Significance
Gαs protein is classically known as a signaling component of cell surface G protein-coupled receptor transduction pathways. However, Gαs has also been localized on endosomes, where it plays a role in receptor sorting to lysosomes. How Gαs regulates this trafficking step is unclear. Although Gαs structure has been known for years, we found a motif in Gαs that allows its interaction with ubiquitin, a key signal for cargo sorting to the lysosomal pathway. We show that disrupting the ubiquitin-interacting motif (UIM) in Gαs impairs its interaction with components of the endosomal sorting machinery and the lysosomal degradation of epidermal growth factor receptor. This study provides molecular insights on the mechanism by which Gαs is involved in this noncanonical function.
Keywords: Gαs, ubiquitin-interacting motif, endosomal sorting complex required for transport, multivesicular bodies, epidermal growth factor receptor
Abstract
The Gαs subunit is classically involved in the signal transduction of G protein-coupled receptors (GPCRs) at the plasma membrane. Recent evidence has revealed noncanonical roles for Gαs in endosomal sorting of receptors to lysosomes. However, the mechanism of action of Gαs in this sorting step is still poorly characterized. Here, we report that Gαs interacts with ubiquitin to regulate the endosomal sorting of receptors for lysosomal degradation. We reveal that the N-terminal extremity of Gαs contains a ubiquitin-interacting motif (UIM), a sorting element usually found in the endosomal sorting complex required for transport (ESCRT) machinery responsible for sorting ubiquitinated receptors into intraluminal vesicles (ILVs) of multivesicular bodies (MVBs). Mutation of the UIM in Gαs confirmed the importance of ubiquitin interaction for the sorting of epidermal growth factor receptor (EGFR) into ILVs for lysosomal degradation. These findings demonstrate a role for Gαs as an integral component of the ubiquitin-dependent endosomal sorting machinery and highlight the dual role of Gαs in receptor trafficking and signaling for the fine-tuning of the cellular response.
Heterotrimeric G proteins are classically known for their role in transducing signals from the extracellular environment across the plasma membrane (1). However, it is becoming increasingly apparent that these signaling molecules also have noncanonical roles in other cellular locations (2). Recent findings have shown the presence of the Gαs subunit on endosomes, where it plays a role in receptor signaling and trafficking (3–5). In fact, some G protein-coupled receptors (GPCRs), in addition to activating Gαs at the plasma membrane, appear to elicit a “second wave” of Gαs activation after ligand-induced internalization in endosomes (6). Along with its endosomal signaling function, Gαs has also been reported to regulate the endosomal trafficking and down-regulation of single and seven transmembrane receptors. Indeed, cellular depletion of Gαs specifically delays the lysosomal degradation of epidermal growth factor receptor (EGFR) and GPCRs (CXCR4 and DOPr) by disrupting their transfer into the intraluminal vesicles (ILVs) of multivesicular bodies (MVBs) (4, 5). However, the precise and likely intricate mechanisms by which Gαs regulates the endosomal sorting of receptors remain unresolved.
The endosomal sorting of receptors to lysosomes is coordinated by the endosomal sorting complex required for transport (ESCRT) machinery, which consists of four distinct complexes, ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III, together with several accessory components (7). The ESCRT complexes act in a sequential and coordinated manner to bind and sort receptors from the early endosome limiting membranes to ILVs of MVBs (7, 8). The MVBs eventually fuse with lysosomes to degrade the internal vesicles and their protein cargo (9). For most receptors (including receptor tyrosine kinases and GPCRs), the covalent attachment of ubiquitin serves as a key sorting signal for entry into the MVB/lysosomal pathway (10, 11). In fact, components of the three first ESCRT complexes (ESCRT-0, ESCRT-I, and ESCRT-II) harbor ubiquitin-binding domains (UBDs) that interact with ubiquitin moieties and specifically catalyze the sorting of ubiquitinated receptors to lysosomes (12, 13). Other contenders for ubiquitin-sorting receptors have also been identified, including eps15b, Tom1, Bro1, and GGA3, which bind ubiquitin and ESCRT components and act conjointly with the ESCRT machinery to sort receptors into MVBs (10, 14).
The fact that Gαs is involved in endosomal sorting of ubiquitinated receptors into MVBs and has been shown to interact with the ESCRT-0 component (4, 5) led us to postulate that Gαs also participates in receptor endosomal sorting through an interaction with ubiquitin. Here, we report seminal data demonstrating that Gαs binds directly to ubiquitin through an N-terminal ubiquitin-interacting motif (UIM), an event that is crucial for Gαs interaction with the ESCRT-0 component and EGFR sorting into ILVs of MVBs for lysosomal degradation. These findings demonstrate that Gαs is an integral component of the ubiquitin-dependent endosomal sorting machinery and provide concrete molecular insights on the molecular mechanism by which Gαs could be involved in this noncanonical endosomal sorting step.
Results and Discussion
Gαs Binds Ubiquitin.
