Significance
We compared the patterns of neural activity in cells in the lateral prefrontal cortex (PFC) with cells in three areas within the medial temporal lobes (MTL) and visual area TE in nonhuman primates during the encoding phase of a temporal-order memory task. While many cells in both regions signaled information about item identity, temporal order, and the combination of the two, cells in these regions used largely distinct encoding strategies. PFC signaled specific information about the conjunction of item and temporal order and prominent stimulus-selective delay activity that is well suited for planning actions. By contrast, MTL areas exhibited item-based signals modulated by temporal order as well as prominent incremental timing signals that are well suited for episodic encoding.
Keywords: temporal-order memory, medial temporal lobe, prefrontal cortex, episodic memory, working memory
Abstract
Neuropsychological and neurophysiological studies have emphasized the role of the prefrontal cortex (PFC) in maintaining information about the temporal order of events or items for upcoming actions. However, the medial temporal lobe (MTL) has also been considered critical to bind individual events or items to their temporal context in episodic memory. Here we characterize the contributions of these brain areas by comparing single-unit activity in the dorsal and ventral regions of macaque lateral PFC (d-PFC and v-PFC) with activity in MTL areas including the hippocampus (HPC), entorhinal cortex, and perirhinal cortex (PRC) as well as in area TE during the encoding phase of a temporal-order memory task. The v-PFC cells signaled specific items at particular time periods of the task. By contrast, MTL cortical cells signaled specific items across multiple time periods and discriminated the items between time periods by modulating their firing rates. Analysis of the temporal dynamics of these signals showed that the conjunctive signal of item and temporal-order information in PRC developed earlier than that seen in v-PFC. During the delay interval between the two cue stimuli, while v-PFC provided prominent stimulus-selective delay activity, MTL areas did not. Both regions of PFC and HPC exhibited an incremental timing signal that appeared to represent the continuous passage of time during the encoding phase. However, the incremental timing signal in HPC was more prominent than that observed in PFC. These results suggest that PFC and MTL contribute to the encoding of the integration of item and timing information in distinct ways.
Memory for the temporal order of items in an episode plays an essential role in planning future actions, a function linked to the prefrontal cortex (PFC), as well as for episodic memory, which is strongly linked to the structures of the medial temporal lobe (MTL). Neuropsychological studies have consistently shown that memory for temporal-order information is impaired in patients with lesions in the frontal lobe (1–6). Lesion studies in rodents support the idea that the hippocampus (HPC) is also critical for temporal-order memory (7–9), although fewer human neuropsychological studies have reported disproportionate impairments of temporal-order memory beyond recognition memory impairments in patients with damage to the MTL (3, 10, 11). Consistent with these findings, a large number of fMRI studies have reported activation of both PFC (12–19) and MTL (18–22) during tasks of long-term temporal-order memory. While the vast majority of these studies focused on neural activity during recall of temporal-order information, only a few studies have examined PFC and MTL activation during the encoding of temporal-order memory (18, 22).
Jenkins and Ranganath (18) reported that activity in the parahippocampal cortex during encoding predicted subsequent memory for fine-scale temporal-order information while coarse-scale temporal accuracy was predicted by activity in both PFC and HPC. Tubridy and Davachi (22) examined encoding of temporal-order memory for triplet pairs and reported that bilateral activations in HPC and parahippocampal cortex, but not in PFC, predicted subsequent temporal-order memory. While both task and methodological differences could explain these differential results, it leaves open the question of the precise contributions of PFC and MTL during the encoding phase of temporal-order memory.
Findings from recent neurophysiological studies in animal model systems provide insight to this question. In rodents, recent studies have described cells in HPC that fire consistently at successive time points in a memory task, suggesting that these cells represent the passage of time within a trial or episode (23–25). Using a temporal-order task requiring working memory, we reported that monkey HPC cells provide a prominent incremental timing signal during the delay period between two to-be-remembered visual-cue stimuli (26, 27). By contrast, the perirhinal cortex (PRC) signaled the conjunction of object and temporal-order information (26). In a similar task requiring working memory for temporal-order information, Ninokura et al. (28) and Warden and Miller (29) reported that PFC neurons carried information about the specific sequence of objects during the delay period before the choice phase of the task using either a frequency code or a phase code (30). Together, these results suggest that neurons in both PFC and MTL signal temporal-order memory. However, because the specific task and analysis strategies differed across these studies, the precise contributions of PFC and MTL areas to the encoding of temporal-order information remain unclear.
