Significance
Individuals who have experienced high levels of stress in early childhood are at risk for a wide range of behavioral problems, yet the neurobiological processes underlying these associations are poorly understood. In this experiment, we uncover a potential mechanism leading to maladaptive decision making: altered brain activation during anticipation of rewards and losses. Individual differences in neural responses to cues of potential reward or loss were associated with early life stress and both laboratory and real-world measures of risk-taking behaviors. These effects were predicted only by childhood stress exposure, not by current levels of stress in the participants’ adult lives. Early life stress was assessed prospectively, when the participants were children, thereby avoiding the potential biases associated with adult retrospective recall.
Keywords: childhood development, social behavior, early life stress, reward, decision making
Abstract
Individuals who have experienced chronic and high levels of stress during their childhoods are at increased risk for a wide range of behavioral problems, yet the neurobiological mechanisms underlying this association are poorly understood. We measured the life circumstances of a community sample of school-aged children and then followed these children for a decade. Those from the highest and lowest quintiles of childhood stress exposure were invited to return to our laboratory as young adults, at which time we reassessed their life circumstances, acquired fMRI data during a reward-processing task, and tested their judgment and decision making. Individuals who experienced high levels of early life stress showed lower levels of brain activation when processing cues signaling potential loss and increased responsivity when actually experiencing losses. Specifically, those with high childhood stress had reduced activation in the posterior cingulate/precuneus, middle temporal gyrus, and superior occipital cortex during the anticipation of potential rewards; reduced activation in putamen and insula during the anticipation of potential losses; and increased left inferior frontal gyrus activation when experiencing an actual loss. These patterns of brain activity were associated with both laboratory and real-world measures of individuals’ risk taking in adulthood. Importantly, these effects were predicated only by childhood stress exposure and not by current levels of life stress.
Individuals who have experienced chronic and high levels of early life stress (ELS) exposure are at increased risk for a wide range of behavioral problems that begin in childhood and continue to increase throughout their lives. These correlates of extreme childhood adversity include health-related issues such as drug and alcohol abuse and threats to well-being that range from teen pregnancy to criminality (1–3). Although the association between early adversity and later maladaptive behaviors has been well-documented, critical gaps remain in our understanding about how and why these problems emerge and persist. One challenge to addressing this issue has been a lack of understanding about why early childhood stress exposure seems to create risk for a very broad range of maladaptive behaviors. A second issue is that it has been difficult to parse the effects of early versus cumulative life experience, given that most people who experience highly stressful childhoods tend to continue to live in stressful situations through adolescence and adulthood (4). For these reasons, in part, we have yet to identify neurobiological mechanisms through which children’s environmental experiences might lead to the emergence and maintenance of a broad range of maladaptive behaviors. Knowledge of specific mechanisms can improve our understanding of why extreme childhood stress exposure leads to a broad array of maladaptive behaviors, provide future biomarkers of early risk, and guide the development of effective interventions for these children. Here, we test a potential mechanism that could account for the poor decisions and choices that stress-exposed individuals often make. These suboptimal decisions contribute to a broad range of social and health-related behaviors often associated with childhood stress exposure.
To address this issue, we first measured the life circumstances of a community sample of elementary school-aged children. These children had a range of life experiences from relatively low or normative life stress to extremely high levels of adversity. We followed these children for a decade and invited those from the highest and lowest quintiles of childhood stress exposure to return to our laboratory as young adults. This group of 54 included 29 individuals (17 female) who had extremely high and verifiable levels of stress during childhood, and 25 individuals (11 female) who had relatively low levels of stress during their early childhoods. When these individuals returned in adulthood, we reassessed their life circumstances and also measured their reward processing and decision making.
