criteria are met, the sensitivity and specificity are 79% and 82%, respectively (positive likelihood ratio: 4.3) (26). Moreover, in an interventional trial, the implementation of a treatment algorithm incorporating these criteria (box 1) lowered the mortality of high-risk patients of this type from 24% (in historical controls) to 6% (25). The evaluation of organ dysfunction due to sepsis must be supplemented by the structured assessment of potentially unstable comorbid conditions; among these, cardiovascular complications such as acute myocardial infarction or left-heart decompensation are of particular prognostic significance (6). An ensuing suggestion for in-hospital, management-based risk stratification is shown in Figure 2.
BOX 1. Minor criteria: if more than 2 of these 9 criteria are met, a medical emergency is present (10).
Severe acute respiratory insufficiency (partial pressure of oxygen = 55 mmHg or = 7 kPa on room air)
Respiratory rate = 30/min
Multilobar infiltrates on chest x-ray
New impairment of concsiousness
Systemic hypotension requiring aggressive volume resuscitation
Acute renal failure (blood urea nitrogen = 20 mg/dL)
Leukopenia (leukocyte count <4000 cells/µL)
Thrombocytopenia (platelet count <100 000 cells/µL)
Hypothermia (body temperature <36°C)
Figure 2.
Risk stratification in the emergency room (from [21])
FiO2, inspired oxygen concentration; paO2, arterial partial oxygen pressure
The management of acute organ dysfunction
Risk factors.
Systemic hypotension, acute respiratory insufficiency, and a decompensated cardiac comorbidity represent clinical emergencies.
In patients with CAP and acute organ dysfunction, rapid, individualized fluid management and the immediate initiation of intravenous broad-spectrum antibiotic treatment improve the clinical outcome (24, 25). Treatment based on so-called sepsis bundles is recommended in the sepsis guidelines (27) (box 2). Their implementation in the emergency room, including treatment by a physician with experience in intensive-care medicine, can improve outcomes in patients with severe CAP as well and lowers the probability that the patient will later need to be transferred to an intensive care unit (25, e3). The serum lactate concentration should also be measured (e4): if it is elevated, rapid, individualized fluid management with re-checking at close intervals is needed, the goal being normalization of the lactate concentration. In patients with acute hypercapnic respiratory insufficiency or accompanying pulmonary edema, non-invasive ventilation should be initiated (10). A recent study showed that nasal high-flow oxygen therapy for patients with primary hypoxic respiratory failure tends to lessen the need for intubation and significantly lowers mortality (28).
BOX 2. Bundle of measures for severe community-acquired pneumonia (10).
To be performed as rapidly as possible within the first 3 hours:
Serum lactate measurement
Blood cultures
Initiation of appropriate (generally combined) broad-spectrum intravenous antibiotic treatment, within one hour if possible
In patients with arterial hypotension or elevated serum lactate, rapid intravenous administration of crystalloids
Evaluation (including blood-gas analysis) and treatment, if needed, of acute respiratory insufficiency
To be performed within the first 6 hours:
Administration of vasopressors if the response to volume administration is inadequate
Repetition of lactate measurement if the initial value was elevated
Re-evaluation of blood gases
Microbiological diagnostic evaluation in the hospital
It is recommended in the German guideline that, as soon as a patient is hospitalized for CAP, blood cultures should be drawn (before the first dose of antibiotic is given, if at all possible), Legionella and pneumococcal antigens should be measured in the urine, and purulent sputum (if present) should be examined microscopically and cultured (10). If the epidemiological setting is suggestive, patients with severe CAP should also be tested for influenza viruses with the polymerase chain reaction (PCR). If mycoplasma pneumonia is suspected, mycoplasma IgM or PCR can be measured; however, the measurement of mycoplasma IgG and IgA is of no diagnostic value, and Chlamydia serology is of no diagnostic value either (e5, e6). If there is a pleural effusion that can be punctured and aspirated, a rapid diagnostic puncture followed by microbiological evaluation and pH measurement is indicated.
Empirical antibiotic treatment
CAP and organ dysfunction.
In patients with CAP and acute organ dysfunction, rapid, individualized fluid management and intravenous broad-spectrum antibiotics improve the clinical outcome.
