Abstract
Objective
MicroRNAs (miRNAs) play an important role in the pathogenesis of autoimmune diseases such as primary Sjögren’s syndrome (pSS). This study is the first to investigate miRNA expression patterns in purified T and B lymphocytes from patients with pSS using a high-throughput quantitative PCR (qPCR) approach.
Methods
Two independent cohorts of both patients with pSS and controls, one for discovery and one for replication, were included in this study. CD4+ T cells and CD19+ B cells were isolated from peripheral blood mononuclear cells by magnetic microbeads and expression of miRNAs was profiled using the Exiqon Human miRNome panel I analysing 372 miRNAs. A selection of differentially expressed miRNAs was replicated in the second cohort using specific qPCR assays.
Results
A major difference in miRNA expression patterns was observed between the lymphocyte populations from patients with pSS and controls. In CD4 T lymphocytes, hsa-let-7d-3p, hsa-miR-155–5 p, hsa-miR-222–3 p, hsa-miR-30c-5p, hsa-miR-146a-5p, hsa-miR-378a-3p and hsa-miR-28–5 p were significantly differentially expressed in both the discovery and the replication cohort. In B lymphocytes, hsa-miR-378a-3p, hsa-miR-222–3 p, hsa-miR-26a-5p, hsa-miR-30b-5p and hsa-miR-19b-3p were significantly differentially expressed. Potential target mRNAs were enriched in disease relevant pathways. Expression of B-cell activating factor (BAFF) mRNA was inversely correlated with the expression of hsa-miR-30b-5p in B lymphocytes from patients with pSS and functional experiments showed increased expression of BAFF after inhibiting hsa-miR-30b-5p.
Conclusions
This study demonstrates major miRNAs deregulation in T and B cells from patients with pSS in two independent cohorts, which might target genes known to be involved in the pathogenesis of pSS.
Keywords: Sjögren’s syndrome, microRNAs, CD4+ T lymphocytes, CD19+ B lymphocytes, epigenetics
Introduction
Primary Sjögren’s syndrome (pSS), also referred to as autoimmune epithelitis, is a complex systemic autoimmune disease affecting 0.01% to 0.3% of the general population.1 2 Lymphoid infiltration of lacrimal and salivary glands leading to xerophthalmia and xerostomia, as well as enhanced activation of polyclonal B lymphocytes, represent the hallmarks of the disease. In spite of progress in the past 10 years, the pathogenesis of the disease remains largely to be elucidated.3
Genome-wide association studies (GWAS) have identified genetic variation in and outside the major histocompatibility complex/human leukocyte antigen (MHC/HLA) region associated with pSS.4–6 Recent studies also demonstrated the key role of epigenetic regulation in the pathogenesis.7–9 Interestingly, a number of the differentially methylated regions overlapped with the loci identified in the GWAS. Furthermore, many differentially methylated genes were found in CD19+ B lymphocytes, while only few changes were present in CD4+ T lymphocytes from patients with pSS,7 emphasising the importance of using purified cell population for epigenetic studies.
MicroRNAs (miRNAs) are evolutionarily conserved key players for cellular and developmental processes in eukaryotic organisms and regulate gene expression mainly at the post-transcriptional level.10 The effect of miRNAs on gene regulation may occur either through direct mRNA degradation or preventing mRNA from being translated, depending on the presence of a complete or incomplete match between the miRNA and the 3′untranslated region (UTR) of the target mRNA sequence. MiRNAs play important roles in immune tolerance and prevention of autoimmunity.11 Differential miRNA expression patterns have been demonstrated in different autoimmune diseases including pSS,11 12 analysing either salivary glands13 or peripheral blood mononuclear cells (PBMCs).14–16 However, these results may be flawed by the analysis of a heterogeneous population of cells that might differ in their composition between patients with pSS and controls. So far, only one study has been performed investigating miRNA expression patterns in purified peripheral blood cell population from patients with pSS, which has focused on CD14+ monocytes.17
Due to the previously reported large differences of DNA methylation patterns in T and B cells from patients with pSS,7 we investigated miRNA expression profiles in sorted T and B cells using a quantitative PCR (qPCR)-based profiling method, which has recently been identified as the most accurate and sensitive qPCR-based method for the analysis of miRNAs18 and validated differentially expressed miRNAs in a second independent cohort in each cell population.
Patients and methods
Patients
Two independent cohorts of female patients with pSS and controls were included in this study. Patients with pSS and age and ethnicity-matched controls recruited in a tertiary national reference centre for pSS in France were included in this study. The discovery cohort comprised 17 Caucasian patients with pSS (sufficient material available for 17 patients for T cells and 16 for B cells) and 15 age and ethnicity-matched controls (sufficient material available for 15 patients for T cells and 12 for B cells). The replication cohort comprised 27 Caucasian patients with pSS (sufficient material available for 27 patients for T cells and 25 for B cells) and 12 age and ethnicity-matched controls (sufficient material available for 12 patients for T cells and 12 for B cells). The characteristics and clinical features of the patients are shown in table 1. Anti-SSA and SBB autoantibody status was assessed in both patients and controls, and controls were all negative for these autoantibodies.
Table 1.
Characteristics of the analysed cohorts with primary Sjögren’s syndrome (pSS) and healthy controls
| Discovery cohort 1 | Replication cohort 2 | |||
| Patients with pSS (n=17) | Controls (n=15) | Patients with pSS (n=27) | Controls (n=12) | |
| Age, mean±SD years | 56.4±16 | 60.8±16.5 | 64.2±13.4 | 65.6±16.9 |
| Female sex (%) | 100 | 100 | 100 | 100 |
| Negative anti-SSA and anti-SSB (%) | 7 (41.2%) | 100 | 6 (22.2%) | 100 |
| Positive anti-SSA (%) | 10 (58.8%) | – | 21 (77.8%) | – |
| Positive anti-SSA and anti-SSB (%) | 5 (29.4%) | – | 14 (51.9%) | – |
| ESSDAI, mean±SD (range) | 3.8±8.2 (0–19) | – | 6.4±7.2 (0–29) | – |
| Treatments | ||||
| Methotrexate (15 mg/week) (%) | 2 (11.7%) | – | 3 (11.1%) | – |
| Hydroxychloroquine (400 mg/day) (%) | 5 (29.4%) | – | 9 (33.3%) | – |
ESSDAI, EULAR Sjögren’s Syndrome Disease Activity Index.