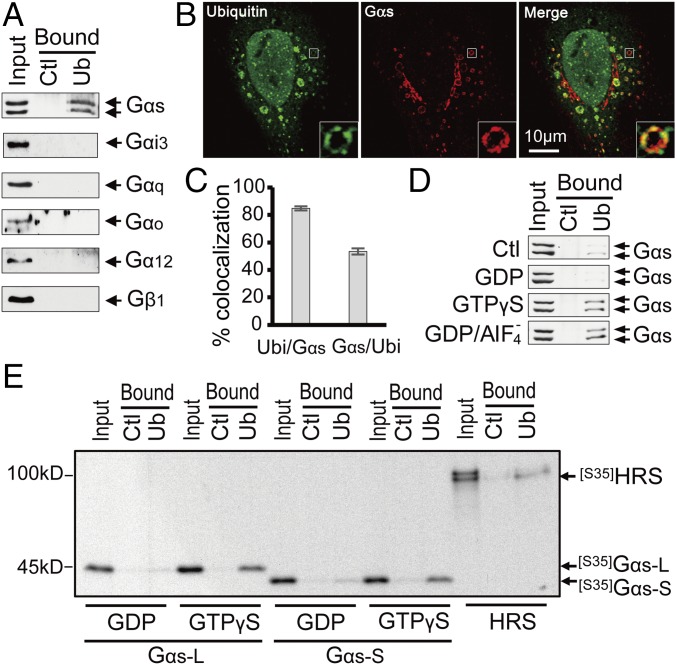
Because ubiquitin is key in the endosomal sorting pathway, we reasoned that Gαs might interact with ubiquitin. To investigate whether human Gαs has the ability to bind ubiquitin, pull-down assays with ubiquitin-agarose beads were performed on extracts of HEK293 cells expressing endogenous Gα proteins. Gαs, but not Gαi3, Gαq, Gαo, Gα12, or Gβ1, was pulled down by ubiquitin beads (Fig. 1A), demonstrating that ubiquitin interacts specifically with Gαs. Consistent with these results, Gαs and ubiquitinated proteins colocalized extensively on Rab5-labeled endosomes (Fig. S1). We next took advantage of the enlarged endosomes produced by expressing Rab5QL, a GTPase-defective mutant of Rab5 (15), to better visualize the spatial relationship of Gαs and ubiquitin on endosomal membranes (Fig. 1B). A strong colocalization between Gαs and conjugated ubiquitin proteins was observed in subdomains of enlarged endosomes (Fig. 1 B and C). Thus, Gαs not only interacts with ubiquitin but also colocalizes with ubiquitinated proteins in microdomains of early endosomes.
Fig. 1.
Gαs interacts directly with ubiquitin and colocalizes with ubiquitinated proteins on endosomal microdomains. (A) Gαs is specifically pulled down by ubiquitin beads. HEK293 cell extracts were incubated with ubiquitin-agarose beads (Ub) or control protein G-agarose beads (Ctl). (B) Gαs colocalizes with ubiquitinated proteins on endosomal microdomains. HeLa cells transfected with Gαs and Rab5-QL (to create enlarged endosomes) were subjected to confocal immunofluorescence microscopy with antibodies specific for ubiquitin and Gαs (also Fig. S1). Insets represent enlarged views of the boxed regions (magnification: 4×). (C) Quantification of the fraction of Gαs colocalizing with ubiquitin (Gαs/Ubi) and the fraction of ubiquitin colocalizing with Gαs (Ubi/Gαs) on enlarged endosomal membranes (as shown in B). Data are represented as the mean ± SEM of n = 53 endosomes. (D) Interaction of Gαs with ubiquitin is altered by Gαs activation status. HEK293 cell extracts pretreated with GDP (to mimic the inactive state) or either GTPγS or GDP/AIF4− (to mimic the active state) were incubated with Ub or Ctl beads (also Fig. S2). (E) Comparison of the direct interaction between ubiquitin and the active and inactive forms of Gαs. In vitro-translated 35S-labeled Gαs short (S) and long (L) forms were pretreated with GDP or GTPγS and incubated with Ub or Ctl beads. HRS, a known ubiquitin-binding protein, served as a positive control.
To determine whether the GTPase activity of Gαs affects its binding with ubiquitin, pull-down assays were performed with HEK293 cell lysates pretreated with GDP (to mimic the inactive state of Gαs) or either GDP/AlF4− or GTPγS (to mimic the active state of Gαs). The interaction between endogenous Gαs and ubiquitin was increased with the latter treatment compared with GDP treatment or no treatment (Fig. 1D). Similar results were obtained using cell lysates expressing constitutively active and inactive Gαs mutants (Fig. S2A), suggesting that ubiquitin binds preferentially to the active form of Gαs. These results were confirmed using 35S-labeled in vitro-translated long or short form of Gαs (Fig. 1E). This in vitro pull-down assay demonstrated that both 35S-labeled Gαs isoforms interact directly with ubiquitin, which was confirmed using purified Gαs proteins (Fig. S2B), and that this interaction is increased with the active (GTPγS-pretreated) form of Gαs (Fig. 1E). Together, these findings suggest that ubiquitin binds directly and preferentially to the active form of Gαs.
The N-Terminal Extremity of Gαs Contains a UIM.
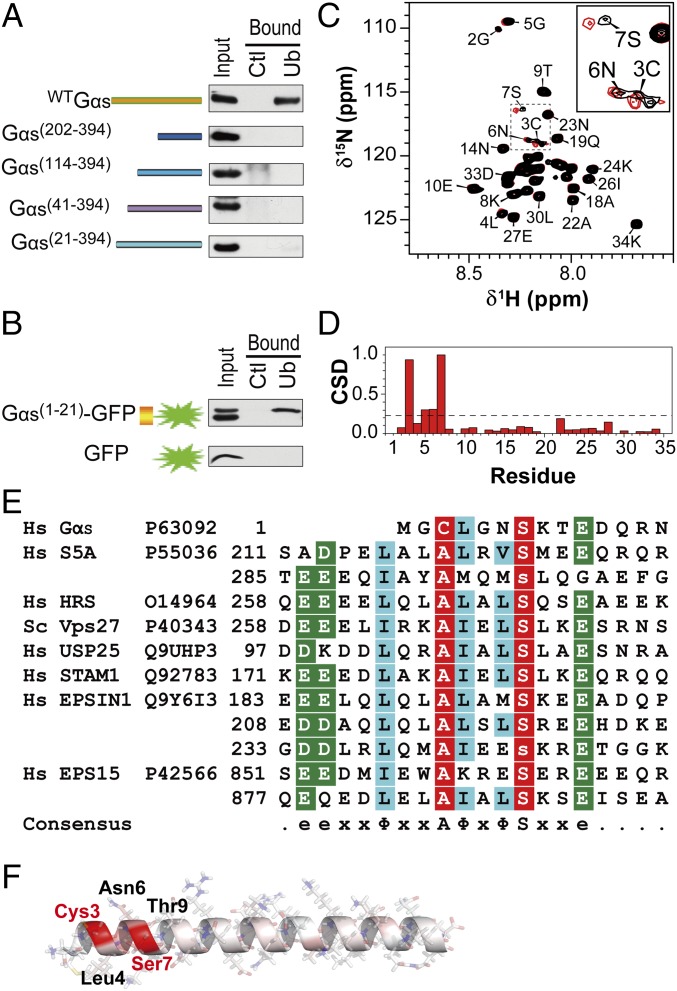
To delineate the ubiquitin-binding region in Gαs, ubiquitin-agarose pull-down assays were performed with a series of truncation mutants of Gαs. Removal of the first 21 residues (Met1 to Glu21) abolished Gαs binding to ubiquitin (Fig. 2A). In concordance, a fusion protein of these 21 residues (Gαs1-21) to GFP enabled it to bind ubiquitin (Fig. 2B), indicating that this region (residues 1–21) of the Gαs N terminus is necessary and sufficient for ubiquitin binding.