In patients with overt organ dysfunction, empirical antimicrobial treatment should be initiated as soon as possible after the diagnosis is made, as this improves the outcome; for all other patients, the initiation of treatment within 8 hours is recommended (10). The initial treatment should be given parenterally for at least 48 hours in a sufficiently high dose and should cover pneumococci, H. influenzae, S. aureus, and enterobacteria; the agent of choice is an intravenous beta-lactam antibiotic (table 3). Even in patients with renal failure, the recommended maximum dose should be given at least for the first 24 hours. For patients with CAP and overt organ dysfunction, the additional administration of a macrolide as part of the initial treatment is recommended, both because this improves outcomes (absolute reduction of mortality from 24% to 21%) (29) and because it covers Legionella in particular. If there is a clinical response and no atypical pathogens are demonstrated, the macrolide drug can be stopped after 3 days of treatment. For hospitalized patients without acute organ dysfunction, an additional macrolide drug is optional, as no clear improvement of the outcome has been demonstrated in prospective, placebo-controlled clinical trials (clinical stability on the 7th day of treatment, 66% vs. 59%, p = 0.07; 30-day mortality, 3.4% vs. 4.8%, p = 0.4) (30, e7).
Table 3. Empirical initial treatment for in-hospital therapy of community-acquired pneumonia (10).
| Severity class | Primary treatment (standard dose) | Alternative treatment (standard dose) |
| Moderately severe CAP (no acute organ dysfunction) |
Beta-lactam IV – Amoxicillin/clavulanic acid (2.2 g q8h) – Ampicillin/sulbactam (3 g q8h) – Cefuroxime (1.5 g q8h) – Ceftriaxone (2 g qd) – Cefotaxime (2 g q8h) + Optional* macrolide IV or po for 3 days – Clarithromycin (500 mg q12h) – Azithromycin (500 mg qd) |
Fluoroquinolone IV or po Moxifloxacin (400 mg qd) Levofloxacin (500 mg qd or q12h) |
| Severe CAP (acute organ dysfunction) |
Beta-lactam IV – Piperacillin/tazobactam (4.5 g q6-8h) – Ceftriaxone (2 g qd) – Cefotaxime (2 g q6-8h) + Macrolide IV for 3 days – Clarithromycin (500 mg q12h) – Azithromycin (500 mg qd) |
Fluoroquinolone IV Moxifloxacin (400 mg qd) Levofloxacin (500 mg q12h) (no monotherapy in patients with septic shock) |
* The additional administration of a macrolide is optional because prospective, placebo-controlled trials have not clearly shown that they improve the outcome
Pseudomonas aeruginosa causes CAP extremely rarely, (<1% of cases in Germany). Risk factors include severe chronic obstructive pulmonary disease (COPD), bronchiectasis, or an indwelling percutaneous endoscopic gastrostomy tube (PEG) (e8). Multiresistant organisms enter into the differential diagnosis only in patients with individual risk factors, such as:
Antimicrobial treatment.
The initial treatment should be given parenterally for at least 48 hours in a sufficiently high dose. The agent of choice is an intravenous beta-lactam antibiotic.
Prior hospitalization (in which case the recommendations for nosocomial pneumonia apply)
Prior prolonged broad-spectrum antibiotic treatment
Prior travel to countries in which multiresistant organisms are prevalent (10).
Additional empirical antiviral treatment with oseltamivir should be considered for hospitalized patients with acute organ dysfunction and elevated risk (comorbid conditions, pregnancy) during the influenza season; oseltamivir should be discontinued as soon as a negative viral test result is obtained by PCR (e9). The recommendations for initial empirical treatment for patients treated in the hospital are shown in Table 3.
Clinical stability and treatment failure
Antiviral treatment.
Additional empirical antiviral treatment with oseltamivir should be considered for hospitalized patients with acute organ dysfunction and elevated risk during the influenza season.
Because of the dynamic nature of septic organ dysfunction, all patients who meet the criteria for severe CAP or who have abnormal vital signs need continuous monitoring of their disease course until they show clinical improvement. The greatest risk of worsening of organ function is within the first 72 hours after admission to the hospital (7, 8). Moreover, laboratory testing for particular types of organ dysfunction is mandatory in patients who have corresponding comorbid conditions. A minimum standard for all hospitalized patients is the re-evaluation of stability criteria once per day (box 3) (10). If all of these criteria are met, the risk of repeated acute organ dysfunction is low. In addition, another CRP or procalcitonin (PCT) measurement after 3–4 days is recommended; if the value fails to come down after an adequate latency (24–48 hours for PCT, 48–72 hours for CRP), the patient must be clinically re-evaluated for treatment failure.
BOX 3. Criteria for clinical stability (10).