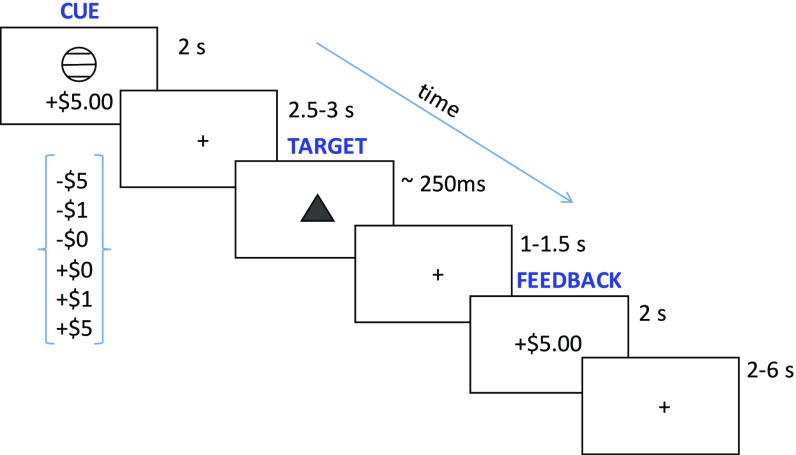
Prior nonhuman animal studies have shown that chronic stress during development can lead to long-term alterations in certain reward-related behaviors. For example, stress-exposed animals evince less motivation to work toward a reward, and this appears to be mediated, in part, by alterations of neuronal function in the basal ganglia (5–7). Based on these data, we tested our participants on a Monetary Incentive Delay (MID) task, which taps activation of the brain’s reward circuitry, including the basal ganglia (8–10). In this task, participants were first presented with a cue indicating a possible monetary gain, loss, or no gain–loss. After a variable delay, a target appeared, and participants were instructed to press a button as quickly as possible while the target was on the screen. If the button was pressed during the target, the participant either won money or avoided losing money; a press too early or late resulted in no win or a loss. Feedback (win–loss) was provided after the response. The duration of the target was dynamically adjusted for each trial based on the performance of prior trials so that each participant maintained a success (hit) rate of 67% for each cue (Fig. 1). This task allowed us to dissociate the anticipation and motivation to work toward a reward (or away from a loss) from the response to actually experiencing a reward or loss. In this manner, the present experiment tests whether alterations in how individuals process potential rewards or losses could explain the increased propensity of individuals who have experienced high childhood stress to engage in frequent maladaptive behaviors.
Fig. 1.
Schematic of the MID task used in this study. Participants were presented with a cue indicating the amount of potential monetary gain or loss. A triangle (“TARGET”) briefly appeared on the screen, and the participant had to press a button while the triangle was on the screen to win or avoid losing money. Pressing the button too early or too late resulted in no win or a loss. Feedback was then provided to indicate success or failure on that trial.
We sought to isolate various stages of reward processing because prior animal and human studies suggest that stress exposure may differentially affect the anticipation of, versus the response to, rewards and losses. For example, marmosets that experienced early maternal deprivation showed reduced motivation to obtain a reward, but no alterations in their consummatory behavior (11). This type of dissociation is consistent with the theory that reward-related brain activation consists of multiple processes. One process, an incentive salience or motivational process, is critical during the anticipation of a reward (or loss) and reflects an individual “wanting” an outcome. The other process, a later hedonic response to reward (or loss), is reflective of “liking” an outcome (12). We reasoned that brain activity during the anticipation of potential rewards and losses would index the individual’s ability to effectively use environmental cues to guide subsequent successful behavioral choices. In contrast, brain activity in response to receipt of a reward or loss could be critical to subsequent learning in anticipation of future choices. There is increasing evidence that individuals who have experienced high levels of childhood stress exposure have altered responses to the actual receipt of rewards (13). Furthermore, maladaptive risk-taking behavior (and, by implication, poor decision making) is associated with deficits in both the ability to process potential rewards and the ability to anticipate potential losses (14–16). For these reasons, we separately evaluated anticipation and receipt of both rewards and losses.
We hypothesized that individuals who had very high levels of early childhood adversity would show abnormalities in processing the initial cues signaling potential reward and loss. More specifically, based on prior studies, we hypothesized that individuals with high levels of early childhood adversity would show decreased activation in reward-related brain areas following cues signaling potential rewards and less activation following cues that could warn them of potential losses (13, 17). We also planned on examining how these individuals process the receipt of a reward or a loss. Reward processes are associated with a network of regions that include the ventral and dorsal striatum of the basal ganglia (including the nucleus accumbens, putamen, and globus pallidus), the thalamus, and the insula; therefore, we examined neural activation of these regions. To better understand possible links between brain activity and behavior, we also tested these participants on behavioral tasks assessing decision making and queried their daily risk-taking behaviors. Our hypothesis was that reduced activation during the anticipation of potential rewards and losses (a fundamental aspect of reward processing assessed by our neuroimaging task) would be correlated with poorer reward-related decision making in a laboratory gambling task and increased maladaptive risk-taking behaviors in these individuals’ everyday lives. Finally, we examined whether childhood experiences accounted for any resulting patterns of behavior above and beyond measures of participants’ current levels of stress in adulthood.
Results
Is Early Life Stress Associated with Altered Behavior in Reward-Motivated Tasks?