Fig. 2.
Gαs contains a UIM. (A) Mapping the Gαs domain responsible for binding to ubiquitin. HEK293 cell extracts expressing various Gαs N-terminal–truncated mutants were incubated with ubiquitin-agarose beads (Ub) or control protein G-agarose beads (Ctl). (B) Gαs N-terminal residues 1–21 are sufficient for ubiquitin binding. Pull-downs were performed on HEK293 cells expressing GFP alone or fused to the 1–21 region of Gαs. (C) NMR chemical shift mapping of the residues of a Gαs1-34 peptide interacting with ubiquitin. The 1H-15N-HSQC spectrum of Gαs1-34 in the absence (black) and presence (red) of ubiquitin at a 1:10 ratio is shown. Fully assigned spectra are shown in Fig. S3A. (D) Normalized weighted CSDs observed in Gαs1-34 upon ubiquitin binding. Significant displacements are assigned to cross-peaks that have CSD values of 1 SD above the mean (dashed line). (E) Alignment of the 1–14 region of Gαs with the sequences of the UIMs found in various ubiquitin-binding proteins (also Fig. S4). e, acidic residue; x, any amino acid; Φ, large hydrophobic residue. Nearly invariant Ala and Ser positions are colored red, and highly similar amino acids are colored blue and green. Hs, Homo sapiens. (F) Structure of Gαs1-34 peptide modeled by Swiss-PdbViewer and SWISS-MODEL, showing in red the residues with the greatest changes in chemical shift upon addition of ubiquitin.
To define this interaction at a residue-specific level, NMR spectroscopy was employed. Given that the addition of flanking residues to ubiquitin-binding regions had been shown to stabilize the conformation of other ubiquitin-binding peptides and increase ubiquitin-binding efficiency (16), a peptide containing residues 1–34 of Gαs (Gαs1-34) was double-labeled with 13C and 15N and used for NMR analysis. The ubiquitin-binding residues of Gαs1-34 were defined by detection of the 1H and 15N amide chemical shift displacements (CSDs) in 2D heteronuclear single quantum coherence spectroscopy (HSQC) spectra upon the addition of unlabeled ubiquitin (Fig. 2C and Fig. S3A). Consistent with the results obtained on the Gαs truncation mutants, residues displaying the most disturbances upon binding are localized exclusively at the N-terminal region of the Gαs peptide, including, more specifically, the Cys3-Ser7 region (Fig. 2D). In particular, Cys3 and Ser7 display the highest CSDs, suggesting their importance in the interaction with ubiquitin.
Interestingly, sequence alignment shows that the Gαs N-terminal region (Cys3 to Glu10) has homology to the UIMs found in other ubiquitin-binding proteins, such as the ESCRT-0 component HRS and the proteasome subunit 5A (S5a) (17) (Fig. 2E). The UIM in Gαs is conserved among different species (Fig. S4A) but absent in other Gα proteins (Fig. S4B). Classically, UIMs comprise a 15-residue consensus sequence e-e-x-x-Φ-x-x-Ala-Φ-x-Φ-Ser-x-x-e (where e, x, and Φ represent acidic residue, any amino acid, and large hydrophobic residue, respectively) (17, 18) in which two conserved residues, Ala and Ser, are crucial for ubiquitin binding (16, 18, 19). In the Gαs UIM, Ser is conserved, whereas Ala is replaced by Cys, which has similar hydrophobic features. In concordance, NMR results indicated that Cys3 and Ser7 are indeed the main ubiquitin-interacting residues in the Gαs UIM. Another difference in the Gαs UIM is the absence of the upstream Φ and negatively charged residues, which have been proposed to mediate favorable interactions (16, 20, 21), and may thus result in lower affinity for ubiquitin. In summary, the N-terminal extremity of Gαs harbors a UIM that is shorter than the classical UIMs but contains the central conserved hydrophobic residues (Cys3 and Ser7) critical for the ubiquitin-binding capacity of these motifs.
Most UIMs consist of a single α-helix structure (22). Even though the 3D structure of Gαs has been elucidated, no discernable structure was observed in the first eight residues of the N-terminal region, indicating that this region is highly flexible and does not adopt a fixed structure in the crystal (23, 24). Therefore, a structure of the Gαs1-34 peptide was modeled using Swiss-PdbViewer, in conjunction with SWISS-MODEL (25). The predicted structure exhibits a high α-helical content and similarities to the helical secondary structure of the UIM of S5a protein (Fig. 2F). Interestingly, Cys3 and Ser7 of the Gαs UIM are located on the same side of the α-helix surface (Fig. 2F), as previously reported for Ala219 and Ser223 in the UIM of S5a (26) or Ala266 and Ser270 in the UIM of HRS (27), enabling their interaction with the conserved Leu8-Ile44-Val70 hydrophobic patch of ubiquitin (18, 19, 27, 28).