To define the specific contributions of PFC and MTL in encoding memory for temporal-order information, in the present study, we recorded from PFC, MTL areas, and visual area TE of the same monkeys as they performed a memory task requiring the retention of a specific sequence of two visual objects (Fig. 1). Based on previous neurophysiological studies, we targeted the dorsal and ventral regions of the lateral PFC (d-PFC and v-PFC). While both regions have been implicated in various aspects of cognitive control of behavior including temporal-order memory (28–30), they reportedly signal different kinds of information (e.g., monitoring/motor planning for d-PFC vs. space/object for v-PFC) (31, 32). We show that, while PFC and MTL encode information about both specific items and temporal order, these two regions signal the integration of item and temporal order in different ways. Some of the data from the MTL recordings have been reported previously (26).
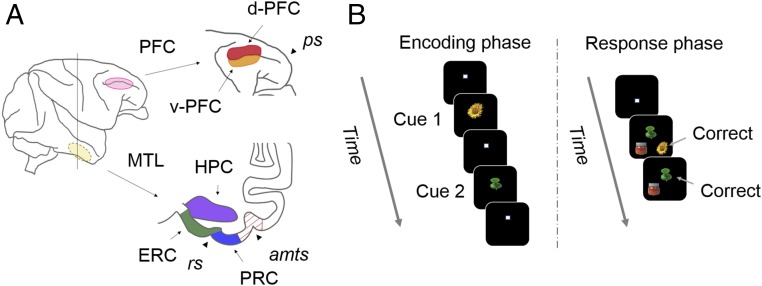
Fig. 1.
(A) Illustration of the target areas. The recording regions in lateral PFC and MTL are shown in a lateral view of macaque brain (Left). The recording sites of PFC included dorsal (d-PFC) and ventral (v-PFC) parts along the principal sulcus (Right Top). Anterior–posterior level of the coronal section indicating MTL (Right Bottom) corresponds to the vertical line of the lateral view. The recording sites of MTL include HPC, ERC, and PRC. TE was recorded as a control region. amts, anterior middle temporal sulcus; rs, rhinal sulcus. (B) Schematic diagram of the temporal-order task. A sequence of two cue stimuli was presented in the encoding phase. The two cue items and one distracter were presented at three different positions randomly in the response phase. The three stimuli were pseudorandomly chosen from a pool of eight well-learned visual stimuli at each trial.
Results
The temporal-order task (Fig. 1B) started with an encoding phase during which animals were first shown a sequence of two items, randomly chosen from a pool of eight items (possible sequences: 8 × 7 = 56). During the response phase, the two stimuli shown during the encoding phase along with a distraction image were presented on the screen, and the animal was required to first touch the first item in the sequence followed by the second item in the sequence (and avoid the distractor item) to get a reward. Following behavioral training and during the recording experiments, two monkeys performed the task on average at 86.3 ± 6.4% correct (mean ± SD; monkey B, n = 599) and 87.3 ± 6.3% (monkey G, n = 330) for the first choice, and 87.3 ± 6.3% (monkey B) and 88.1 ± 6.0% (monkey G) for the second choice.
As the monkeys performed the temporal-order memory task, we recorded from 127 neurons from the PFC and compared these responses to the responses of 802 neurons recorded in the same two monkeys throughout the MTL [n = 232 in HPC, 166 in entorhinal cortex (ERC), and 317 in PRC] as well as in TE (n = 87) (Fig. 1A).
PFC Item-Selective Responses.