The signs of clinical stability are defined as:
Heart rate = 100/min
Respiratory rate = 24/min
Systolic blood pressure = 90 mm Hg
Body temperature = 37.8°C
Ability to take food by mouth
Normal state of consciousness
No hypoxemia (pO2= 60 mmHg, SaO2= 90%)
pO2, partial pressure of oxygen; SaO2, oxygen saturation
Continuous monitoring of disease course.
All patients who meet the criteria for severe CAP or who have abnormal vital signs need continuous monitoring of their disease course until they show clinical improvement.
If clinical stability does not ensue or if there is clinical worsening after 3–5 days of appropriate treatment, the patient must be evaluated for treatment failure. The important clinical distinction must be drawn between a mere delay in the achievement of stability on the one hand and clinically progressive pneumonia on the other. Progressive pneumonia carries a poor prognosis with an up to tenfold increase in mortality. Thus, clinical progression necessitates an immediate diagnostic assessment comprising re-evaluation of severity criteria and organ functions, following up of the inflammatory parameters, and the exclusion of complications such as an abscess or pleural effusion. Care on an intensive care unit for stabilization of organ function and the rapid, appropriate tailoring of the antimicrobial therapy are the main beneficial measures. There should also be a repeated microbiological evaluation, including bronchoscopy if indicated, as well as a meticulous evaluation for extrapulmonary infectious and non-infectious differential diagnoses and complications, such as pulmonary embolism or hitherto unrecognized immunosuppressed state (including HIV infection). Antibiotic treatment in patients with progressive infection should cover any gaps in the antimicrobial spectrum of the initial treatment and should always be given as intravenous combination therapy in adequate doses (10).
Narrowing or de-escalation of antibiotic treatment
If there is a clinical response, it should be determined whether the antibiotic treatment can be narrowed or de-escalated. For example, in patients being initially treated with a macrolide as part of combination therapy, the macrolide can be discontinued if there is a clinical response and the absence of atypical pathogens is demonstrated. If pneumococci are clearly revealed by blood culture and/or a urinary antigen test, and clinical improvement takes place under empirical treatment, it is recommended that the antibiotic treatment should be narrowed to penicillin (31). Once the criteria for clinical stability have been met, the treatment can be switched to the oral administration of a preparation with good oral bioavailability (e.g., amoxicillin/clavulanic acid) and it should be determined whether the patient is now fit for discharge from the hospital. As long as there are no complications, the treatment should be stopped 2–3 days after clinical stability has been reached; thus, 5–7 days of treatment usually suffice even for severe CAP (32). The implementation of “antibiotic stewardship” may help implementing these steps (33).
Adjuvant treatment: cardiac complications and steroids
Treatment failure.
If clinical stability does not ensue or if there is clinical worsening after 3–5 days of appropriate treatment, the patient must be evaluated for treatment failure. Progressive pneumonia carries an up to tenfold increase in mortality.
Acute cardiovascular complications arise in up to 14–25% of all patients hospitalized with CAP (6, e10). In a small, prospective randomized trial, the administration of acetylsalicylic acid (ASA) lowered cardiovascular mortality in 185 hospitalized patients with CAP from 4% (4 patients) to 0% (p = 0.04) (34). In an observational study, preexisting medication with ASA was associated with lower in-hospital mortality (e11). Although these preliminary data do not support any general recommendation for the adjuvant administration of ASA to patients with CAP, they do underscore the need to evaluate patients with preexisting cardiac disease repeatedly for potential cardiac complications, as well as the need to re-assess the indication for ASA treatment independently of CAP and to keep giving ASA to patients who were taking it before they developed CAP. Moreover, prophylactic antithrombotic treatment is recommended for all immobilized patients.
Corticosteroid administration as a potential adjuvant treatment for patients hospitalized with CAP has been studied in multiple clinical trials. The available evidence for a clinically relevant effect is not convincing. Two randomized trials demonstrated no benefit from steroid administration with respect to hard endpoints such as mortality or organ failure (35, e12). No general indication for corticosteroid treatment can be derived from these data (10). Systemic corticosteroids are indicated for additionally exacerbated obstructive airway disease or for septic shock that does not respond to catecholamine treatment.
Post-discharge care and secondary prevention
Cardiovascular complications.
Patients with pre-existing cardiac disease need repeated evaluation for potential cardiac complications and reassessment of the indication for ASA treatment.