All patients with pSS fulfilled the American European Consensus Group 2002 criteria for the disease.19 Activity of the disease was assessed by the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) score.20 Controls were either healthy subjects or patients suffering from a mechanical rheumatologic condition (back pain, sciatica or osteoarthritis) without any sign of autoimmunity nor cancer nor inflammation. The study was approved by the local ethics committee (CCP Ile de France VII No. CO-10–003), and informed written consent was obtained from all patients and controls.
Cell isolation and RNA preparation
T and B lymphocytes were purified from PBMCs by direct magnetic labelling with CD4 and CD19 microbeads (Miltenyi Biotec, Paris, France), and their purity was analysed on a BD FACSCanto (BD Biosciences, San Jose, California, USA) and confirmed to be higher than 95% in all cases. RNA was extracted using the miRNeasy mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol.
Analysis of microRNA and gene expression by real-time qPCR
MicroRNA expression was analysed using the Human miRNome panel I (Version 3, Exiqon, Vedbaek, Denmark) in the discovery cohort and a custom Pick & Mix panel for the validation cohort. MiRNAs with a p value of less than 0.05 (two-tailed t-test) between patients with pSS and controls were considered to be significantly differentially expressed in each cohort.
Expression of BAFF by real-time qPCR
Amplification primers for BAFF were BAFF, 5’ -TGAAACACCAACTATACAAAAAG-3’ and 5’ -TCAATTCATCCCCAAAGACAT-3’. ATP5B, CYC1 and EIF4A2 were used for normalisation. The relative expression of hsa-miR-30b-5p and BAFF was calculated using the ∆∆Cq method compared with the expression of the reference miRNAs or genes, respectively.21 Functional impact of hsa-miR30b-5p on BAFF expression was evaluated transfecting THP-1 cells (American Type Culture Collection (ATCC)) with a 3`-fluorescein labelled miRCURY LNA Power microRNA inhibitor (Exiqon) or a fluorescent miRNA inhibitor control (scrambled sequence), isolation of transfected cells by fluorescence-activated cell sorting (FACS) followed by RNA isolation and expression analysis of BAFF.
More details on experimental procedures and data analysis are given in online supplementary material material.
annrheumdis-2017-211417supp001.docx (35KB, docx)
Results
In this study, we analysed the expression level of 372 miRNAs in purified blood cell population from patients with Sjögren’s syndrome and controls and validated our findings in an independent replication cohort. In T cells from the discovery cohort, 203±9 miRNAs could be reliably detected, while in B cells 200±13 miRNAs were detectable.
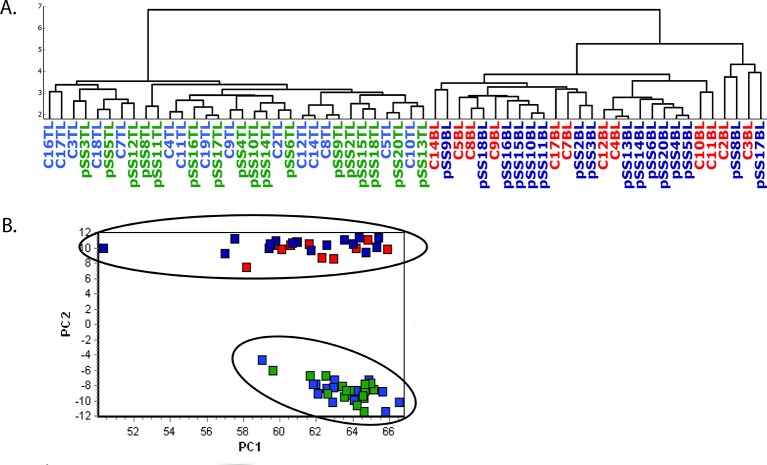
Following normalisation using the global mean expression of all valid miRNAs,22 hierarchical clustering and principal component analysis of the miRNA expression patterns in T and B lymphocytes of all patients and control samples were performed (figure 1). There was a major difference in miRNA expression patterns between the two lymphocyte populations from both patients with pSS and controls separating the samples into major clusters. Inside each cell population, no clustering of patients with pSS and controls was observed. Similar results were also observed using the normalisation approach based on reference miRNAs (data not shown).
Figure 1.
Hierarchical clustering analysis (A) and principal component analysis (B) of microRNAs detected by quantitative PCR (Cq <37). pSS: patient with primary Sjögren’s syndrome, C: control, TL: CD4+ T lymphocytes, BL: CD19+ B lymphocytes, light blue: control T cells; green: pSS T cells; red: control B cells; dark blue: pSS B cells.
Differentially expressed miRNAs in T and B lymphocytes from patients with pSS compared with controls
In total, 21 miRNAs including 9 upregulated and 12 downregulated miRNAs were found significantly differentially expressed in T cells from patients with pSS compared with controls by both normalisation methods (table 2). In B lymphocytes, 24 differentially expressed miRNAs were identified reproducibly by the two different normalisation methods, of which 11 miRNAs were upregulated and 13 miRNAs were downregulated (table 3). Differentially expressed miRNAs did not seem to be regulated through DNA methylation at the promoters of the miRNAs (online supplementary material). To avoid potential confounding through medication, we reanalysed the data excluding patients under methotrexate or hydroxychloroquine treatment. Results confirmed the overall fold changes and significantly expressed miRNAs with the exception of miR-22–3 p and let-7i-5p in T cells and miR-142–5 p, miR-151a-5p, miR-502–5 p and miR-484 in B cells, which were no longer significant.
Table 2.