We began by confirming that behavioral aspects of decision making are associated with childhood stress exposure. To do so, we administered the Cambridge Gambling Task (CGT; Cambridge Cognition Ltd.), a well-validated measure of risk taking in the context of decision making. In this task, participants are presented with a row of 10 red and blue boxes. The ratio of red to blue boxes varies for each trial (e.g., four blue boxes and six red boxes or three blue boxes and seven red boxes, etc.). The participant must guess whether a yellow token is hidden in a red box or a blue box. Participants select a proportion of their points to gamble based on their confidence about the location of the hidden token. The points that can be bet are presented alternately in ascending and descending orders; the reaction time difference between these two conditions can then be compared to determine the patience that the participant has in placing a higher- or lower-value bet. We found that individuals with higher levels of early life stress were more averse to waiting to place their bets, making their decisions about how much money to bet more quickly than those who had less childhood stress (P < 0.01). Those who had more stressful childhoods also made poorer decisions compared with the individuals with low childhood stress exposure; those with high child stress exposure chose targets that had a lower likelihood of winning (P < 0.05; this variable is called “Quality of Decision Making” in the Cambridge Gambling Task). Of most relevance to the hypotheses being tested here, individuals who experienced high child stress exposure had difficulty adjusting their subsequent behavior as they accumulated more experience and feedback during the task. Specifically, even after receiving more information about risk through previous experiences of losing in the task, those who had experienced high childhood stress continued to place large bets on trials with high probabilities of loss (P < 0.05; this variable is called “Risk Adjustment” in the Cambridge Gambling Task). In contrast, individuals who had lower levels of childhood stress benefited more from their experiences and began to place lower bets on trials with high probability of loss. Values for these variables are presented in SI Appendix, Fig. S1.
In sum, individuals who had experienced high levels of childhood stress exposure made poor decisions with regard to risk taking compared with individuals with lower early stress exposure, and those with high early life stress exposure appeared unable to effectively learn from loss trials to improve their subsequent risk assessments.
Does Brain Activation During Anticipation or Response to Reward–Loss Account for the Association Between Early Life Stress and Poor Decision Making?
We next sought to better understand why individuals who experienced early life stress engaged in poor-quality decision making. First, we examined individuals’ patterns of brain activation on the Monetary Incentive Delay task (8). Fig. 1 depicts a schematic of the task, and SI Appendix, Fig. S2, shows the regressors used to model the task-related activation.
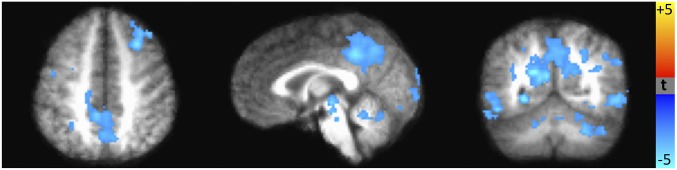
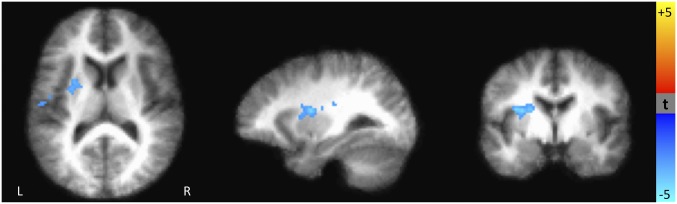
The anticipation of a reward or loss—the period of time between the cue and the target, assessed before participants know whether or not they have gained or lost money—provides a window into how these individuals use predictive cues from the environment. Across all participants, the anticipation of a potential reward versus no reward was associated with greater activation in numerous brain regions including the caudate, putamen, globus pallidus, and nucleus accumbens (SI Appendix, Fig. S3). However, as shown in Fig. 2, individuals who experienced higher levels of childhood stress showed less brain activation during the anticipation of potential rewards (compared with no rewards). These differences (measured by comparing brain activity in the potential reward condition to the no-reward condition) emerged in the posterior cingulate/precuneus, middle temporal gyrus, lingual gyrus, right middle frontal gyrus, and cerebellum (SI Appendix, Table S1). Participants with higher levels of childhood stress also displayed less brain activity during the anticipation of potential losses (compared with no losses). Specifically, those with higher childhood stress had less activity in the putamen and insula before losses (Fig. 3) compared with those with lower stress exposure.
Fig. 2.
Brain areas where the difference between the anticipation of potential large rewards (+$5) vs. no rewards (+$0) is significantly correlated with early life stress. Blue regions indicate areas where the brain activation during the anticipation of potential rewards vs. no rewards is negatively correlated with early life stress. That is, the participants with higher early life stress showed lower activation in the precuneus, middle temporal gyrus, and cerebellum during the anticipation of potential rewards (P < 0.05).
Fig. 3.
Brain areas where the difference between the anticipation of potential large losses (−$5) vs. no loss (−$0) is significantly correlated with early life stress. Blue regions indicate areas where the brain activation during the anticipation of potential losses vs. no loss is negatively related to early life stress. That is, subjects with higher early life stress showed lower activation in the insula and putamen during the anticipation of potential losses (P < 0.01).