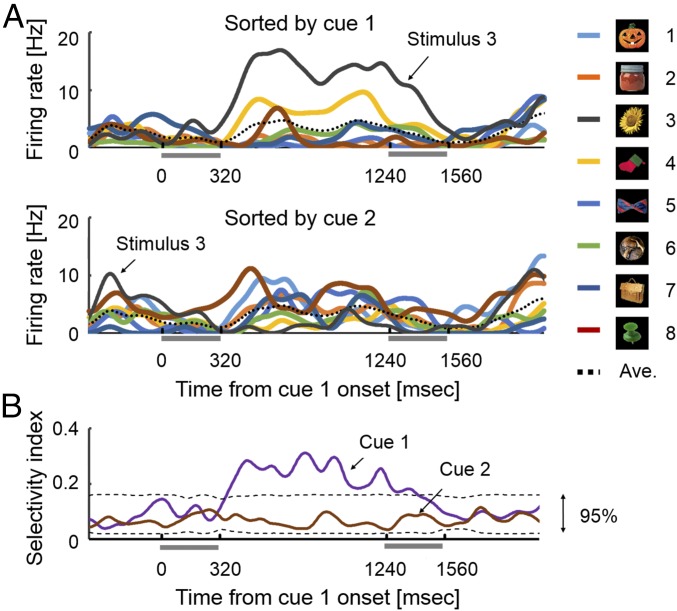
We first examined the pattern of item-selective neural responses in PFC. An example of stimulus-selective response in v-PFC is shown in Fig. 2A. This v-PFC cell exhibited a clear stimulus-selective response for stimulus 3 only when it was shown as cue 1, but no selective response to the same visual images shown as cue 2 (t = 2.98, df = 10, P = 0.014 for cue 1 and t = 0.84, df = 13, P = 0.41 for cue 2, two-tailed paired t test). To examine the effect of all eight stimuli on the stimulus selectivity of this cell at each time point, we calculated how much of the variance in firing rate at a given time point, t, could be accounted for by the factor of stimulus [selectivity index (STI)] (33). The STI value for cue 1 for this sample v-PFC cell became significant soon after the cue 1 offset (dashed line, 95% confidence level in Fig. 2B) and was sustained until the midpoint of the cue 2 presentation through the delay period. In contrast to the robust stimulus selectivity for cue 1, the STI value for cue 2 did not reach significance at any point through the encoding phase.
Fig. 2.
An example of an v-PFC item cell showing stimulus selectivity to the cue 1 stimulus. (A) Spike density function (SDF, σ = 20 ms) to each cue 1 stimulus (Top) and to each cue 2 stimulus (Bottom) in the encoding phase. (B) Time courses of stimulus selectivity for the item cell in A are indicated for cue 1 and cue 2. Dashed lines indicate ranges of 95% confidence level estimated by a simulation test (1,000 times of shuffles).
In v-PFC, 33% of the neurons (n = 24/78) showed significant (P < 0.05, one-way ANOVA) stimulus-selective responses during the cue presentation and following the delay period (80–840 ms from cue stimulus onset) to either cue 1 or cue 2 (Table S1). We refer to these stimulus-selective cells as “item cells.” Of the 24 item cells, 22 neurons showed a stimulus selectivity exclusively for one of the two cue presentations in the encoding phase (n = 14 for cue 1, n = 8 for cue 2). The remaining two neurons responded selectively to both cue 1 and cue 2. This result indicated that the vast majority of item cells in v-PFC (22/24) signal item information selectively for a particular time point in the trial [e.g., firing in response to the flower only when it was presented as the first but not the second cue in the encoding phase (Fig. 2)]. We refer to these cells as “exclusive-or-item cells.” Consistent with previous studies (34), we found fewer cells that showed stimulus-selective responses in d-PFC (3/49 for cue 1 or 5/49 for cue 2) relative to v-PFC, and all of them showed a stimulus selectivity exclusively for one of the two cue presentations. This area-specific distribution of item cells in PFC was reasonable from anatomical findings using retrograde transsynaptic transport of rabies virus; while v-PFC receives inputs from the inferotemporal cortex, d-PFC receives inputs from the MTL (35).
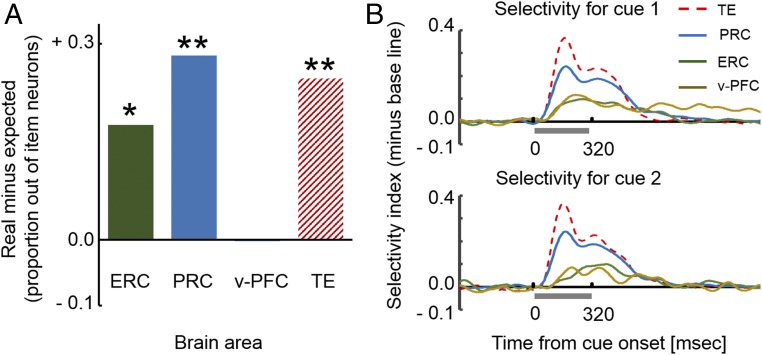
Given the striking pattern of the stimulus-selective activity of v-PFC cells, we compared the number of cells that showed stimulus selectivity across the two cue periods, a pattern of activity that we refer to as that of “conjunctive-item cells” (e.g., n = 2 in v-PFC), with the expected value in that area calculated as a product of the proportion of neurons with significant stimulus selectivity for cue 1 and for cue 2 (e.g., 16/78 × 10/78 × 78 = 2.05) (Fig. 3A). We found that the actual number did not differ significantly from the expected value in v-PFC (P > 0.68, permutation test, Fig. S2). The presence/absence of the stimulus-selective response to cue 1 and cue 2 were not dependent (P = 0.32, Fisher’s exact test). These results indicate that the v-PFC signal combined information about both item and temporal order by coding stimulus selectivity for cue 1 and cue 2 separately.