The UIM of Gαs Interacts with the Ile44 Hydrophobic Patch of Ubiquitin.
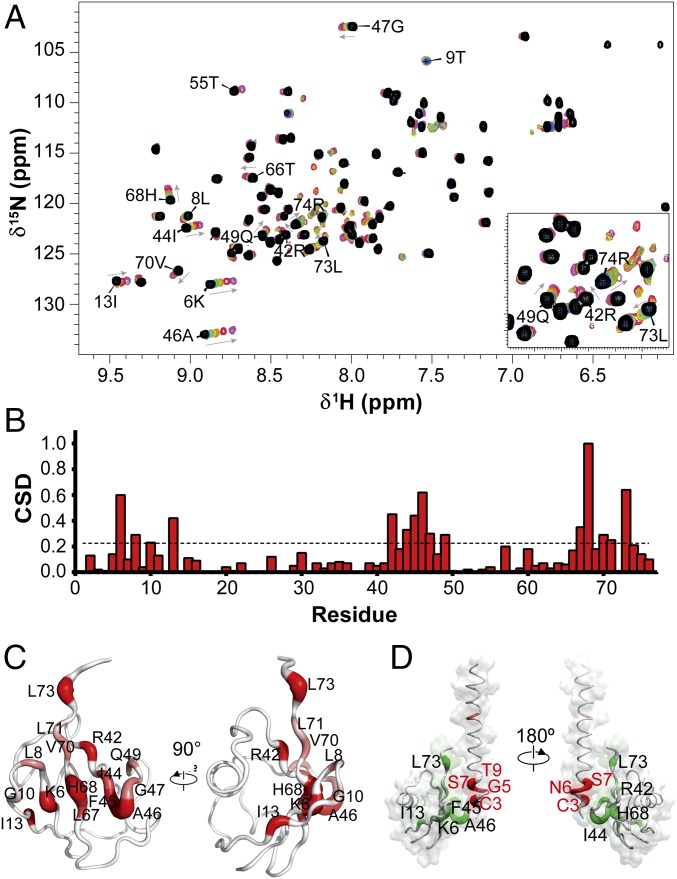
NMR spectroscopy was subsequently employed to define the corresponding Gαs-binding surfaces in ubiquitin. The 15N-labeled ubiquitin was titrated with unlabeled Gαs1-34 peptide, and the resulting 1H-15N-HSQC spectra were analyzed to detect localized CSDs (Fig. 3A and Fig. S3B) and identify specific residues on ubiquitin involved in the interaction (Fig. 3B). The residues with the most shifted cross-peaks are clustered around the C-terminal strands of the ubiquitin β-sheet, corresponding to the Ile44 hydrophobic surface of ubiquitin (Fig. 3C). In addition to basic residues Lys6, Arg42, and His68, which may interact with the acidic residues of Gαs UIM, the Gαs surface binding on ubiquitin comprises mainly exposed hydrophobic residues (Leu8, Ile44, Phe45, Leu67, Val70, Leu71, and Leu73) and suggests a complementary hydrophobic surface interaction with Gαs (Fig. 3D). The polar residue Gln49 also displays considerable displacement upon Gαs binding. However, the CSDs of remote residues like Gly10 and Ile13 most likely originate from differential interactions with residues disturbed by the direct interaction. Importantly, the identified Gαs-binding surfaces in ubiquitin correspond very closely to ubiquitin-binding sites that were mapped for other UIMs (e.g., HRS, hS5a) (27, 28) (Fig. S5). This similarity lends further credence to the idea that Gαs contains a UIM that binds ubiquitin in a very similar way.
Fig. 3.
Ubiquitin-binding surface of Gαs. (A) NMR chemical shift mapping of the residues in ubiquitin interacting with the Gαs1-34 peptide. The 1H-15N-HSQC spectra of ubiquitin in the absence (black) and increasing ratios (1:1, 1:2.5, 1:5, 1:10, 1:25, 1:50, and 1:100) of Gαs peptide (blue to purple) are shown. Fully assigned spectra are shown in Fig. S3B. (B) Normalized weighted CSD of ubiquitin residues observed upon binding to Gαs1-34 peptide. (C) Surface/worm representation of ubiquitin showing in red the residues with the greatest CSD upon addition of Gαs1-34 peptide. Worm radii are proportional to CSD values, and regions of higher perturbations (red) have a thicker backbone (also Fig. S5). (D) Interaction model mapping the interaction residues on ubiquitin structure and Gαs1-34 peptide. Worm radii are proportional to CSD values and are coded in a white-to-red (Gαs peptide) or white-to-green (ubiquitin) gradient.
The NMR analysis also indicated that the ubiquitin–Gαs peptide interactions are in the fast exchange regime, with an estimated dissociation constant (Kd) ranging from 0.49 to 1.32 mM (average of 1.10 ± 0.25 mM) (Fig. S6 and Table S1). Such binding affinity is in good agreement with the Kd values previously reported for other UIM–ubiquitin complexes (varying from 0.1 to 2 mM) (10, 22).
Ubiquitin Binding Is Important for the Role of Gαs in Endosomal Sorting.