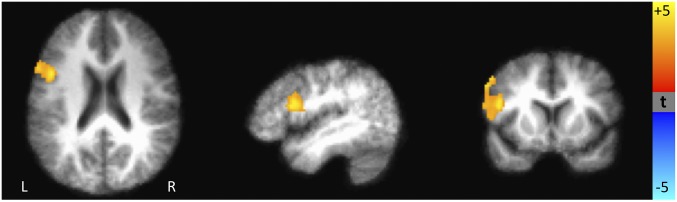
Although our primary interest was in how individuals used predictive cues to guide their behavior, we also examined patterns of activation once participants knew that they had actually received or lost rewards. Participants who had experienced higher childhood stress showed greater activation in the left inferior frontal gyrus in response to losses (Fig. 4); these individuals also had decreased activation when they avoided a loss, observed in the cingulate gyrus, right precentral gyrus, and right middle temporal gyrus. No significant correlations with childhood stress emerged during the response to successfully responding to or missing a reward trial.
Fig. 4.
Brain areas where differences in the response to missing the target on a loss vs. no-loss trial (i.e., response to losing money) is correlated with early life stress. Yellow/orange regions show brain areas where the activation during the response to loss vs. no-loss trials is positively correlated with early life stress. Significantly greater activation was observed in the putamen and inferior frontal gyrus in subjects with higher early life stress.
In sum, individuals with high levels of childhood stress exposure failed to fully engage the same neural circuitry as those with lower levels of childhood stress exposure during the encoding and processing of cues that signaled potential reward or loss. After not effectively engaging these predictive cues, those individuals who experienced high levels of childhood stress displayed higher levels of neural activation when they did in fact lose.
To better understand how the neural processing of reward–punishment cues was related to overt behavior, we compared fMRI data with the individuals’ performance on the laboratory gambling task. This allowed us to test whether the neural measures of reward and loss processing were related to the behavioral measures of the quality of the participants’ risk assessment and decision making. Brain activation during anticipation of potential loss was associated with successful risk adjustment on the Cambridge Gambling Task (P < 0.01). Risk adjustment reflects an individual’s ability to update their behavior based on more information from their previous experience. In other words, the more neural activity that an individual had when processing cues signaling potential loss, the better improvement they showed in terms of making good choices on the gambling task. This relationship was most evident in the putamen: Individuals who showed greater activation of the putamen in response to cues of potential losses were also more likely to adjust their risk assessment across the experiment. Finally, the overall quality of participants’ decision making on the Cambridge Gambling Task (i.e., placing bets on the most likely outcome) was related to their brain responses to cues indicating potential rewards and losses. Both greater activation in the precuneus, middle temporal gyrus, and right middle frontal gyrus during the anticipation of rewards and greater activation of the putamen during the anticipation of potential losses were associated with better “quality of decision making” during the laboratory gambling task (P < 0.04). Furthermore, participants with greater activation in this network of brain areas during the anticipation of potential rewards and losses in the fMRI task took significantly less time to place their bet (“deliberation time”) in the laboratory gambling task (P < 0.05).
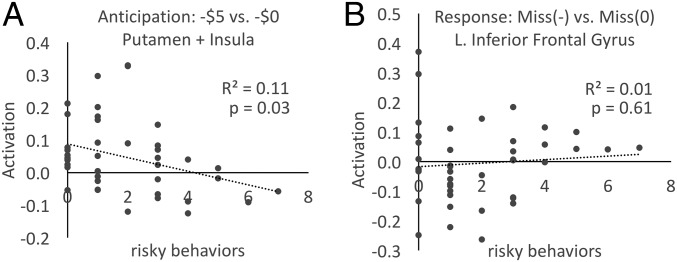
Given these consistent patterns of associations between the participants’ levels of childhood stress exposure, the behavioral measures of their decision making on the Cambridge Gambling Task, and the neural indices of reward–loss processing, we examined whether these laboratory measures were related to behaviors in the participants’ actual daily lives. To do so, we queried participants about the frequencies with which they engaged in behaviors such as driving without wearing a seatbelt or not seeking medical attention for injuries (SI Appendix). As shown in Fig. 5, participants who had experienced high levels of stress in their childhoods engaged in more maladaptive risky behaviors as adults (r = 0.54, P < 0.001). Higher levels of these maladaptive risky behaviors were associated with less activation of the putamen during the anticipation of potential losses (r = −0.34, P < 0.03; Fig. 6A) in adulthood. This relationship did not reflect a global level of less activation among the high childhood stress group, but was specific to anticipating a potential loss: No significant correlation was found between individual differences in maladaptive risk-taking behavior and brain activation during the anticipation or receipt of rewards, or following an actual loss (Fig. 6B and SI Appendix, Fig. S12).
Fig. 5.