Patients who have been treated in the hospital for CAP have a statistically significant long-term elevation of mortality compared to matched controls (OR 1.65 over 10 years) (e13). This is accounted for in large measure by deaths from comorbid conditions (9, 36). Routine insurance data from Germany reveal high mortality (4.7%) in the interval between hospital discharge and 30 days after hospital admission; the patients who died were mainly old (mean age 84) and multimorbid (suffering mostly from cardiac and neurological disease) (2). These findings underscore the need for structured post-discharge care of patients with CAP even after they have reached clinical stability and been discharged from the hospital. There is, however, a lack of scientific evidence and guideline recommendations on this topic. A chest x-ray at some time (>2 weeks) after discharge from the hospital to rule out non-infectious pathological findings is recommended for smokers and for all elderly patients (>65 years) and those with comorbid diseases (10). It seems reasonable as well for patients with chronic accompanying illnesses, such as congestive heart failure, COPD, renal or hepatic insufficiency, or diabetes mellitus, to undergo individually adapted clinical follow-up at close intervals to check for possible organ decompensation, progression, or complications. Moreover, CAP having been identified as an independent long-term risk factor for cardiovascular events (37), a structured evaluation of all of the recognized cardiovascular risk factors and appropriately directed treatment (if needed) are indicated as well.
Long-term elevation of mortality.
Patients who have been treated in the hospital for CAP have a statistically significant long-term elevation of mortality compared to matched controls.
In view of these facts, primary and secondary prevention also play an important role in the post-discharge care of patients with CAP. Anti-influenza and antipneumococcal vaccination is recommended as standard prevention for all persons over age 60 and as indicated prevention for those with comorbidities. Data on the preventive efficacy of vaccination against influenza in patients with CAP are sparse, but the recommendation to vaccinate is nonetheless justified because of its demonstrated efficacy against influenza and the significance of influenza for the incidence and severity of CAP. With regard to antipneumococcal vaccination, there are data from a large-scale, high-quality, prospective randomized trial showing that a 13-valent conjugated vaccine (PCV13) lessens the frequency of pneumococcal pneumonia due to the serotypes covered by the vaccine by 45%, and the frequency of the invasive form infection by 75% (38). The German Federal Standing Committee on Immunization currently recommends the vaccination of all persons aged 60 or older, and of all persons with chronic comorbidities, with the 23-valent polysaccharide vaccine PSV23; sequential vaccination with PCV13 followed by PSV23 is reserved for immunosuppressed persons and those with hepatic or renal failure. On the other hand, the S3 guideline contains a general recommendation for the 13-valent conjugate vaccine. This discrepancy arises from differences in the assessment of the efficacy of the PSV23 vaccine and of the significance of childhood vaccination for the serotypes affecting adults that are amenable to prevention by vaccination (39, 40). Further important matters include smoking cessation and the strict reevaluation of the indications for all drugs that have been identified as risk factors for CAP, including sedatives, antipsychotic drugs, and inhaled steroids (in COPD) (10). Optimal oral hygiene and the evaluation and treatment of possible dysphagia in patients with risk factors for it are further important measures for pneumonia prevention whose importance may be underestimated in routine practice (e14, e15).
Further Information On Cme.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.This CME unit can be accessed until 4 March 2018, and earlier CME units until the dates indicated:
“ Impingement Syndrome of the Shoulder” (Issue 45/17) until 4 February 2018,
“Fitness to Drive in Cardiovascular Disease” (Issue 41/17) until 7 January 2018,
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the most appropriate answer.
Question 1
When a patient is admitted to the hospital via the emergency room for community-acquired pneumonia, what should be done first?
isolation of the patient on the hospital ward
individualized fluid management
initiation of noninvasive ventilation
identification of high-risk patients
administration of normal saline
Question 2
Which of the following is a component of risk assessment in outpatients with suspected CAP, according to the CRB-65 criteria?
laboratory tests
reddish skin discoloration on the chest
respiratory frequency
clinical course over the ensuing 24 hours
risk factors in the patient’s home situation
Question 3
How is CAP generally distinguished from acute bronchitis that does not require treatment with antibiotics?
by a focal auscultatory abnormality
by the elevated body temperature
by spirometry
by the demonstration of an infiltrate
by measurement of the C-reactive protein
Question 4
Which of the following findings in a hospitalized patient with community-acquired pneumonia is an indication for intensified monitoring?
respiratory rate 12–18/min
oxygen saturation >95%
pH 7.36–7.44
leukocyte count <6000 cells/µL
platelet count <100 000 cells/µL
Question 5
A 42-year-old man is given the diagnosis of CAP in the emergency room. He wants to be treated as an outpatient. His temperature is 38.2°C, his blood pressure is 130/80 mmHg, his respiratory rate is 24/min, and his oxygen saturation is 96%. What is the appropriate decision?