Fig. 3.
(A) Proportions of conjunctive-item cells with stimulus selectivity to both cue 1 and cue 2 from item cells with stimulus selectivity to either cue 1 or cue 2. The ratios of conjunctive-item cells were significantly larger than the expected values in ERC, PRC, and TE but not in v-PFC. *P < 0.0012; **P < 0.0002. (B) Time courses of population-averaged stimulus selectivity to cue 1 (Top) and to cue 2 (Bottom).
MTL Item-Selective Responses.
We next applied the same item-selectivity analysis to HPC, ERC, and PRC in the MTL and TE. Consistent with our earlier analysis using a shortened time window [80–400 ms from cue stimulus onset from Naya and Suzuki (26)], we found substantial numbers of item cells in all areas except for the HPC (Fig. S1 and Table S1) and conducted further analysis for ERC, PRC, and TE. In contrast to v-PFC, we found that there were more conjunctive-item cells than expected by chance in ERC (P < 0.0012, permutation testing), PRC (P < 0.0002), and TE (P < 0.0002) (Fig. 3A and Fig. S2). The presence or absence of a stimulus-selective response to cue 1 and cue 2 was dependent on each other in these areas (P = 0.0017, Fisher’s exact test for ERC; χ2 = 84.8 and 33.3, P < 0.0001, χ2 test for PRC and TE). These results indicate that the MTL item cells tended to show stimulus selectivity regardless of the temporal order.
Temporal Dynamics of the Integrated Signal in PFC and MTL.
We next compared the temporal dynamics of stimulus-selective signals at the population level in PFC and MTL. Fig. 3B shows population-averaged time courses of STI (t) for item cells with stimulus selectivity for cue 1 presentation (Top) and for cue 2 presentation (Bottom) in each area. TE (106 ms and 91 ms for half peak time from cue 1 onset and cue 2 onset) and PRC (106 ms and 101 ms) started to show selective activity earlier than v-PFC (146 ms and 136 ms) and ERC (121 ms and 206 ms) for both cue 1 and cue 2. This tendency did not depend on the magnitude of the selectivity index in each area (Fig. S7). Because the stimulus-selectivity signals for the cue 1 and cue 2 presentations were carried by independent groups of item cells in v-PFC, this suggests that the specific signal combining item and temporal order emerged as soon as the cells responded to either cue 1 or cue 2. Similarly, our previous study showed that PRC cells signaled both item and temporal-order information by firing at different rates in response to the same item when it was presented in a different temporal order (Fig. S6), and this differential response between cue 1 and cue 2 appeared as soon as the PRC item cells started to respond to the cue stimuli (figure S6 in ref. 26). Thus, by simply comparing the time courses of stimulus-selective response in PRC and v-PFC, we can estimate those of the combined signals for item and temporal order in these two areas. This comparison suggests that the integrated signal developed in PRC before developing in v-PFC during both cue 1 and cue 2 periods of the task (Fig. 3B).
Incremental Timing in the Delay Period.