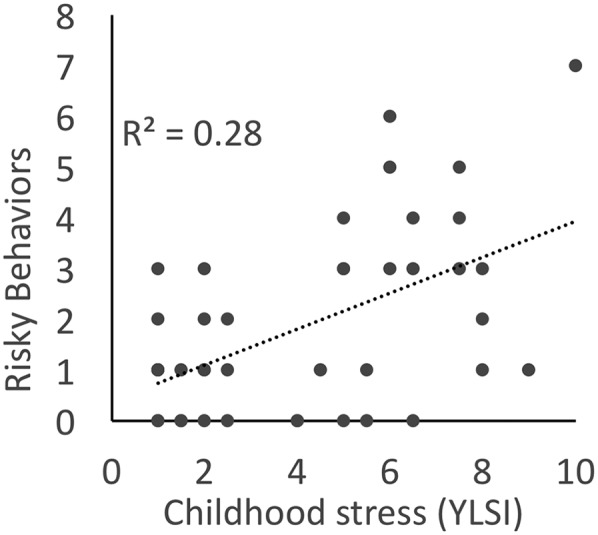
Individual differences in risky behaviors, measured from the Youth Risk Behavior Survey, are correlated with measures of early life stress (assessed using the YLSI; P < 0.0003). This effect held even after controlling for current life stress (assessed using the LSI; P < 0.05).
Fig. 6.
Across participants, an index of risky behaviors (measured from the Youth Risk Behavior Survey) is significantly correlated with the activation of the putamen during the anticipation of potential loss in the MID task (A), but not with the activation in the left inferior frontal gyrus during the response to the loss (B).
We next used a mediation analysis to determine if individual differences in brain activation during the reward-processing task accounted for the relationships between childhood stress and laboratory measures of reward-related decision making. Across participants, activation in the precuneus during the anticipation of potential rewards mediated the relationship between childhood stress exposure and the deliberation time during the laboratory gambling task (P < 0.04). SI Appendix, Table S2, describes other brain regions that appear to mediate the relationship between childhood stress and laboratory tests of behavior, but that fell below our a priori threshold for significance. Potentially meaningful among these results were that activation of the putamen during anticipation of potential losses mediated the relationship between childhood stress and the quality of decision making (P < 0.06) as well as the ability to adjust risk in the laboratory gambling task (P < 0.06). Finally, we examined whether the measures of brain activation mediated the relationship between childhood stress and real-life measures of adult risk-taking behaviors. The activation in the lingual gyrus and precuneus during the anticipation of potential rewards as well as the activation in the left inferior frontal gyrus during the response to losses significantly mediated the association between early life stress and real-life maladaptive risk-taking behaviors (P < 0.04).
Are These Findings Specific to Early Life Stress?
The last question that we sought to address has been one of the most vexing in studies of the effects of childhood adversity. It is well known that individuals who have stressful childhoods are likely to encounter cumulative stressors throughout their lives. For this reason, it is often difficult to garner data that are informative about the relative role of early childhood life events. Consistent with the view that stressful childhoods are associated with stressful adulthoods, we observed a correlation between the Youth Life Stress Interview (YLSI), completed during the participants’ childhoods, and their reported levels of current life stress when we assessed them in early adulthood with the University of California at Los Angeles Life Stress Interview (UCLA LSI) (R2 = 0.28) (SI Appendix, Fig. S13). To determine the relative roles of stress early in participants’ lives versus the effects of their current stress in adulthood, we repeated all of the analyses described above, substituting the measure of the participants’ current life stress for the measure of childhood stress. The mediation analyses were no longer significant, and none of the key relationships between brain measures and behavior emerged when the measures of adult stress were used. Activation of the putamen during the anticipation of losses and activation of the precuneus, middle occipital cortex, middle frontal gyrus, and middle temporal gyrus during the anticipation of potential rewards were not significantly correlated with levels of current life stress; these relationships held only when based on childhood stress exposure. As expected, participants current real-life maladaptive risk-taking behaviors were associated with both childhood stress (P < 0.0003) and current life stress (P < 0.0001), but the measures of childhood stress contributed variance above and beyond the measures of current life stress (P < 0.05).
Discussion
The present experiment sought to test the role of specific mechanisms that might account for some of the manifold negative life outcomes that have been observed in people who experienced high levels of adversity and stress in their early childhoods. Traditionally, a problem in addressing this question has been the potential confound of asking adults who are currently experiencing a high level of life difficulties to retrospectively recall their childhood experiences. This is because their current contexts or mental states may cause them to have a negative bias of their lives as children. Conversely, studying individuals only in childhood cannot address how these individuals will function in adulthood. Therefore, we combined these approaches to gain an objective assessment of stress exposure during childhood and then tested neurocognitive mechanisms and overt behavior in these same individuals later in development. By doing so, we observed that individuals who experienced high levels of childhood stress displayed significant alterations in fundamental aspects of reward processing compared with those with lower levels of childhood stress. Specifically, those with very high levels of early life stress were much slower to make decisions on a laboratory gambling task; yet, despite spending more time on their choices, those individuals ended up making poorer decisions. For example, the high-childhood-stress participants consistently placed large bets on trials that had a low chance of winning, failed to place large bets on trials with a good chance of winning, and—perhaps most importantly—failed to change this behavior after repeated losses. In addition, these behaviors meaningfully converged with patterns of brain activity when these individuals were presented with environmental cues signaling potential positive and negative outcomes.