Differentially expressed miRNAs with p value less than 0.05 in T cells from patients with pSS compared with the controls in the discovery cohort (two different normalisation ways were used: global mean and reference miRNAs)
| (pSS TL) vs (ctrl TL)/global mean normalisation | (pSS TL) vs (ctrl TL)/reference miRNAs normalisation | Common miRNAs/global mean and reference miRNAs | ||||
| miRNAs | Fold change | p Value | miRNAs | Fold change | p Value | |
| hsa-let-7d-3p | −2.53 | 5.07E-07 | hsa-let-7d-3p | −2.58 | 1.97E-07 | hsa-let-7d-3p |
| hsa-miR-28–5 p | 1.36 | 3.78E-05 | hsa-miR-28–5 p | 1.34 | 3.81E-04 | hsa-miR-28–5 p |
| hsa-miR-378a-3p | −1.33 | 7.32E-04 | hsa-miR-378a-3p | −1.36 | 1.15E-04 | hsa-miR-378a-3p |
| hsa-miR-26a-5p | −1.28 | 9.50E-04 | hsa-miR-26a-5p | −1.30 | 1.23E-03 | hsa-miR-26a-5p |
| hsa-miR-425–3 p | −1.33 | 1.04E-03 | hsa-miR-425–3 p | −1.36 | 1.09E-03 | hsa-miR-425–3 p |
| hsa-miR-486–5 p | 1.56 | 6.45E-03 | hsa-miR-486–5 p | 1.53 | 0.013 | hsa-miR-486–5 p |
| hsa-miR-181c-5p | 1.43 | 7.33E-03 | hsa-miR-181c-5p | 1.40 | 0.022 | hsa-miR-181c-5p |
| hsa-miR-30b-5p | −1.17 | 0.010 | hsa-miR-30b-5p | −1.19 | 0.024 | hsa-miR-30b-5p |
| hsa-miR-140–3 p | 1.14 | 0.013 | hsa-miR-140–3 p | 1.12 | 0.043 | hsa-miR-140–3 p |
| hsa-miR-296–5 p | 1.27 | 0.013 | hsa-miR-296–5 p | 1.25 | 0.044 | hsa-miR-296–5 p |
| hsa-miR-30c-5p | −1.12 | 0.014 | hsa-miR-30c-5p | −1.14 | 8.19E-03 | hsa-miR-30c-5p |
| hsa-miR-155–5 p | 1.30 | 0.014 | hsa-miR-155–5 p | 1.27 | 0.013 | hsa-miR-155–5 p |
| hsa-miR-31–3 p | −1.43 | 0.015 | hsa-miR-31–3 p | −1.46 | 0.017 | hsa-miR-31–3 p |
| hsa-miR-598 | −1.31 | 0.015 | hsa-miR-598 | −1.34 | 0.017 | hsa-miR-598 |
| hsa-miR-222–3 p | 1.22 | 0.018 | hsa-miR-222–3 p | 1.19 | 0.046 | hsa-miR-222–3 p |
| hsa-miR-18a-3p | 1.37 | 0.028 | hsa-miR-18a-3p | 1.34 | 0.043 | hsa-miR-18a-3p |
| hsa-let-7e-5p | −1.28 | 0.032 | hsa-let-7e-5p | −1.30 | 0.020 | hsa-let-7e-5p |
| hsa-miR-194–5 p | 1.13 | 0.036 | ||||
| hsa-miR-361–3 p | 1.32 | 0.037 | ||||
| hsa-miR-152 | 1.32 | 0.037 | ||||
| hsa-miR-374a-5p | −1.21 | 0.037 | hsa-miR-374a-5p | −1.24 | 0.041 | hsa-miR-374a-5p |
| hsa-miR-22–3 p | 1.34 | 0.038 | ||||
| hsa-miR-665 | −1.42 | 0.039 | hsa-miR-665 | −1.44 | 0.029 | hsa-miR-665 |
| hsa-let-7g-5p | 1.10 | 0.039 | ||||
| hsa-let-7i-5p | 1.12 | 0.040 | ||||
| hsa-miR-107 | 1.18 | 0.041 | ||||
| hsa-miR-27b-3p | −1.27 | 0.041 | hsa-miR-27b-3p | −1.30 | 0.034 | hsa-miR-27b-3p |
| hsa-miR-150–5 p | 1.15 | 0.045 | ||||
| hsa-miR-16–5 p | 1.14 | 0.045 | hsa-miR-16–5 p | 1.12 | 0.037 | hsa-miR-16–5 p |
| hsa-miR-454–3 p | 1.15 | 0.045 | ||||
| hsa-miR-324–3 p | 1.12 | 0.047 | ||||
| hsa-miR-23b-3p | −1.22 | 0.038 | ||||
| hsa-miR-505–3 p | −1.36 | 0.044 | ||||
| hsa-miR-766–3 p | −1.17 | 0.029 | ||||
‘MiRNAs in grey’ represent those found differentially expressed by one way of normalisation only. Nominal p values without correction for multiple comparisons are shown.
miRNA, microRNA; pSS, primary Sjögren’s syndrome; TL, T lymphocytes.
Table 3.
Differentially expressed miRNAs (p value<0.05) in B cells from patients with pSS compared with the controls in the discovery cohort (two different normalisation ways were used: global mean and reference miRNAs)
| (pSS BL) vs (ctrl BL)/global mean normalisation | (pSS BL) vs (ctrl BL)/reference miRNAs normalisation | Common miRNAs/global mean and reference miRNAs | ||||
| miRNAs | Fold change | p Value | miRNAs | Fold change | p Value | |
| hsa-miR-30b-5p | −1.52 | 4.08E-04 | hsa-miR-30b-5p | −1.56 | 5.01E-03 | hsa-miR-30b-5p |
| hsa-miR-222–3 p | 1.46 | 4.16E-04 | hsa-miR-222–3 p | 1.43 | 2.29E-04 | hsa-miR-222–3 p |
| hsa-miR-107 | 1.35 | 1.07E-03 | hsa-miR-107 | 1.32 | 2.35E-03 | hsa-miR-107 |
| hsa-miR-18a-3p | 1.94 | 1.79E-03 | hsa-miR-18a-3p | 1.89 | 3.51E-03 | hsa-miR-18a-3p |
| hsa-miR-324–5 p | 1.64 | 1.81E-03 | hsa-miR-324–5 p | 1.6 | 2.60E-03 | hsa-miR-324–5 p |
| hsa-miR-151a-3p | 1.4 | 1.96E-03 | hsa-miR-151a-3p | 1.37 | 4.71E-03 | hsa-miR-151a-3p |
| hsa-miR-324–3 p | 1.22 | 2.21E-03 | hsa-miR-324–3 p | 1.19 | 0.026 | hsa-miR-324–3 p |
| hsa-miR-19b-3p | −1.39 | 3.35E-03 | hsa-miR-19b-3p | −1.43 | 0.014 | hsa-miR-19b-3p |
| hsa-miR-26a-5p | −1.34 | 5.38E-03 | hsa-miR-26a-5p | −1.37 | 0.016 | hsa-miR-26a-5p |
| hsa-miR-20a-5p | −1.17 | 5.81E-03 | hsa-miR-20a-5p | −1.2 | 0.014 | hsa-miR-20a-5p |
| hsa-miR-141–3 p | −1.86 | 6.54E-03 | hsa-miR-141–3 p | −1.9 | 5.64E-03 | hsa-miR-141–3 p |
| hsa-miR-29b-2–5 p | 1.39 | 7.51E-03 | hsa-miR-29b-2–5 p | 1.35 | 0.010 | hsa-miR-29b-2–5 p |
| hsa-miR-374a-5p | −1.54 | 8.90E-03 | hsa-miR-374a-5p | −1.58 | 0.017 | hsa-miR-374a-5p |
| hsa-miR-484 | 1.45 | 0.012 | hsa-miR-484 | 1.41 | 0.019 | hsa-miR-484 |
| hsa-miR-195–5 p | −1.57 | 0.012 | hsa-miR-195–5 p | −1.61 | 0.019 | hsa-miR-195–5 p |
| hsa-miR-210 | 1.33 | 0.012 | hsa-miR-210 | 1.3 | 0.039 | hsa-miR-210 |
| hsa-miR-32–5 p | −1.63 | 0.013 | hsa-miR-32–5 p | −1.67 | 0.027 | hsa-miR-32–5 p |
| hsa-miR-582–5 p | −1.67 | 0.014 | hsa-miR-582–5 p | −1.72 | 0.018 | hsa-miR-582–5 p |
| hsa-let-7d-3p | −1.65 | 0.022 | hsa-let-7d-3p | −1.7 | 8.83E-03 | hsa-let-7d-3p |
| hsa-miR-378a-3p | −1.35 | 0.027 | hsa-miR-378a-3p | −1.38 | 0.020 | hsa-miR-378a-3p |
| hsa-miR-505–3 p | −1.59 | 0.027 | hsa-miR-505–3 p | −1.63 | 0.020 | hsa-miR-505–3 p |