Risk stratification implies that outpatient treatment is possible.
He should not be treated as an outpatient because of the elevated risk of an impairment of consciousness.
He should not be treated as an outpatient because he is febrile.
Outpatient treatment is not yet possible because of the risk of respiratory insufficiency.
The serum procalcitonin concentration must be measured before any decision is taken.
Question 6
What positive effect was obtained in an interventional trial by the use of the so-called minor criteria in patients with severe CAP?
The patients were discharged from the hospital an average of 2 days earlier.
On long-term follow-up, 92% of the patients were still complying with the recommended preventive measures against CAP.
Antibiotic consumption was markedly reduced.
Mortality was lowered from 24% to 6%.
Timely measurement of the lactate concentration resulted in a reduction of the number of patients who had to be transferred to the intensive care unit.
Question 7
For which patients with CAP is a macrolide drug recommended as initial antibiotic treatment, in addition to a beta-lactam?
patients aged 65 and older
patients with acute organ dysfunction
patients undergoing ambulatory treatment
patients with accompanying COPD
patients with a markedly elevated CRP concentration
Question 8
When might the administration of acetylsalicylic acid to a patient with community-acquired pneumonia be indicated?
If clinical stability has not been achieved after 3-5 days of appropriate treatment.
If influenza virus is demonstrated to be present.
If the patient does not tolerate the additional administration of a macrolide.
If a hospitalized patient develops cardiovascular complications.
If the systolic blood pressure is below 90 mmHg.
Question 9
What drug should be given as initial therapy for severe CAP, and in what dose?
amoxicillin 250 mg q12h
cefuroxime 0,5 g q12h
penicillin 400 mg qd
piperacillin/tazobactam 4.5 g q6-8h
ciprofloxacin 500 mg q12h
Question 10
How high is the in-hospital mortality of patients with CAP who are treated as inpatients?
ca. 33%
ca. 25%
ca. 19% in women and 22% in men
less than 1%
ca. 13%
Case illustration: community-acquired pneumonia.
A 56-year-old man presented to the emergency room with acute malaise, fever to 38.4°C, dyspnea, and a productive cough with yellowish sputum. He had no other serious medical conditions and was taking no medications. He stated that he had smoked approximately 15 cigarettes a day for the past 20 years.
On examination, his general condition was somewhat impaired, but he was fully alert and oriented. His respiratory rate was 26/min, his blood pressure was 120/55 mmHg, and his heart rate was 100/min (slight tachycardia). Inspiratory rales were heard over the inferior and middle lung fields on the right. The physical examination was otherwise unremarkable.
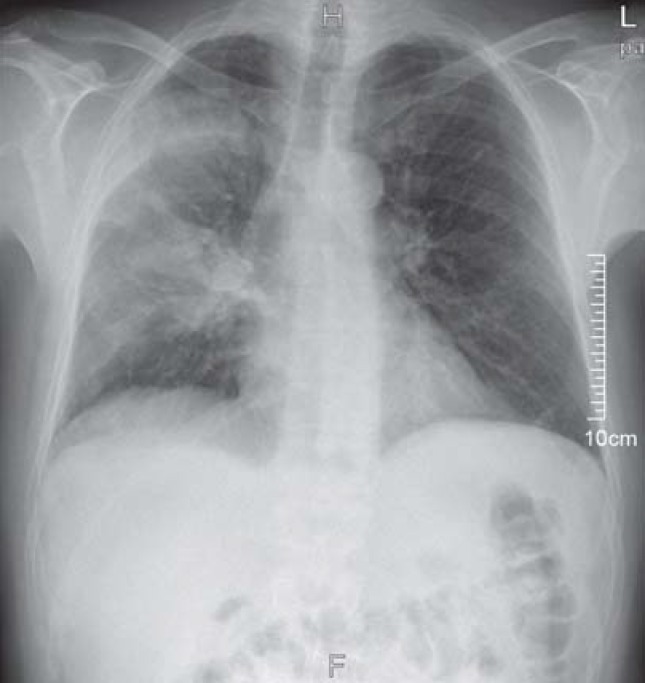
A chest x-ray showed marked consolidation in the right upper and middle lobes and linear paracardiac opacities in the left lower lobe (Figure). Capillary blood-gas analysis showed an arterial partial oxygen pressure (paO2) of 78 mmHg and an arterial partial carbon dioxide pressure (PaCO2) of 32 mmHg on room air.
These findings established the diagnosis of bilateral community-acquired pneumonia. Risk stratification yielded a CRB-65 score of 1 (diastolic hypotension), implying an elevated risk of death.