The neuroimaging data revealed that individuals who experienced high levels of childhood stress had reduced activation in a number of brain areas when they were presented with cues that could have helped them anticipate future rewards and losses. In contrast, individuals with lower levels of childhood stress showed greater neural activation when they were presented with a cue that signaled the potential for a larger loss; those who experienced higher childhood stress did not show this modulation. One interpretation of this finding is that extremely high levels of childhood stress exposure affect development of the mechanisms that allow individuals to avoid a loss by attending to the magnitude of potential negative consequences. An alternative explanation for our data might be that those with more stressful childhood experiences have some sort of generalized deficit in cognitive functioning. But the lower levels of brain activation that we observed did not occur in all brain areas activated in the task; rather, group differences were restricted to key brain regions commonly involved in processing rewards and losses. Moreover, the high-childhood-stress group performed equivalently to the low-stress group on neurocognitive tasks not related to decision making. In addition, individuals with high childhood stress show greater left inferior frontal activation in response to actually receiving a loss, suggesting that their difficulties do not stem from an inability to process or experience rewards and punishments. Increased activation within this frontal brain region has been found in a previous study using the monetary incentive delay task and was suggested to be associated with altered action-contingent learning or performance monitoring (18). Another study using a different gambling task also found increased activation within this region to be associated with regret and disappointment (19). Therefore, there is some convergence of findings in this regard.
One further possibility is that a higher level of emotional response experienced by individuals with high childhood stress following a loss may further undermine their ability to learn from the loss and update their behavior on subsequent events. While we have no direct measure of participants’ emotional reactions during the MID task, prior studies using this task have found that participants have affective reactions to winning and losing money (e.g., ref. 20). If the increased left inferior frontal gyrus activation does reflect greater negative emotions (a relationship that we can only infer), this would be consistent with this view as well. In other words, both poor initial learning of, and heightened emotional reactivity to, loss might leave high-stress-exposed individuals less able to modify and better use the anticipation of (and motivation to avoid) subsequent potential future losses. It is also interesting to note that reduced activation of the putamen when participants were presented with cues about potential future losses was associated with the frequency of their real-world maladaptive risk-taking behaviors and judgments in their daily lives. This again underscores the importance of the activation during the anticipation of reward–loss period in guiding individuals adaptive and maladaptive behavioral choices.
Individuals with low levels of childhood stress showed robust activation in the posterior precuneus (BA31), middle temporal gyrus, and middle frontal gyrus during the anticipation of potential rewards compared with no-reward trials, while those with high childhood stress failed to showed this increased activation in these regions in rewarded versus nonrewarded trials. Among other functions, these regions of the precuneus, parietal cortex, and middle temporal gyrus are involved in visual attention (21, 22). It is difficult to ascribe specific functional roles to individual regions of detected activation—both because of the challenges of reverse inference and because brain function typically involves coordinated networks of activity. However, one possibility is that individuals with relatively low childhood stress devote more attention to signals of reward, while those with high childhood stress do not show this attentional modulation. Such a finding converges with extant studies demonstrating alterations in attention to emotional cues among young children living in stressful environments (23). In the present task, differences in attention were manifested more in visuospatial attention regions including the precuneus and parietal cortex. This may be because the Monetary Incentive Delay task, which requires rapidly pressing a button when a target appears, places particularly strong demands on visuospatial attention.
Similarly, individual differences in activation of these networks were also related to differences in real-world risk-taking behaviors. Furthermore, individual differences in the amplitude of this activation were associated with differences in participants’ ability to delay their decisions about how much money to bet in the laboratory “gambling” task. This ability to delay a decision could be interpreted as impulsivity, but an alternative view is that this variable reflects differences in the value or importance that an individual assigns to actually placing a bet. The distinct associations observed between brain activation and laboratory measures of reward processing also suggest different pathways by which increased risk-taking behaviors in the real world emerge, which are mediated by different brain structures.
Our observed associations between activations in the Monetary Incentive Delay task and early life stress appear distinct from patterns that have been observed following acute stress. For example, Kumar et al. (24) report that in a typical community sample, acute stress increased activation during the anticipation of reward versus no-reward trials in the MID task and decreased the activation during the response to the reward. This is consistent with prior findings that show opposite effects from acute versus chronic stress (e.g., acute stress increases incentive-triggered motivation, while chronic stress abolishes this effect) (25).