| hsa-miR-30c-5p | −1.17 | 0.028 | ||||
| hsa-miR-491–5 p | 1.57 | 0.029 | hsa-miR-491–5 p | 1.53 | 0.036 | hsa-miR-491–5 p |
| hsa-miR-502–5 p | 1.74 | 0.029 | hsa-miR-502–5 p | 1.69 | 0.048 | hsa-miR-502–5 p |
| hsa-miR-151a-5p | 1.2 | 0.035 | ||||
| hsa-miR-148b-3p | −1.23 | 0.038 | hsa-miR-148b-3p | −1.26 | 0.012 | hsa-miR-148b-3p |
| hsa-miR-130b-3p | 1.47 | 0.041 | ||||
| hsa-miR-15a-5p | −1.21 | 0.043 | ||||
| hsa-miR-29b-3p | −1.48 | 0.044 | ||||
| hsa-miR-296–5 p | 1.64 | 0.044 | ||||
| hsa-miR-142–5 p | −1.53 | 0.046 | ||||
| hsa-miR-192–5 p | −1.28 | 0.048 | ||||
| hsa-miR-374b-5p | −1.38 | 0.049 | ||||
‘MiRNAs in grey’ represents those found differentially expressed by one way of normalisation only. Nominal p-values without correction for multiple comparisons are shown.
BL, B lymphocytes; miRNA, microRNA; pSS, primary Sjögren’s syndrome.
Pathway analysis
Analysing the differentially expressed miRNAs in T cells from patients with pSS identified by both normalisation methods, pathway in cancers (p=8.27×10−37), Phosphatidylinositol-3-kinase - Protein Kinase B (PI3K-Akt) signalling pathway (p=1.11×10−36) and transforming growth factor (TGF)-beta signalling pathway (p=1.68×10−28) were found as the top canonical pathways. In B lymphocytes, the Wnt signalling pathway (p=3.24×10−35), pathway in cancers (p=2.41×10−32) and PI3K-Akt signalling pathway (p=1.23×10−31) were identified as the top canonical pathways. MiRNAs contributing to the enrichment are shown in online supplementary tables S1 and S2.
annrheumdis-2017-211417supp002.docx (378KB, docx)
Expression of miRNAs depending on the presence of anti-SSA autoantibodies
As the presence of autoantibodies is a major factor for the presence of altered DNA methylation profiles in B cells from patients with pSS,7 we investigated whether there was a correlation of deregulated miRNAs expression with the presence of autoantibodies using the global mean normalised data. Patients were divided based on the production of anti-SSA autoantibodies into two subgroups: anti-SSA− patients, anti-SSA+ patients. In T cells, 22 miRNAs were found differentially expressed in patients with anti-SSA+ pSS compared with controls, including 14 downregulated and eight upregulated miRNAs; 16 miRNAs were found differentially expressed in anti-SSA− patients, among which six miRNAs (hsa-let-7d-3p, hsa-miR-378a-3p, hsa-miR-28–5 p, hsa-miR-425–3 p, hsa-miR-486–5 p and hsa-miR-31–3 p) were also found in anti-SSA+ patients with pSS (online supplementary table S3). However, in B cells 3.5 times more deregulated miRNAs were found in seropositive patients with pSS (41 miRNAs) compared with controls than those in seronegative patients with pSS (12 miRNAs), including hsa-miR-30b-5p, hsa-miR-222–3 p, hsa-miR-151a-3p, hsa-miR-18a-3p, hsa-miR-107, hsa-miR-324–3 p and hsa-miR-582–5 p that were found in both anti-SSA− and anti-SSA+ patients (online supplementary table S4). When anti-SSA-positive and negative patients with pSS were directly compared, 10 miRNAs were found downregulated and 15 miRNAs were found upregulated in B cells from patients with anti-SSA+ pSS (online supplementary table S5).
As among the 17 patients with pSS included in the discovery cohort of this study, only 3 patients with pSS had a moderate or high ESSDAI score (≥5), we could not reliably investigate in this cohort any association between miRNA expression and disease activity. Similarly only four patients were positive for anti-SSB+ antibodies precluding a statistical analysis on the influence of SSB autoantibodies on miRNA expression profiles.
Replication of differentially expressed miRNAs
Eleven and nine differentially expressed miRNAs in T cells and B cells, respectively (online supplementary table S6) identified by the screen using the human panel I in the discovery cohort were tested in a new independent replication cohort of 27 patients and 12 controls as well as in the samples of the discovery cohort using a custom-designed Pick-&-Mix microRNA PCR Panel. We added in the replication hsa-miR-146a-5p, which was close to statistical significance in the discovery cohort (p=0.07), since it has been reported as deregulated in other pSS studies analysing mainly PBMCs.14–16 23 As shown in table 4, hsa-let-7d-3p, hsa-miR-155–5 p, hsa-miR-222–3 p, hsa-miR-30c-5p, hsa-miR-146a-5p, hsa-miR-378a-3p and hsa-miR-28–5 p were confirmed to be differentially expressed in T cells from both the discovery and the replication cohort (online supplementary figure S1). Hsa-miR-378a-3p, hsa-miR-222–3 p, hsa-miR-26a-5p, hsa-miR-30b-5p and hsa-miR-19b-3p were confirmed to be significantly deregulated in the B cells in both cohorts (online supplementary figure S2). When performing a combined analysis on both cohorts, all miRNAs except one for B and one for T cells showed significant expression differences when compared with controls (table 4). When focusing only on differences between anti-SSA-positive patients and controls, the statistical significance of the six validated miRNAs in B cells increased by at least on order of magnitude for all six miRNAs, but none of the other miRNAs included in the validation step reached statistical significance (data not shown). Restricting the analyses to anti-SSA-positive patients had little impact on the results in T cells.