The patient was admitted to the hospital. Intensified monitoring was indicated as recommended in the 2016 S3 guideline; documentation of the respiratory rate and hemodynamic status every three hours was ordered. Two sets of blood cultures were taken, and urine antigen tests were performed for pneumococci and for Legionella pneumophila serogroup 1. Sputum could not be obtained for analysis. The antigen test for pneumococci was positive. The blood cultures were negative. These findings established the diagnosis of pneumococcal pneumonia.
The CRP concentration was 15.1 mg/dL, and the serum potassium level was mildly low at 3.5 mmol/L. All other laboratory findings were unremarkable; in particular, there was no leukocytosis and no renal dysfunction.
The patient was treated with ampicillin/sulbactam 3 g IV q8h and clarithromycin 500 mg po bid, as well as with normal saline IV at 40 ml/hr.
The patient’s blood pressure became stable within three hours. His condition improved rapidly and permanently; after the first 24 hours, he was no longer febrile. The CRP concentration fell to 8.0 mg/dL and the potasssium concentration returned to normal.
At this point, intravenous ampicillin/sulbactam and oral clarithromycin were stopped and amoxicillin 1 g po tid was given.
The patient was discharged home after 5 days in the hospital with instructions to keep taking amoxicillin for two more days (7 days of antibiotics total). An outpatient follow-up chest x-ray four weeks after admission showed only mild residual opacities.
Comment
This is the typical course of moderately severe community-acquired pneumococcal pneumonia. Hypotonia and multilobar infiltrates implied an elevated risk of death, but early and appropriate antibiotic treatment led to prompt improvement. Intensified monitoring was indicated so that impending sepsis could be recognized and treated without delay.
Chest x-ray: marked consolidation in the right upper and middle lobes, linear paracardiac opacities in the left lower lobe.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
PD Dr. Kolditz has served as a paid consultant for Astra-Zeneca, Bayer, and Basilea. He has received third-party funding for a research project from Pfizer, reimbursement of travel expenses from Pfizer und Astra-Zeneca, and lecture honoraria from Pfizer, Astra-Zeneca, Bayer and Roche.
Prof. Ewig has served as a paid consultant for Pfizer.
References
- 1.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 2.Kolditz M, Tesch F, Mocke L, Hoffken G, Ewig S, Schmitt J. Burden and risk factors of ambulatory or hospitalized CAP: a population based cohort study. Respir Med. 2016;121:32–38. doi: 10.1016/j.rmed.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Breitling LP, Saum KU, Schottker B, Holleczek B, Herth FJ, Brenner H. Pneumonia in the noninstitutionalized older population. Dtsch Arztebl Int. 2016;113:607–614. doi: 10.3238/arztebl.2016.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewig S, Birkner N, Strauss R, et al. New perspectives on community-acquired pneumonia in 388 406 patients Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64:1062–1069. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolditz M, Bauer TT, Konig T, Rohde G, Ewig S. 3-day mortality in hospitalised community-acquired pneumonia: frequency and risk factors. Eur Respir J. 2016;47:1572–1574. doi: 10.1183/13993003.00113-2016. [DOI] [PubMed] [Google Scholar]
- 6.Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125:773–781. doi: 10.1161/CIRCULATIONAHA.111.040766. [DOI] [PubMed] [Google Scholar]
- 7.Dremsizov T, Clermont G, Kellum JA, Kalassian KG, Fine MJ, Angus DC. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129:968–978. doi: 10.1378/chest.129.4.968. [DOI] [PubMed] [Google Scholar]
- 8.Phua J, Ngerng WJ, Lim TK. The impact of a delay in intensive care unit admission for community-acquired pneumonia. Eur Respir J. 2010;36:826–833. doi: 10.1183/09031936.00154209. [DOI] [PubMed] [Google Scholar]
- 9.Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162:1059–1064. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 10.Ewig S, Hoffken G, Kern WV, et al. [Management of adult community-acquired pneumonia and prevention—update 2016] Pneumologie. 2016;70:151–200. doi: 10.1055/s-0042-101873. [DOI] [PubMed] [Google Scholar]