Despite much convergence with extant literatures, our findings differ slightly from a recent report using a similar type of task (17). Whereas Dillon et al. (17) found reduced striatal activation during the anticipation of potential reward in participants with higher life stress, we did not observe significant effects in this region. Furthermore, we found alterations in both the anticipation and the response to losses among our high-childhood-stress participants, while Dillion et al. reported no differences during these conditions as a function of early stress exposure. In considering how to reconcile these findings, we observed that Dillon et al. used a slightly different task. Key differences included: (i) the hit/miss ratio of these researchers was fixed at 50/50 rather than being tied to the subject’s actual behavior, (ii) they used a narrower range of monetary gain and losses, and (iii) they used a smaller amount for the monetary loss relative to the amount of potential gain, whereas we kept the amount of potential rewards and losses equivalent. For these reasons, it is possible that the larger loss amounts used in the present experiment resulted in greater salience of these cues for participants, and thus more brain activation during the anticipation and response to those greater losses.
In some ways, it could have been informative to have collected fMRI measures of individuals’ decision making during childhood and to have been able to compare these measures over time. However, such a design would also have limitations. For example, young children could not be expected to be able to complete the same decision-making tasks as adults; therefore, any alteration of these tasks to make them equally appropriate for both young children and adults could well have major implications for the types of neural systems activated in a task. Such a design would also bring up concerns about whether the rewards and losses used (whether money, points, or prizes) would be expected to have comparable salience across age. In brief, if we had collected such data during our participants’ childhoods, some issues could be addressed, but other methodological concerns would emerge. In future research, it would also be informative to assess whether the money offered as rewards and losses held different salience across participants. It may be that $5 meant more to those with stressful lives compared with those with less stressful lives. However, if this were the case, we might expect those who valued the rewards more to devote even greater attention to cues signaling reward possibilities, and this did not occur.
Finally, it is meaningful that all of the associations reported here between life stress, brain activation, and reward-related decision making and behavior—especially the ability to anticipate and benefit from cues signaling potential rewards and losses—was accounted for by childhood stress exposure, not by the current levels of stress in these young adults’ lives. Although it is unlikely that the relationships between brain and complex social behaviors would rely on any single mechanism or factor, the ability to distinguish here between early versus cumulative life stress does help focus attention on the role of childhood experience in the development of these systems.
Conclusions
The present experiment reveals that very high levels of stress early in development are associated with significant alterations in reward and loss processing in adulthood. The data reported here indicate that individuals who experienced very high levels of stress in their early childhoods appear to have problems as adults in effectively using cues in the environment that signal rewards and losses. These data provide a window into understanding how and why patterns of poor decisions and maladaptive risk-taking behaviors that are known to create manifold health and social risks are common among those individuals who experienced very high levels of child adversity.
Methods
Participants.
Fifty-four individuals ranging in age from 19.0 y to 23.7 y (mean age: 20.5 y) participated in the current study. Within this group of 54 participants, 29 individuals (17 female) were assessed as having had high levels of stress during early childhood, and 25 individuals (11 female) were assessed as having had relatively low levels of childhood stress. The current study was approved by the University of Wisconsin Institutional Review Board, and all participants provided informed consent. A number of participants had to be excluded from the data analysis due to claustrophobia, excessive head motion, or significant mental health issues, resulting in a final group of 42 participants (19 low stress, 23 high stress) for fMRI analysis. For further details, see SI Appendix.
Procedures.
Participants had their life stress measured in our laboratory when they were children (mean age: 10.2 y) and were recontacted ∼10 y later. They returned to our laboratory as young adults (mean age: 20.6 y) and underwent an MRI scan during which they performed a reward-processing (MID) task. Following the MRI scan, participants had their current life stress re-evaluated, completed a battery of neuropsychological tests including the Cambridge Gambling Task, and reported their current risk-taking behaviors.
Behavioral Measures.
Childhood stress exposure was assessed using the YLSI, a semistructured interview using trained interviewers and an independent team of raters. Current life stress was evaluated using the UCLA LSI (26). Neuropsychological functioning in reward processing was assessed through the CGT, a subtest of the Cambridge Neuropsychological Test Automated Battery (Cambridge Cognition Ltd.). To measure actual risky behaviors in participants’ daily lives, participants completed a modified version of the Youth Risk Behavior Survey (27) developed and used by the Centers for Disease Control and Prevention. These measures are described in greater detail in SI Appendix.
fMRI Tasks.
Brain activation related to reward processing was assessed using an MID task (8, 9). This task allows separate measurement of both the anticipation of reward or loss and the response to the receipt of reward–loss. A schematic of the paradigm is presented in Fig. 1. This task has been shown to reliably activate reward-processing regions (ventral striatum, insula, thalamus, medial prefrontal cortex) and allows the dissociation of reward anticipation and outcome.
MRI Data Acquisition.