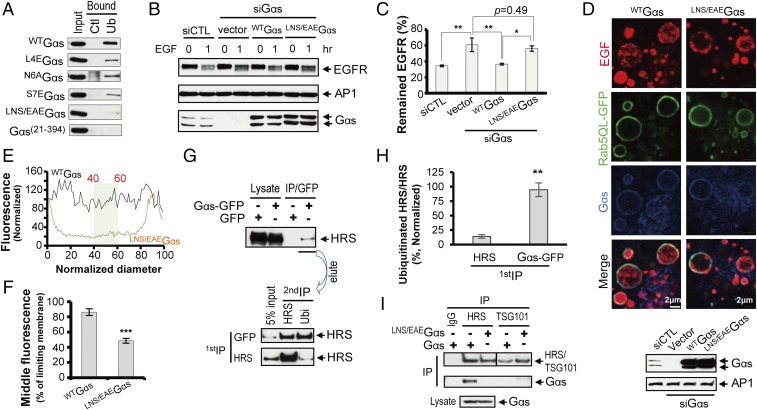
To determine the functional relevance of ubiquitin binding by Gαs, a UIM-deficient Gαs was generated. Based on the NMR data (Fig. 2 C and D), we selected the Leu4-to-Ser7 sequence for mutagenesis and ubiquitin-binding studies. Even if Cys3 was identified as a potential key determinant for ubiquitin binding (Fig. 2D), it was not substituted, given that it is a palmitoylation site responsible for Gαs membrane recruitment (29, 30). In concordance with our NMR results and previous studies mutating the UIM consensus sequence (16, 31), which predict Ser7 as a key ubiquitin-binding residue, mutating Ser7 to Glu (S7EGαs) dramatically reduced the ability of Gαs to bind ubiquitin (Fig. 4A). Although Leu4 and Asn6 have only a modest CSD on the HSQC spectrum (Fig. 2D), their mutations (L4EGαs and N6AGαs) clearly alter Gαs binding to ubiquitin (Fig. 4A). A possible explanation is that these changes affect the adjacent residues Cys3 and Ser7, and prevent their interaction with ubiquitin. Moreover, previous mutational studies suggested that the bulky hydrophobic residue usually located on the C-terminal side of Ala in the UIM motif is an important affinity determinant (20, 21). In contrast, mutation of Gly5 (G5EGαs), whose CSD was similar to Asn6, did not alter ubiquitin binding (Fig. S7). Thus, Gly5 CSD must be caused by an indirect effect, such as stabilization of the α-helical motif upon ubiquitin binding. Indeed, based on the Gαs orientation in complex with ubiquitin (Figs. 2F and 3D), Gly5 is oriented on the opposite side of the binding interface and may not be involved in the interaction. In summary, the single mutations of Leu4, Asn6, and Ser7 reduced Gαs binding to ubiquitin, but residual binding still remained (Fig. 4A), possibly due to incomplete inactivation of the UIM. Therefore, a mutant of the three residues Leu4, Asn6, and Ser7 was generated (LNS/EAEGαs), which showed a stronger defect in ubiquitin association (Fig. 4A) and was thus defined as UIM-deficient. Importantly, given that previous reports showed the importance of the Gαs N-terminal region in regulating Gαs activity (32), we used a bioluminescence resonance energy transfer-based assay to confirm that LNS/EAEGαs did not alter the rate of cAMP production (Fig. S8). Therefore, we ruled out that the effect of this Gαs mutant on ubiquitin binding is due to an alteration in Gαs activity.
Fig. 4.
Loss of ubiquitin binding inhibits Gαs interaction with HRS and delays EGFR sorting into ILVs of MVBs for lysosomal degradation. (A) Comparison of the contributions of the different UIM residues to ubiquitin binding using pull-down assays (also Fig. S7). Ctl, control protein G-agarose beads; Ub, ubiquitin-agarose beads. (B) Gαs-UIM mutation delays EGFR degradation. Levels of EGFR in Gαs-depleted HeLa cells transfected with siRNA-resistant forms of wtGαs or UIM-deficient Gαs (LNS/EAEGαs) and incubated with or without 100 nM EGF are shown. (C) Quantification of EGFR degradation (as shown in B). Data are presented as the percentage of EGFR expression compared with unstimulated cells (T0) and as the mean ± SEM from three independent experiments. *P < 0.05; **P < 0.01 (one-way ANOVA). (D, Top) Mutating the Gαs UIM alters the transfer of EGFR to the ILVs of MVBs. Gαs-depleted HeLa cells were transfected as described in B, together with Rab5QL-GFP. Cell-surface EGFRs labeled by Alexa Fluor 647-EGF were internalized for 30 min and then processed for confocal microscopy. (D, Bottom) Expression of Gαs proteins were checked by Western blot. (E) Representative line scan analysis to quantify EGFR localization to the ILVs of endosomes. The normalized diameter represents the diameter of the endosome, where 0 and 100 correspond to the pixel distances with the first and second maximum pixel intensities of Rab5QL-GFP, corresponding to the limiting membranes of the endosomes. The hatched box shows the normalized fluorescence values of pixels from 40 to 60% of the normalized diameter that were used to determine the mean intraluminal fluorescence for each endosome. (F) Compiled results of the line scan analysis for wtGαs or NLS/EAEGαs. Data are represented as the mean ± SEM of n ≥ 180 endosomes. ***P < 0.001 (Student’s t test). (G) Gαs interacts preferentially with ubiquitinated HRS. HEK293 cell extracts expressing GFP-Gαs and HRS were immunoprecipitated with either anti-GFP or HRS antibodies (first IP). Bound material was eluted and immunoprecipitated with either antiubiquitin or anti-HRS antibodies (second IP). (H) Quantification of the level of ubiquitinated HRS in the Gαs-GFP IP eluate and the HRS IP eluate (as shown in G). Data are presented as the percentage of ubiquitinated HRS versus total HRS and as the mean ± SEM from five independent experiments. **P < 0.01 (Student’s t test). (I) Gαs interaction with HRS is inhibited by Gαs UIM mutation. HEK293 cell extracts expressing wtGαs or UIM-deficient Gαs (LNS/EAEGαs) were incubated with HRS or TSG101 antibodies and analyzed by Western blotting using specific antibodies.