Table 4.
Validation of the differentially expressed miRNAs in T and B cells by quantitative PCR
| Discovery cohort | Replication cohort | Combined analysis | ||||
| miRNAs | Fold change | p Value | miRNAs | p Value | miRNAs | p Value |
| T cells | ||||||
| hsa-let-7d-3p | −2.36 | 5.58×10−7 | hsa-let-7d-3p | 0.008 | hsa-let-7d-3p | 1.32×10−8 |
| hsa-miR-28-5p | 1.14 | 0.014 | hsa-miR-28-5p | 0.030 | hsa-miR-28-5p | 6.23×10−4 |
| hsa-miR-378a-3p | −1.50 | 9.93×10−5 | hsa-miR-378a-3p | 0.029 | hsa-miR-378a-3p | 9.94×10−6 |
| hsa-miR-26a-5p | −1.15 | 0.075 | hsa-miR-26a-5p | 0.046 | hsa-miR-26a-5p | 0.008 |
| hsa-miR-30b-5p | −1.19 | 0.036 | hsa-miR-30b-5p | 0.378 | hsa-miR-30b-5p | 0.026 |
| hsa-miR-140-3p | 1.13 | 0.018 | hsa-miR-140-3p | 0.389 | hsa-miR-140-3p | 0.018 |
| hsa-miR-30c-5p | −1.10 | 0.019 | hsa-miR-30c-5p | 0.019 | hsa-miR-30c-5p | 0.006 |
| hsa-miR-155-5p | 1.31 | 0.004 | hsa-miR-155-5p | 0.009 | hsa-miR-155-5p | 2.17×10−4 |
| hsa-miR-31-3p | −1.44 | 0.043 | hsa-miR-31-3p | 0.312 | hsa-miR-31-3p | 0.011 |
| hsa-miR-222-3p | 1.22 | 0.025 | hsa-miR-222-3p | 0.009 | hsa-miR-222-3p | 6.60×10−4 |
| hsa-miR-16-5p | 1.18 | 7.26×10−4 | hsa-miR-16-5p | 0.829 | hsa-miR-16-5p | 0.669 |
| hsa-miR-146a-5p | 1.30 | 0.017 | hsa-miR-146a-5p | 0.021 | hsa-miR-146a-5p | 0.001 |
| B cells | ||||||
| hsa-miR-30b-5p | −1.59 | 0.003 | hsa-miR-30b-5p | 0.012 | hsa-miR-30b-5p | 4.16×10−5 |
| hsa-miR-222-3p | 1.32 | 0.002 | hsa-miR-222-3p | 0.005 | hsa-miR-222-3p | 1.62×10−5 |
| hsa-miR-18a-3p | 1.28 | 0.027 | hsa-miR-18a-3p | 0.137 | hsa-miR-18a-3p | 0.613 |
| hsa-miR-19b-3p | −1.38 | 0.012 | hsa-miR-19b-3p | 0.028 | hsa-miR-19b-3p | 4.87×10−4 |
| hsa-miR-26a-5p | −1.37 | 0.013 | hsa-miR-26a-5p | 0.006 | hsa-miR-26a-5p | 9.85×10−5 |
| hsa-miR-20a-5p | −1.14 | 0.082 | hsa-miR-20a-5p | 0.019 | hsa-miR-20a-5p | 0.002 |
| hsa-miR-32-5p | −1.75 | 0.014 | hsa-miR-32-5p | 0.416 | hsa-miR-32-5p | 0.038 |
| hsa-let-7d-3p | −2.03 | 2.12×10−4 | hsa-let-7d-3p | 0.452 | hsa-let-7d-3p | 0.024 |
| hsa-miR-378a-3p | −1.29 | 0.062 | hsa-miR-378a-3p | 3.11×10−4 | hsa-miR-378a-3p | 5.49×10−6 |
| hsa-miR-146a-5p | −1.35 | 0.024 | hsa-miR-146a-5p | 0.157 | hsa-miR-146a-5p | 0.008 |
Differentially expressed miRNAs found in the discovery cohort and in the replication cohort are shown. MiRNAs in black depict those with a p value<0.05 and miRNAs in grey depict those with p value>0.05. Nominal p values without correction for multiple comparisons are shown.
miRNA, microRNA; pSS, primary Sjögren’s syndrome.
Expression of BAFF is inversely correlated with the expression of hsa-miR-30b-5p in B cells from patients with pSS
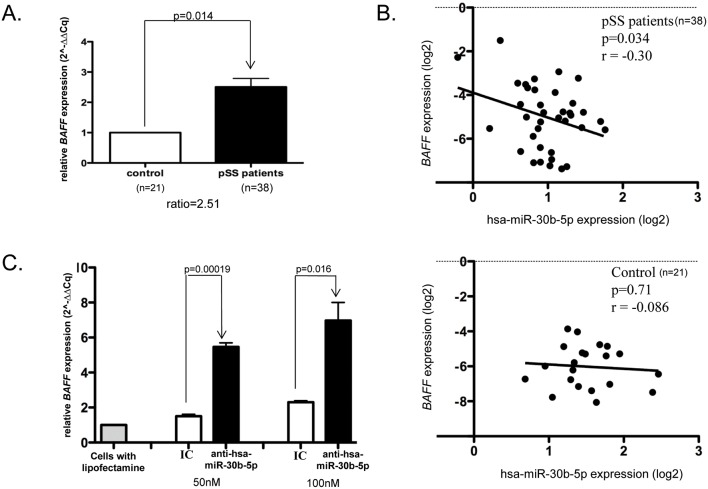
The BAFF has been reported upregulated in B lymphocytes infiltrating the salivary glands of patients with pSS24 and hsa-miR-30a-3p, another member of the hsa-miR-30 family, has been reported to negatively regulate BAFF expression in systemic sclerosis and fibroblasts from patients with rheumatoid arthritis.25 As we identified hsa-miR-30b-5p to be significantly downregulated in our study and a potential binding site for hsa-miR-30b-5p in the 3′UTR of BAFF was identified (online supplementary figure S3), we further investigated the expression of BAFF and a possible correlation between the expression of hsa-miR-30b-5p and BAFF in B cells from patients with pSS. The expression of BAFF was increased in peripheral B cells from patients with pSS compared with controls (figure 2A). In contrast to controls, in which no correlation between the expression of hsa-miR-30b-5p and BAFF was observed, there was a significant inverse correlation between the expression of hsa-miR-30b-5p and BAFF in B cells from patients with pSS (figure 2B). Transfection of THP-1 cells with an antagomir (miRNA inhibitor) for hsa-miR-30b-5p led to a strong increase in BAFF expression compared with a fluorescent miRNA inhibitor control (scrambled sequence) (figure 2C), providing further evidence that the loss of hsa-miR-30b-5p could contribute to the increased expression of BAFF in patients with pSS.