- 11.Ewig S, Welte T, Chastre J, Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis. 2010;10:279–287. doi: 10.1016/S1473-3099(10)70032-3. [DOI] [PubMed] [Google Scholar]
- 12.Van Vugt SF, Broekhuizen BD, Lammens C, et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: diagnostic study. BMJ. 2013;346 doi: 10.1136/bmj.f2450. f2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SM, Fahey T, Smucny J, Becker LA. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2014;3 doi: 10.1002/14651858.CD000245.pub3. CD000245. [DOI] [PubMed] [Google Scholar]
- 14.Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;9 doi: 10.1002/14651858.CD007498.pub2. CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pletz MW, Rohde G, Schutte H, Bals R, von BH, Welte T. [Epidemiology and aetiology of community-acquired pneumonia (CAP)] Dtsch Med Wochenschr. 2011;136:775–780. doi: 10.1055/s-0031-1275806. [DOI] [PubMed] [Google Scholar]
- 16.Dumke R, Schnee C, Pletz MW, et al. Mycoplasma pneumoniae and chlamydia spp infection in community-acquired pneumonia, Germany, 2011-2012. Emerg Infect Dis. 2015;21:426–434. doi: 10.3201/eid2103.140927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer TT, Ewig S, Marre R, Suttorp N, Welte T. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260:93–101. doi: 10.1111/j.1365-2796.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 18.Ewig S, Bauer T, Richter K, et al. Prediction of in-hospital death from community-acquired pneumonia by varying CRB-age groups. Eur Respir J. 2013;41:917–922. doi: 10.1183/09031936.00065212. [DOI] [PubMed] [Google Scholar]
- 19.Choudhury G, Chalmers JD, Mandal P, et al. Physician judgement is a crucial adjunct to pneumonia severity scores in low-risk patients. Eur Respir J. 2011;38:643–648. doi: 10.1183/09031936.00172910. [DOI] [PubMed] [Google Scholar]
- 20.Kolditz M, Ewig S, Schutte H, Suttorp N, Welte T, Rohde G. Assessment of oxygenation and comorbidities improves outcome prediction in patients with community-acquired pneumonia with a low CRB-65 score. J Intern Med. 2015;278:193–202. doi: 10.1111/joim.12349. [DOI] [PubMed] [Google Scholar]
- 21.Kolditz M, Braeken D, Ewig S, Rohde G. Severity assessment and the immediate and long-term prognosis in community-acquired pneumonia. Semin Respir Crit Care Med. 2016;37:886–896. doi: 10.1055/s-0036-1592127. [DOI] [PubMed] [Google Scholar]
- 22.Creutz P, Kothe H, Braun M, et al. Failure of ambulatory treatment in CAP patients leading to subsequent hospitalization and its association to risk factors—prospective cohort study. J Pulmon Resp Med. 2013;3 [Google Scholar]
- 23.Kolditz M, Ewig S, Klapdor B, et al. Community-acquired pneumonia as medical emergency: predictors of early deterioration. Thorax. 2015;70:551–558. doi: 10.1136/thoraxjnl-2014-206744. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 25.Lim HF, Phua J, Mukhopadhyay A, et al. IDSA/ATS minor criteria aid pre-intensive care unit resuscitation in severe community-acquired pneumonia. Eur Respir J. 2014;43:852–862. doi: 10.1183/09031936.00081713. [DOI] [PubMed] [Google Scholar]
- 26.Salih W, Schembri S, Chalmers JD. Simplification of the IDSA/ATS criteria for severe CAP using meta-analysis and observational data. Eur Respir J. 2014;43:842–851. doi: 10.1183/09031936.00089513. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 28.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 29.Sligl WI, Asadi L, Eurich DT, Tjosvold L, Marrie TJ, Majumdar SR. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med. 2014;42:420–432. doi: 10.1097/CCM.0b013e3182a66b9b. [DOI] [PubMed] [Google Scholar]
- 30.Garin N, Genne D, Carballo S, et al. Beta-lactam monotherapy vs beta-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174:1894–1901. doi: 10.1001/jamainternmed.2014.4887. [DOI] [PubMed] [Google Scholar]
- 31.Cremers AJ, Sprong T, Schouten JA, et al. Effect of antibiotic streamlining on patient outcome in pneumococcal bacteraemia. J Antimicrob Chemother. 2014;69:2258–2264. doi: 10.1093/jac/dku109. [DOI] [PubMed] [Google Scholar]
- 32.Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176:1257–1265. doi: 10.1001/jamainternmed.2016.3633. [DOI] [PubMed] [Google Scholar]
- 33.Murray C, Shaw A, Lloyd M, et al. A multidisciplinary intervention to reduce antibiotic duration in lower respiratory tract infections. J Antimicrob Chemother. 2014;69:515–518. doi: 10.1093/jac/dkt362. [DOI] [PubMed] [Google Scholar]