The functional role of the UIM-deficient Gαs in endosomal sorting was next evaluated on the degradation of EGFR, the prototypical receptor used to study ubiquitin-dependent endosomal sorting. Gαs-depleted HeLa cells were transfected with siRNA-resistant forms of wild-type Gαs (wtGαs) or ubiquitin-binding mutant (LNS/EAEGαs) (Fig. 4 B and C). As previously reported (5), Gαs depletion delays EGFR degradation. Reintroducing wtGαs in these cells restored the level of EGFR degradation to levels not significantly different from those of control cells. In contrast, reintroducing LNS/EAEGαs did not reverse the effect of Gαs depletion on the levels of EGFR degradation (Fig. 4C). These results indicate that EGFR was not efficiently delivered to the lysosomes in cells expressing the UIM-deficient LNS/EAEGαs.
Based on the colocalization of Gαs with ubiquitinated proteins on early endosome membranes (Fig. 1B) and our previous findings that Gαs plays a crucial role in the sorting of receptors in the ILVs of MVBs (4), we examined whether UIM-deficient Gαs altered the sorting of EGFR into ILVs of Rab5QL-enlarged endosomes. Cell-surface EGFRs were labeled with Alexa Fluor 647-EGF and internalized for 30 min (33). In Gαs-depleted HeLa cells rescued with wtGαs, Alexa Fluor 647-EGF was mainly located in ILVs of enlarged endosomes (Fig. 4D). In contrast, in LNS/EAEGαs-rescued cells, Alexa Fluor 647-EGF localized predominantly to the limiting membranes of enlarged endosomes, with most endosomes exhibiting little intraluminal fluorescence (Fig. 4D). Quantification of EGFR distribution across the endosomes by line scan analysis of confocal cross-sections (4, 33) (Fig. 4E) revealed a 40% reduction of Alexa Fluor 647-EGF fluorescence in the endosomal lumen of LNS/EAEGαs-rescued cells compared with wtGαs-rescued cells (Fig. 4F). These results clearly demonstrate that mutations in the UIM of Gαs cause defects in the sorting of EGFR in ILVs of MVBs.
Finally, to gain some mechanistic insight into the role of Gαs-UIM in EGFR endosomal sorting, we next examined whether the mutation of UIM altered Gαs interaction with the ESCRT machinery, a central player in EGFR sorting into ILVs of MVBs. Because Gαs has been previously shown to interact with HRS, an ESCRT-0 component (4, 5), and because many ESCRT components, including HRS and tumor susceptibility gene 101 (TSG101), can themselves be ubiquitinated (10, 34, 35), we hypothesized that Gαs interacts with the ubiquitinated form of HRS via its UIM. To support this idea, the level of ubiquitinated HRS interacting with Gαs was assessed by sequential double-immunoprecipitation (IP). This procedure consists in an initial IP of either Gαs or HRS, followed by elution of bound proteins and a second round of IP with antibodies against either ubiquitin to determine the amount of ubiquitinated HRS in the eluate or against HRS to determine the total amount of HRS in the eluate (Fig. 4G). Higher levels of ubiquitinated HRS were found in Gαs co-IP compared with HRS IP (Fig. 4G). Indeed, quantitative analysis (Fig. 4H) indicated that the ratio of ubiquitinated HRS/total HRS in the Gαs IP eluate (94.6 ± 11.8%) is 6.8-fold greater than the one found in the HRS IP eluate (14 ± 3.2%), demonstrating that Gαs binds preferentially to ubiquitinated HRS. We next investigated whether the mutation of Gαs UIM altered the interaction with HRS. wtGαs, but not the UIM-deficient LNS/EAEGαs, coimmunoprecipitated with endogenous HRS (Fig. 4I). However, neither wtGαs nor LNS/EAEGαs coimmunoprecipitated with the ESCRT-I component TSG101. Together, these results indicate that Gαs interacts preferentially with ubiquitinated HRS and that the UIM in Gαs is key for this interaction. This is consistent with the idea that Gαs UIM is involved in intermolecular interaction with specific ubiquitinated ESCRT components, further supporting that Gαs is an integral component of the ubiquitin-dependent endosomal sorting machinery.
In summary, these findings demonstrate that mutations in the UIM of Gαs prevent the interaction of Gαs with HRS, which delays the sorting of EGFR in ILVs of MVBs and subsequent lysosomal degradation. Interestingly, these results on EGFR degradation are phenotypically similar to those observed when Gαs was depleted (5), suggesting that the interaction of Gαs with ubiquitin, via its UIM, is crucial for its interaction and action on EGFR endosomal sorting machinery.
Conclusions
In this report, a conserved and bona fide UIM has been identified at the N-terminal extremity of Gαs. Like other known UIMs, the Gαs UIM is a single-sided motif that interacts with the classical hydrophobic surface patch of ubiquitin with low affinity. Among several Gα proteins tested, only Gαs binds ubiquitin and contains a UIM (Fig. S4B), which is consistent with the finding that Gαs can regulate endosomal sorting. Whereas other UBDs have been reported in close proximity to the lipid-binding domain or to have the ability to bind both ubiquitin and phospholipids (36, 37), the Gαs UIM is a UBD containing a palmitoylation site (Cys3) in its sequence. This posttranslational modification influences the capacity of Gαs to bind to ubiquitin. Indeed, an attenuation of the degree of Gαs palmitoylation in cells treated with the palmitoylation inhibitor 2-bromopalmitate increases Gαs binding to ubiquitin (Fig. S9). Interestingly, activation of Gαs has been reported to cause its rapid depalmitoylation (38), which could explain our observation that the active form of Gαs binds preferentially to ubiquitin. However, in the in vitro binding assays using nonpalmitoylated Gαs, ubiquitin binds preferentially to the active form of Gαs (Fig. 1E), indicating that the conformational change induced by activation has additional effects, perhaps through a better exposure of the N-terminal region containing the UIM. Of note, the insertion of a tag (GST or GFP) at the N terminus of Gαs alters both its ability to properly interact with ubiquitin and the capacity of its active form to influence ubiquitin binding (Fig. S10). We believe this explains why the interaction between HRS and Gαs-GFP or GST-Gαs reported in our previous work (4) was weak and independent from the Gαs activation state.