Figure 2.
Regulation of BAFF by hsa-miR-30b-5p. Expression of BAFF (A), inverse correlation with hsa-miR-30b-5p expression (B) in B cells from patients with pSS (n=38) and controls (n=21) and upregulation of BAFF after transfection with an anti-hsa-miR-30b-5p compared with a fluorescent miRNA inhibitor control in the THP-1 cell line (C). Analysis of BAFF expression was performed in triplicate for each patient. Correlation was assessed using the Pearson coefficient. Transfection experiments with 50 or 100 nM of hsa-miR-30b-5p inhibitor or the control inhibitor were performed in triplicate and a t-test was used for significance analysis in all presented analyses. Expression levels were normalised to control cells cultured in parallel exposed to lipofectamine RNAiMAX, but no miRNA inhibitor. BAFF, B-cell activating factor; IC, fluorescent miRNA inhibitor control (scrambled sequence); miRNA, microRNA; pSS, primary Sjögren’s syndrome.
Discussion
In this study, large-scale profiling of miRNAs by qPCR showed highly divergent miRNAs expression patterns between T and B lymphocytes from both patients with pSS and controls. These results are in concordance with our previous study of genome-wide DNA methylation patterns.7 In contrast to the DNA methylation data, however, deregulation of miRNAs was not restricted to B cells, but was also present to a similar extent in CD4+ T cells. Several of the miRNAs found in our study overexpressed in T cells have previously been shown to be overexpressed in salivary glands of patients with pSS such as hsa-miR-16, hsa-miR-150, hsa-miR-222, hsa-miR-30b and hsa-miR-324–3 p where the expression level correlated positively with a high focus score and hsa-miR-181a and hsa-miR-155 overexpressed in T cells and associated with reduced salivary flow in the glands.13 Of note, there was little overlap of the differentially expressed miRNAs in the salivary glands and B cells.
Among validated differentially expressed miRNAs, we identified hsa-miR-146a-5p, an important negative regulator of inflammatory responses and whose loss has been shown to cause spontaneous autoimmunity as well as oncogenic transformation.26 Validated targets of this miRNA include IRF5, STAT1 and IRAK1, which have also been found associated with genetic variation or DNA methylation changes in pSS.4 7 Previous reports have shown upregulation of hsa-miR-146a-5p in PBMCs from patients with pSS14–16 23 preceding clinical symptoms.16 Using sorted cell populations, we show that the direction of the observed changes differs in function of the cell type, with an overexpression in T cells and decreased expression in B cells, which has probably previously been masked by the greater proportion of T cells in PBMCs compared with B cells. Expression of has-miR-146a-5p could thus have different impact depending on the cell types in pSS, with the loss promoting the interferon expression in B cells,27 while sustaining inflammation in the T-cell compartment.28 Lineage-specific modifications of the expression level of miR-146a-5p are required to fully understand the effect of this miR on inflammation and the pathogenic interferon response.
Hsa-miR-155–5 p was previously shown to be upregulated in synovial tissue, macrophages, PBMCs and sera from patients with rheumatoid arthritis and in CD4+ cells of mouse models for experimental autoimmune encephalomyelitis,29–32 which we also confirmed in our study. In contrast, miR-155 was previously found downregulated in PBMCs from patients with pSS15 suggesting a confounding effect of cellular composition in the PBMC data. Taken together, the results on these two extremely well studied miRNAs and our findings of highly divergent patterns of miRNAs expression in T and B cells emphasise the importance of using purified cells for epigenetics studies. It will be desirable in the future to analyse even more specific subpopulation of the B and T cells, which might show a more pronounced deregulation of such as naïve or memory B/T cells, plasmablasts or T helper subsets such as follicular T helper cells, which have shown altered distribution in pSS.33 34 Recent analyses highlight the importance of cytotoxic CD8+ cells,35 which have so far not been analysed for changes in miRNA expression or other epigenetic modifications in pSS. Furthermore, while the number of patients treated with hydroxychloroquine or methotrexate was too small in our discovery cohort to make definite conclusions, we identified some miRNAs that were no longer significant after including only treatment-naïve patients suggesting effects of the medication on miRNA expression, which has previously been reported for both hydroxychloroquine36 or methotrexate.37 38
The significantly differentially expressed miRNAs in our study were enriched for several pathways including the PI3K-Akt signalling pathway. The miRNA-17–92 cluster promotes the survival of B lymphocytes, at least partly through its ability to regulate PI3K signalling and genes expressed downstream of this pathway.39 40 Preliminary evidence suggested a downregulation of this cluster in the salivary glands from patients with pSS,41 which was confirmed in our study. Furthermore, hsa-miR-32–5 p, another validated negative regulator of PTEN (phosphatase and tensin homolog gene),42 was also found downregulated in the B cells in our study. Although further functional experiments are required to confirm the targets of the miRNAs in a cell-type specific manner, the PI3K-AKT pathway has recently attracted interest as a therapeutic target in pSS with multicentric randomised phase 2 studies evaluating a PI3 kinase delta inhibitor in pSS (NCT02610543, NCT02775916) ongoing. Modulating miRNA levels might be an additional tool to change the activity of key components of this signalling pathway.