A series of structural and functional brain images was acquired on a 3T General Electric MR750 MRI scanner using an eight-channel receive-only radiofrequency head coil (General Electric Medical Systems).
fMRI Task Analyses.
All MRI data analyses were performed using the Analysis of Functional NeuroImages analysis package (28), unless otherwise specified. Activation amplitudes were estimated for the anticipation of gains, losses, or no gains or losses, as well as the response to success or failure in hitting the target. Differences in activation as a function of ELS were assessed on a voxel-wise level using a t test including either (i) the YLSI scores from the interview administered when the participants were children or (ii) the LSI scores from the interview conducted in young adulthood on the same day as the scanning session. Voxel-wise t tests were corrected for multiple comparisons by estimating the spatial autocorrelation function from the preprocessed fMRI data and setting a minimum cluster-size threshold based on a Monte Carlo simulation that incorporates this estimated autocorrelation function (29, 30).
Mediation Analysis.
We used a standard multivariate analytic framework (31) to test whether the relationship between ELS and risk taking, as measured by the CGT, is statistically mediated by the brain’s activation during either the anticipation or the receipt of rewards and losses, as measured by the MID task. Significance of mediation was assessed using the Causal Mediation Analysis package in R, using a nonparametric bootstrap resampling with 5,000 iterations.
Supplementary Material
Acknowledgments
We thank Brian Knutson for providing us with the Monetary Incentive Delay task scripts; Joanna Swinarska, Alex Rokni, and Anna Bechner for assistance; and the individuals who agreed to participate in this study for their generous participation. This work was supported by National Institute of Mental Health Grants MH61285 and MH68858 (to S.D.P.) and by a core grant to the Intellectual and Developmental Disabilities Research Center at the Waisman Center from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant P30-HD03352).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.F.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708791114/-/DCSupplemental.
References
- 1.Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health Committee on Early Childhood, Adoption, and Dependent Care Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 2.Norman RE, et al. The long-term health consequences of child physical abuse, emotional abuse, and neglect: A systematic review and meta-analysis. PLoS Med. 2012;9:e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans GW, Li D, Whipple SS. Cumulative risk and child development. Psychol Bull. 2013;139:1342–1396. doi: 10.1037/a0031808. [DOI] [PubMed] [Google Scholar]
- 5.Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- 6.Hollon NG, Burgeno LM, Phillips PE. Stress effects on the neural substrates of motivated behavior. Nat Neurosci. 2015;18:1405–1412. doi: 10.1038/nn.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: The effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 8.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 9.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 10.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry. 2004;56:72–79. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71:670–679. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boecker R, et al. Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS One. 2014;9:e104185. doi: 10.1371/journal.pone.0104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balodis IM, et al. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol Psychiatry. 2012;71:749–757. doi: 10.1016/j.biopsych.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JS, et al. Altered brain activity during reward anticipation in pathological gambling and obsessive-compulsive disorder. PLoS One. 2012;7:e45938. doi: 10.1371/journal.pone.0045938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harms MB, Shannon Bowen KE, Hanson JL, Pollak SD. Instrumental learning and cognitive flexibility processes are impaired in children exposed to early life stress. Dev Sci. October 19, 2017 doi: 10.1111/desc.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon DG, et al. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enzi B, et al. Alterations of monetary reward and punishment processing in chronic cannabis users: An fMRI study. PLoS One. 2015;10:e0119150. doi: 10.1371/journal.pone.0119150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chua HF, Gonzalez R, Taylor SF, Welsh RC, Liberzon I. Decision-related loss: Regret and disappointment. Neuroimage. 2009;47:2031–2040. doi: 10.1016/j.neuroimage.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Knutson B, Greer SM. Anticipatory affect: Neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwisher N, Wojciulik E. Visual attention: Insights from brain imaging. Nat Rev Neurosci. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- 22.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 23.Pollak SD. Multilevel developmental approaches to understanding the effects of child maltreatment: Recent advances and future challenges. Dev Psychopathol. 2015;27:1387–1397. doi: 10.1017/S0954579415000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P, et al. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neurosci. 2014;266:1–12. doi: 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemos JC, et al. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammen C, et al. Children of depressed mothers: Maternal strain and symptom predictors of dysfunction. J Abnorm Psychol. 1987;96:190–198. doi: 10.1037//0021-843x.96.3.190. [DOI] [PubMed] [Google Scholar]
- 27.Eaton DK, et al. Centers for Disease Control and Prevention (CDC) Youth risk behavior surveillance United States, 2011. MMWR Surveill Summ. 2012;61:1–162. [PubMed] [Google Scholar]
- 28.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 29.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. fMRI clustering and false-positive rates. Proc Natl Acad Sci USA. 2017;114:E3370–E3371. doi: 10.1073/pnas.1614961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. fMRI clustering in AFNI: False-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.