- 34.Oz F, Gul S, Kaya MG, et al. Does aspirin use prevent acute coronary syndrome in patients with pneumonia: multicenter prospective randomized trial. Coron Artery Dis. 2013;24:231–237. doi: 10.1097/MCA.0b013e32835d7610. [DOI] [PubMed] [Google Scholar]
- 35.Blum CA, Nigro N, Briel M, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511–1518. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 36.Cangemi R, Calvieri C, Falcone M, et al. Relation of cardiac complications in the early phase of community-acquired pneumonia to long-term mortality and cardiovascular events. Am J Cardiol. 2015;116:647–651. doi: 10.1016/j.amjcard.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 37.Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 39.Ewig S, Pletz MW, Salzberger B. Pneumokokken-Impfung (1): Kritik an den STIKO-Empfehlungen. Dtsch Arztebl. 2017;114:A24–A-27. [Google Scholar]
- 40.Falkenhorst G, Leidel J, Bodgan C. Pneumokokken-Impfung (2): Plädoyer für STIKO-Empfehlungen. Dtsch Arztebl. 2017;114:A28–A-30. [Google Scholar]
- E1.Van Vugt SF, Verheij TJ, de Jong PA, et al. Diagnosing pneumonia in patients with acute cough: clinical judgment compared to chest radiography. Eur Respir J. 2013;42:1076–1082. doi: 10.1183/09031936.00111012. [DOI] [PubMed] [Google Scholar]
- E2.Pakhale S, Mulpuru S, Verheij TJ, Kochen MM, Rohde GG, Bjerre LM. Antibiotics for community-acquired pneumonia in adult outpatients. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD002109.pub4. CD002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Hortmann M, Heppner HJ, Popp S, Lad T, Christ M. Reduction of mortality in community-acquired pneumonia after implementing standardized care bundles in the emergency department. Eur J Emerg Med. 2014;21:429–435. doi: 10.1097/MEJ.0000000000000106. [DOI] [PubMed] [Google Scholar]
- E4.Gwak MH, Jo S, Jeong T, et al. Initial serum lactate level is associated with inpatient mortality in patients with community-acquired pneumonia. Am J Emerg Med. 2015;33:685–690. doi: 10.1016/j.ajem.2015.03.002. [DOI] [PubMed] [Google Scholar]
- E5.Von Baum H, Welte T, Marre R, Suttorp N, Lück C, Ewig S. Mycoplasma pneumoniae pneumonia revisited within the German Competence Network for Community-acquired pneumonia (CAPNETZ) BMC Infect Dis. 2009;9 doi: 10.1186/1471-2334-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Wellinghausen N, Straube E, Freidank H, von Baum H, Marre R, Essig A. Low prevalence of chlamydia pneumoniae in adults with community-acquired pneumonia. Int J Med Microbiol. 2006;296:485–491. doi: 10.1016/j.ijmm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- E7.Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372:1312–1323. doi: 10.1056/NEJMoa1406330. [DOI] [PubMed] [Google Scholar]
- E8.Von Baum H, Welte T, Marre R, Suttorp N, Ewig S. Community-acquired pneumonia through enterobacteriaceae and pseudomonas aeruginosa: diagnosis, incidence and predictors. Eur Respir J. 2010;35:598–605. doi: 10.1183/09031936.00091809. [DOI] [PubMed] [Google Scholar]
- E9.Lehnert R, Pletz M, Reuss A, Schaberg T. Antiviral medications in seasonal and pandemic influenza—a systematic review. Dtsch Arztebl Int. 2016;113:799–807. doi: 10.3238/arztebl.2016.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Aliberti S, Ramirez J, Cosentini R, et al. Acute myocardial infarction versus other cardiovascular events in community-acquired pneumonia. ERJ open research. 2015;1 doi: 10.1183/23120541.00020-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Falcone M, Russo A, Cangemi R, et al. Lower mortality rate in elderly patients with community-onset pneumonia on treatment with aspirin. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001595. e001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- E13.Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia A prospective cohort. Am J Respir Crit Care Med. 2015;192:597–604. doi: 10.1164/rccm.201501-0140OC. [DOI] [PubMed] [Google Scholar]
- E14.Bassim CW, Gibson G, Ward T, Paphides BM, Denucci DJ. Modification of the risk of mortality from pneumonia with oral hygiene care. J Am Geriatr Soc. 2008;56:1601–1607. doi: 10.1111/j.1532-5415.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- E15.Almirall J, Rofes L, Serra-Prat M, et al. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur Respir J. 2013;41:923–928. doi: 10.1183/09031936.00019012. [DOI] [PubMed] [Google Scholar]