Our demonstration of a functional UIM in Gαs provides concrete molecular insights for its unconventional role in receptor endosomal sorting. Ubiquitin has emerged as a key signal for cargo sorting to the lysosomal pathway, and UIMs are recurring motifs found in ESCRT components, which are indispensable for interaction with ubiquitinated cargo to coordinate their transfer to ILVs. Furthermore, many components of the ESCRT sorting machinery, such as HRS, are also subject to monoubiquitination (34, 39). Monoubiquination mediates intermolecular interactions with UIMs present in other ESCRT components or regulatory proteins for proper assembly and coordination of the sorting machinery (10). It can also induce autoinhibitory conformation, such that the ubiquitin moiety interacts with its own UIM, thus preventing ESCRT components from recognizing ubiquitinated cargo and ESCRT from performing its sorting function (10, 33). In this study, we demonstrated that Gαs UIM is crucial for Gαs interaction with HRS and for EGFR sorting into ILVs of MVBs for lysosomal degradation. The possible mechanism by which Gαs could regulate HRS sorting activity is through its interaction with ubiquitinated HRS, which would prevent its autoinhibitory conformation, or by acting as an adaptor for the recruitment of other regulatory proteins, such as ubiquitin ligases or deubiquitinases. These possibilities remain to be tested to further understand the role of Gαs as a component of the ubiquitin-dependent endosomal sorting machinery.
Our findings also highlight the dual role of Gαs in receptor signaling and trafficking, providing an additional piece to our understanding of the intertwined molecular network between these processes. It is becoming increasingly apparent that Gαs has an important function in endosomal signaling, either by eliciting a second wave of G protein activation after GPCR internalization or, as we show herein, by regulating receptor sorting to lysosomes, and thus limiting the time spent on endosomes. Given that the active state of Gαs binds preferentially to ubiquitin, an attractive possibility is that the stimulation of Gαs-coupled GPCRs will increase the endosomal sorting of receptors to lysosomes leading to their down-regulation, thereby modulating the cellular response. Finally, with increasing reports revealing how ubiquitin acts as a versatile scaffold for the recruitment of trafficking and signaling proteins, this UIM motif in Gαs may open new avenues to better understand Gαs recruitment on organelles and its role in nonconventional signaling pathways.
Materials and Methods
Antibodies, Reagents, and Plasmid Constructs.
A detailed list of antibodies, DNA constructs, and other reagents is provided in SI Materials and Methods.
Cell Culture, Transfections, IP, Pull-Down, and Western Blot Analysis.
HeLa cells and HEK293T cells were maintained and transfected as described by Rosciglione et al. (4). Co-IP, pull-down, and immunoblotting assays were performed as described by Rosciglione et al. (4). The EGFR degradation assay was performed as described by Zheng et al. (5). Western blot quantification was performed using ImageJ software (NIH). Experiments were performed in triplicate, and the results are presented as the mean ± SD. The statistical significance of the differences between the samples was assessed using the Student’s t test; a value of P < 0.05 was considered significant. Experimental details are provided in SI Materials and Methods.
Immunofluorescence and Quantification of Alexa Fluor 647-EGF Localization in the Endosomal Lumen.
Immunofluorescence assays were performed as described by Rosciglione et al. (4). For the uptake of Alexa Fluor 647-EGF, HeLa cells treated with control or Gαs siRNA duplex were transfected with siRNA-resistant forms of wtGαs or LNS/EAEGαs, along with GFP-tagged Rab5-Q79L, to create enlarged endosomes. Cell surface receptors were labeled with Alexa Fluor 647-EGF and internalized for 30 min in DMEM at 37 °C, and then fixed and processed for immunofluorescence. The quantification of Alexa Fluor 647 signal in the endosomal lumen was determined as previously described (4).
NMR Spectroscopy and Modeling.
The 15N- or 13C, 15N-double–labeled peptides were purified from Escherichia coli BL21(DE3) transformed with pGEX-KG-Gαs1-34 and incubated with 15N ammonium chloride and 13C glucose. All NMR experiments were performed at 298 K on a Varian INOVA 600-MHz spectrometer equipped with a triple-resonance probe with z-axis pulsed-field gradients. NMR data were processed using NMRPipe (40) and analyzed with CCPNmr Analysis (41). Gαs1-34 peptide was modeled using Swiss-PdbViewer, in conjunction with SWISS-MODEL (25). The HADDOCK2.2 flexible docking server (42) was used for the modeling of biomolecular complexes between Gαs1-34 and ubiquitin (Protein Data Bank ID code 1D3Z) according to residues presenting significant CSD in both structures. Experiment details are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Asuka Inoue (Tohoku University) for the generous gift of Gαs CRIPSR knock-out HEK293 cells. This work was supported by Grant RGPIN-2015-06138 from the Natural Sciences and Engineering Research Council of Canada (to C.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708215114/-/DCSupplemental.