Among the differentially expressed miRNAs, hsa-miR-30b-5p draw our attention as it has been previously reported as among the most significantly differentially expressed miRNAs in minor salivary glands from patients with pSS.13 Furthermore, hsa-miR-30a-3p, an miR of the same family, negatively regulates the expression of BAFF in systemic sclerosis and rheumatoid arthritis fibroblasts.25 BAFF, produced by monocytes, activated T lymphocytes, dendritic cells and epithelial cells in patients with pSS promotes B-cell maturation, proliferation and survival.3 43 44 BAFF levels have been found to be increased in the serum and salivary gland B, T and epithelial cells of patients with pSS compared with controls and correlate with autoantibody production.24 43 45 In the presence of excess levels of BAFF, mice develop manifestations of autoimmune disease with features of SLE and Sjögren’s syndrome and have an increased risk to develop lymphoma at later age.46 On the other hand, blocking of both BAFF and C-X-C motif chemokine ligand (CXCL13) improves salivary gland function.47 The 3′UTR of BAFF is targeted by several miRNAs and genetic variation in the 3′UTR plays a major role in the potential of miRNAs to regulate BAFF expression.48 We confirmed the overexpression of BAFF in the B cells of our cohorts correlating inversely with the expression of hsa-miR-30b-5p. This might be caused by the binding of hsa-miR-30b-5p to the 3′-UTR of BAFF mRNA. This hypothesis was substantiated by our transfection experiments with an inhibitor of hsa-miR-30b-5p leading to a greatly increased BAFF expression. Further studies will be required to confirm the specific binding sites of hsa-miR-30b on the BAFF mRNA as well as to determine the relative importance of hsa-miR-30b compared with the other miRNAs binding to BAFF, as hsa-miR-30b was not tested in a recent study having shown the importance of miRNAs for regulating BAFF expression.48
In summary, we have performed a large-scale analysis of miRNAs in patients with pSS in sorted blood-derived T and B cells and demonstrated cell-type specific miRNA expression patterns, potentially related to the pathophysiology of the disease. Being one of the key molecular changes that initiate pSS development, BAFF was induced by one of the identified miRNAs, hsa-miR-30b-5p.
Acknowledgments
The authors thank Fredric Auvre (IRCM, CEA) and the platform OCCIgen (Organization of Cytometry and Cellular Imaging of GENopole) for help with the cell sorting of transfected cells and Florence Busato (LEE, CNRGH,CEA) for help with graphical representation of the data.
Footnotes
XM and JT contributed equally.
Handling editor: Tore K Kvien
Contributors: JT and XM conceived the study, GN and XM provided clinical data and patient material, SFWR, SB, ER, NS, CD, ABT performed experiments and analysed data, VR performed bioinformatic analyses, SFWR wrote the first draft of the manuscript, JFD, JT and XM critically revised the manuscript. All authors approved the final version of the manuscript.
Funding: The research described in this manuscript was funded by a grant from the French National Research Agency (ANR- 2010-BLAN-1133 01), the Fondation pour la Recherche Medicale (DEQ20150331730 - Sjögren’s syndrome and AutoImmunity associated Lymphomas) and the institutional budget of the CNG.
Competing interests: None declared.
Patient consent: No personalised medical records were used, all data were anonymised. Informed and written consent was obtained from all study participants according to the French law.
Ethics approval: The presented study was approved by the local ethics committee CPP Ile de France VII No. CO-10-003.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Raw data (CT values or processed log-2 fold ratios of miRNA expression data) are available from the corresponding authors upon request.
References
- 1. Bowman SJ, Ibrahim GH, Holmes G, et al. Estimating the prevalence among Caucasian women of primary Sjögren’s syndrome in two general practices in Birmingham, UK. Scand J Rheumatol 2004;33:39–43. 10.1080/03009740310004676 [DOI] [PubMed] [Google Scholar]
- 2. Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:1983–9. 10.1136/annrheumdis-2014-205375 [DOI] [PubMed] [Google Scholar]
- 3. Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren’s syndrome. Nat Rev Rheumatol 2013;9:544–56. 10.1038/nrrheum.2013.110 [DOI] [PubMed] [Google Scholar]
- 4. Lessard CJ, Li H, Adrianto I, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren’s syndrome. Nat Genet 2013;45:1284–92. 10.1038/ng.2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Zhang K, Chen H, et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjögren’s syndrome at 7q11.23. Nat Genet 2013;45:1361–5. 10.1038/ng.2779 [DOI] [PubMed] [Google Scholar]
- 6. Taylor KE, Wong Q, Levine DM, et al. Genome-wide association analysis reveals genetic heterogeneity of Sjögren’s syndrome according to ancestry. Arthritis Rheumatol 2017;69:1294–305. 10.1002/art.40040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miceli-Richard C, Wang-Renault SF, Boudaoud S, et al. Overlap between differentially methylated DNA regions in blood B lymphocytes and genetic at-risk loci in primary Sjögren’s syndrome. Ann Rheum Dis 2016;75:933–40. 10.1136/annrheumdis-2014-206998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altorok N, Coit P, Hughes T, et al. Genome-wide DNA methylation patterns in naive CD4+ T cells from patients with primary Sjögren’s syndrome. Arthritis Rheumatol 2014;66:731–9. 10.1002/art.38264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imgenberg-Kreuz J, Sandling JK, Almlöf JC, et al. Genome-wide DNA methylation analysis in multiple tissues in primary Sjögren’s syndrome reveals regulatory effects at interferon-induced genes. Ann Rheum Dis 2016;75:2029–36. 10.1136/annrheumdis-2015-208659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Rooij E. The art of microRNA research. Circ Res 2011;108:219–34. 10.1161/CIRCRESAHA.110.227496 [DOI] [PubMed] [Google Scholar]
- 11. Garo LP, Murugaiyan G. Contribution of MicroRNAs to autoimmune diseases. Cell Mol Life Sci 2016;73:2041–51. 10.1007/s00018-016-2167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JQ, Papp G, Szodoray P, et al. The role of microRNAs in the pathogenesis of autoimmune diseases. Autoimmun Rev 2016;15:1171–80. 10.1016/j.autrev.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 13. Alevizos I, Alexander S, Turner RJ, et al. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjögren’s syndrome. Arthritis Rheum 2011;63:535–44. 10.1002/art.30131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng L, Ma W, Yi F, et al. MicroRNA profiling in Chinese patients with primary Sjögren syndrome reveals elevated miRNA-181a in peripheral blood mononuclear cells. J Rheumatol 2014;41:2208–13. 10.3899/jrheum.131154 [DOI] [PubMed] [Google Scholar]
- 15. Shi H, Zheng LY, Zhang P, et al. miR-146a and miR-155 expression in PBMCs from patients with Sjögren’s syndrome. J Oral Pathol Med 2014;43:792–7. 10.1111/jop.12187 [DOI] [PubMed] [Google Scholar]
- 16. Pauley KM, Stewart CM, Gauna AE, et al. Altered miR-146a expression in Sjögren’s syndrome and its functional role in innate immunity. Eur J Immunol 2011;41:2029–39. 10.1002/eji.201040757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams AE, Choi K, Chan AL, et al. Sjögren’s syndrome-associated microRNAs in CD14(+) monocytes unveils targeted TGFβ signaling. Arthritis Res Ther 2016;18:95 10.1186/s13075-016-0987-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mestdagh P, Hartmann N, Baeriswyl L, et al. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (miRQC) study. Nat Methods 2014;11:809–15. 10.1038/nmeth.3014 [DOI] [PubMed] [Google Scholar]
- 19. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554–8. 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seror R, Theander E, Brun JG, et al. Validation of EULAR primary Sjögren’s syndrome disease activity (ESSDAI) and patient indexes (ESSPRI). Ann Rheum Dis 2015;74:859–66. 10.1136/annrheumdis-2013-204615 [DOI] [PubMed] [Google Scholar]
- 21. Pfaffl MW. Quantification strategies in real-time PCR, Bustin SA, A-Z of quantitative PCR La Jolla. CA, USA: International University Line, 2004:87–112. [Google Scholar]
- 22. Mestdagh P, Van Vlierberghe P, De Weer A, et al. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol 2009;10:R64 10.1186/gb-2009-10-6-r64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zilahi E, Tarr T, Papp G, et al. Increased microRNA-146a/b, TRAF6 gene and decreased IRAK1 gene expressions in the peripheral mononuclear cells of patients with Sjögren’s syndrome. Immunol Lett 2012;141:165–8. 10.1016/j.imlet.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 24. Daridon C, Devauchelle V, Hutin P, et al. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren’s syndrome. Arthritis Rheum 2007;56:1134–44. 10.1002/art.22458 [DOI] [PubMed] [Google Scholar]
- 25. Alsaleh G, François A, Philippe L, et al. MiR-30a-3p negatively regulates BAFF synthesis in systemic sclerosis and rheumatoid arthritis fibroblasts. PLoS One 2014;9:e111266 10.1371/journal.pone.0111266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boldin MP, Taganov KD, Rao DS, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 2011;208:1189–201. 10.1084/jem.20101823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum 2009;60:1065–75. 10.1002/art.24436 [DOI] [PubMed] [Google Scholar]
- 28. Li J, Wan Y, Guo Q, et al. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res Ther 2010;12:R81 10.1186/ar3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murugaiyan G, Beynon V, Mittal A, et al. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol 2011;187:2213–21. 10.4049/jimmunol.1003952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurowska-Stolarska M, Alivernini S, Ballantine LE, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci U S A 2011;108:11193–8. 10.1073/pnas.1019536108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Filková M, Aradi B, Senolt L, et al. Association of circulating miR-223 and miR-16 with disease activity in patients with early rheumatoid arthritis. Ann Rheum Dis 2014;73:1898–904. 10.1136/annrheumdis-2012-202815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Long L, Yu P, Liu Y, et al. Upregulated microRNA-155 expression in peripheral blood mononuclear cells and fibroblast-like synoviocytes in rheumatoid arthritis. Clin Dev Immunol 2013;2013:1–10. 10.1155/2013/296139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Szabó K, Papp G, Szántó A, et al. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren’s syndrome and systemic lupus erythematosus. Clin Exp Immunol 2016;183:76–89. 10.1111/cei.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verstappen GM, Meiners PM, Corneth OBJ, et al. Abatacept attenuates T follicular helper-cell-dependent B-cell hyperactivity in primary Sjogren’s syndrome. Arthritis & rheumatology 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Tasaki S, Suzuki K, Nishikawa A, et al. Multiomic disease signatures converge to cytotoxic CD8 T cells in primary Sjögren’s syndrome. Ann Rheum Dis 2017;76:1458–66. 10.1136/annrheumdis-2016-210788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chafin CB, Regna NL, Hammond SE, et al. Cellular and urinary microRNA alterations in NZB/W mice with hydroxychloroquine or prednisone treatment. Int Immunopharmacol 2013;17:894–906. 10.1016/j.intimp.2013.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. García-Rodríguez S, Arias-Santiago S, Blasco-Morente G, et al. Increased expression of microRNA-155 in peripheral blood mononuclear cells from psoriasis patients is related to disease activity. J Eur Acad Dermatol Venereol 2017;31:312–22. 10.1111/jdv.13861 [DOI] [PubMed] [Google Scholar]
- 38. Løvendorf MB, Zibert JR, Gyldenløve M, et al. MicroRNA-223 and miR-143 are important systemic biomarkers for disease activity in psoriasis. J Dermatol Sci 2014;75:133–9. 10.1016/j.jdermsci.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 39. Simpson LJ, Ansel KM. MicroRNA regulation of lymphocyte tolerance and autoimmunity. J Clin Invest 2015;125:2242–9. 10.1172/JCI78090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao C, Srinivasan L, Calado DP, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol 2008;9:405–14. 10.1038/ni1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alevizos I, Illei GG. MicroRNAs in Sjögren’s syndrome as a prototypic autoimmune disease. Autoimmun Rev 2010;9:618–21. 10.1016/j.autrev.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu G, Chai J, Ma L, et al. Downregulated microRNA-32 expression induced by high glucose inhibits cell cycle progression via PTEN upregulation and Akt inactivation in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 2013;433:526–31. 10.1016/j.bbrc.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 43. Lavie F, Miceli-Richard C, Quillard J, et al. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjögren’s syndrome. J Pathol 2004;202:496–502. 10.1002/path.1533 [DOI] [PubMed] [Google Scholar]
- 44. Lavie F, Miceli-Richard C, Ittah M, et al. B-cell activating factor of the tumour necrosis factor family expression in blood monocytes and T cells from patients with primary Sjögren’s syndrome. Scand J Immunol 2008;67:185–92. 10.1111/j.1365-3083.2007.02049.x [DOI] [PubMed] [Google Scholar]
- 45. Mariette X, Roux S, Zhang J, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren’s syndrome. Ann Rheum Dis 2003;62:168–71. 10.1136/ard.62.2.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 1999;190:1697–710. 10.1084/jem.190.11.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharma A, Kiripolsky J, Klimatcheva E, et al. Early BAFF receptor blockade mitigates murine Sjögren’s syndrome: Concomitant targeting of CXCL13 and the BAFF receptor prevents salivary hypofunction. Clin Immunol 2016;164:85–94. 10.1016/j.clim.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Steri M, Orrù V, Idda ML, et al. Overexpression of the Cytokine BAFF and Autoimmunity Risk. N Engl J Med 2017;376:1615–26. 10.1056/NEJMoa1610528 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2017-211417supp001.docx (35KB, docx)
annrheumdis-2017-211417supp002.docx (378KB, docx)