References
- 1.Gilman AG. G proteins: Transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Hewavitharana T, Wedegaertner PB. Non-canonical signaling and localizations of heterotrimeric G proteins. Cell Signal. 2012;24:25–34. doi: 10.1016/j.cellsig.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosciglione S, Thériault C, Boily MO, Paquette M, Lavoie C. Gαs regulates the post-endocytic sorting of G protein-coupled receptors. Nat Commun. 2014;5:4556. doi: 10.1038/ncomms5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng B, et al. Regulation of epidermal growth factor receptor degradation by heterotrimeric Galphas protein. Mol Biol Cell. 2004;15:5538–5550. doi: 10.1091/mbc.E04-06-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsvetanova NG, Irannejad R, von Zastrow M. G protein-coupled receptor (GPCR) signaling via heterotrimeric G proteins from endosomes. J Biol Chem. 2015;290:6689–6696. doi: 10.1074/jbc.R114.617951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 9.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piper RC, Dikic I, Lukacs GL. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb Perspect Biol. 2014;6:a016808. doi: 10.1101/cshperspect.a016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy JE, Marchese A. Regulation of GPCR trafficking by ubiquitin. Prog Mol Biol Transl Sci. 2015;132:15–38. doi: 10.1016/bs.pmbts.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raiborg C, et al. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 13.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roxrud I, Raiborg C, Pedersen NM, Stang E, Stenmark H. An endosomally localized isoform of Eps15 interacts with Hrs to mediate degradation of epidermal growth factor receptor. J Cell Biol. 2008;180:1205–1218. doi: 10.1083/jcb.200708115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenmark H, et al. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher RD, et al. Structure and ubiquitin binding of the ubiquitin-interacting motif. J Biol Chem. 2003;278:28976–28984. doi: 10.1074/jbc.M302596200. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann K, Falquet L. A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci. 2001;26:347–350. doi: 10.1016/s0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- 18.Swanson KA, Kang RS, Stamenova SD, Hicke L, Radhakrishnan I. Solution structure of Vps27 UIM-ubiquitin complex important for endosomal sorting and receptor downregulation. EMBO J. 2003;22:4597–4606. doi: 10.1093/emboj/cdg471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu KS, et al. Binding surface mapping of intra- and interdomain interactions among hHR23B, ubiquitin, and polyubiquitin binding site 2 of S5a. J Biol Chem. 2003;278:36621–36627. doi: 10.1074/jbc.M304628200. [DOI] [PubMed] [Google Scholar]
- 20.Polo S, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 21.Young P, Deveraux Q, Beal RE, Pickart CM, Rechsteiner M. Characterization of two polyubiquitin binding sites in the 26 S protease subunit 5a. J Biol Chem. 1998;273:5461–5467. doi: 10.1074/jbc.273.10.5461. [DOI] [PubMed] [Google Scholar]
- 22.Hurley JH, Lee S, Prag G. Ubiquitin-binding domains. Biochem J. 2006;399:361–372. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunahara RK, Tesmer JJ, Gilman AG, Sprang SR. Crystal structure of the adenylyl cyclase activator Gsalpha. Science. 1997;278:1943–1947. doi: 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis. 2009;30(Suppl):S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Young P, Walters KJ. Structure of S5a bound to monoubiquitin provides a model for polyubiquitin recognition. J Mol Biol. 2005;348:727–739. doi: 10.1016/j.jmb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Hirano S, et al. Double-sided ubiquitin binding of Hrs-UIM in endosomal protein sorting. Nat Struct Mol Biol. 2006;13:272–277. doi: 10.1038/nsmb1051. [DOI] [PubMed] [Google Scholar]
- 28.Walters KJ, Kleijnen MF, Goh AM, Wagner G, Howley PM. Structural studies of the interaction between ubiquitin family proteins and proteasome subunit S5a. Biochemistry. 2002;41:1767–1777. doi: 10.1021/bi011892y. [DOI] [PubMed] [Google Scholar]
- 29.Linder ME, et al. Lipid modifications of G proteins: Alpha subunits are palmitoylated. Proc Natl Acad Sci USA. 1993;90:3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degtyarev MY, Spiegel AM, Jones TL. The G protein alpha s subunit incorporates [3H]palmitic acid and mutation of cysteine-3 prevents this modification. Biochemistry. 1993;32:8057–8061. doi: 10.1021/bi00083a001. [DOI] [PubMed] [Google Scholar]
- 31.Shih SC, et al. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol. 2002;4:389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 32.Russell M, Johnson GL. G protein amino-terminal alpha i2/alpha s chimeras reveal amino acids important in regulating alpha s activity. Mol Pharmacol. 1993;44:255–263. [PubMed] [Google Scholar]
- 33.Hanafusa H, et al. Leucine-rich repeat kinase LRRK1 regulates endosomal trafficking of the EGF receptor. Nat Commun. 2011;2:158. doi: 10.1038/ncomms1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeller D, et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol. 2006;8:163–169. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
- 35.Amit I, et al. Tal, a Tsg101-specific E3 ubiquitin ligase, regulates receptor endocytosis and retrovirus budding. Genes Dev. 2004;18:1737–1752. doi: 10.1101/gad.294904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slagsvold T, et al. Eap45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. J Biol Chem. 2005;280:19600–19606. doi: 10.1074/jbc.M501510200. [DOI] [PubMed] [Google Scholar]
- 37.Schuh AL, Audhya A. The ESCRT machinery: From the plasma membrane to endosomes and back again. Crit Rev Biochem Mol Biol. 2014;49:242–261. doi: 10.3109/10409238.2014.881777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedegaertner PB, Bourne HR. Activation and depalmitoylation of Gs alpha. Cell. 1994;77:1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 39.Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci. 2012;125:265–275. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- 40.Delaglio F, et al. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 41.Vranken WF, et al. The CCPN data model for NMR spectroscopy: Development of a software pipeline. Proteins. 2005;59:687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- 42.van Zundert GCP, et al. The HADDOCK2.2 web server: User-friendly integrative modeling of biomolecular complexes. J Mol Biol. 2016;428:720–725. doi: 10.1016/j.jmb.2015.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.