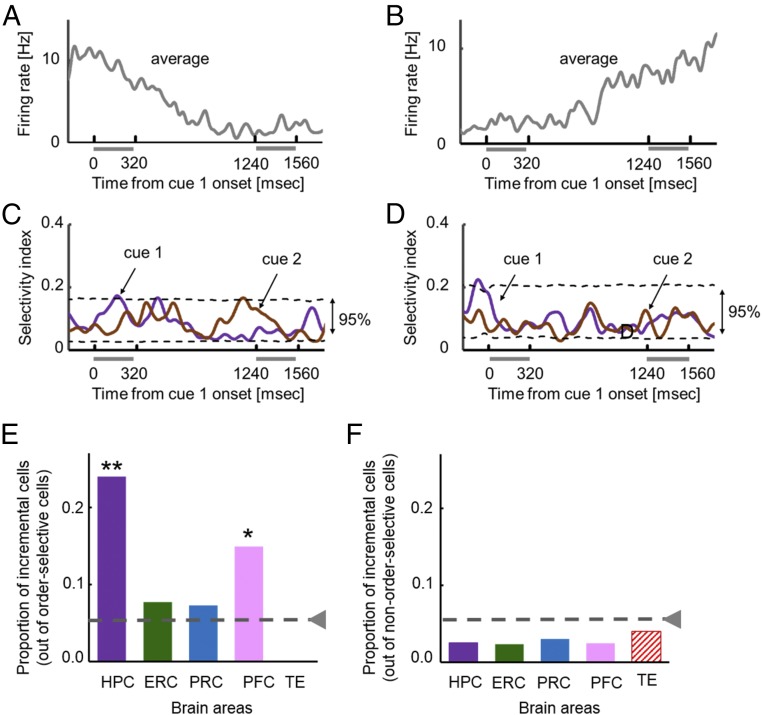
We focused our next analysis on the delay period between the cue 1 and cue 2 presentations of the encoding phase of the task. We previously reported that HPC neurons provide an incremental timing signal that represents the passage of time between the presentations of the cue 1 and cue 2 stimuli (26). This signal was found both at the population level (26) and at the single neuron level (27). To identify these incremental timing signals in PFC, we first defined order-selective cells as cells the responses of which differentiated between the two cue periods on a two-tailed t test (P < 0.05). A substantial number of order-selective cells were observed in both v-PFC and d-PFC as well as MTL areas (Fig. S1). In the present study, some order-selective cells in PFC as well as HPC changed their spike-firing rates monotonically during the delay period between cue 1 and cue 2. Fig. 4 A and B show examples of order-selective PFC cells with monotonic decreasing and increasing activities. None of these cells showed stimulus selectivity to cue stimuli (Fig. 4 C and D and Fig. S8). To assess the monotonicity of this increasing or decreasing pattern of activity, we examined how well a first-order polynomial fit the spike-firing rates during the delay period compared with a zero-order polynomial (26, 27). For the cells shown in Fig. 4, significant improvements were observed in the fittings by a first-order polynomial for both neurons (f = 5.1 and 3.7; P = 0.0002 and 0.002; df0 = 22; df1 = 21), and these fits were not improved by a second-order polynomial (f = 2.0 and 1.19; P = 0.06 and 0.35; df1 = 21; df2 = 20). We referred to the neurons with significant improvements only by a first-order polynomial fitting as incremental-time cells.
Fig. 4.
(A) An example of d-PFC order-selective cells showing greater responses during cue 1 than cue 2. Average SDF (σ = 20 ms) across all trials in the encoding phase is shown. (B) An example of v-PFC order-selective cells showing greater responses during cue 2 than cue 1. (C and D) Time courses of stimulus selectivity for the above order-selective cells. The same formats as Fig. 2B. (E) Proportions of incremental cells from the order-selective cells. Dashed gray line, chance level (0.0475). PFC contains both d-PFC and v-PFC. *P = 0.0011. **P < 0.0001. (F) Proportions of incremental cells from the other recorded neurons (non–order-selective cells).
We examined proportions of incremental-time cells from the order-selective cells in each area (Fig. 4E). Because d-PFC and v-PFC showed a similar tendency (13 and 16%), we merged the data in this analysis. The proportions differed significantly among the areas when we included TE (χ2 = 11.5, df = 4, P = 0.021, χ2 test) and when we excluded TE (χ2 = 9.1, df = 3, P = 0.028). HPC and PFC showed significantly larger proportions of incremental time cells than would be expected by chance (4.75%) in HPC [24%, P < 0.0001, B(75, 0.045), binominal-distribution test)] and in PFC [15%, P = 0.0011, B(47, 0.045)], while the other MTL areas (i.e., ERC and PRC) and TE did not (Fig. 4E). HPC provided the strongest incremental timing signal while smaller but significant neuronal populations in PFC also signaled incremental timing (Fig. 4E and Fig. S9). We also asked if incremental timing signals were seen when neurons did not show differential responses between cue 1 and cue 2 (non–order-selective cells). We found that proportions of the incremental time cells from the non–order-selective cells were below the chance level in all of the tested areas (Fig. 4F).
Stimulus-Selective Delay Activity.
While the incremental-time cells in HPC and PFC represented a continuous passage of time during the delay period between cue 1 and cue 2, the item cells in v-PFC maintained their stimulus-selective activity through the entire delay period (t = 5.68, df = 15, P < 0.0001 for the first 400 ms and t = 3.45, df = 15, P = 0.0036 for the last 400 ms, two-tailed paired t test) (Fig. S4). In contrast, the item cells in MTL (i.e., ERC and PRC) and TE attenuated their stimulus-selective signal in the middle of the delay period between cue 1 and cue 2 (Fig. S4).
Discussion
In this study, we compared the responses of neurons in PFC and MTL in the same animals as they performed a temporal-order memory task. Consistent with previous reports, we confirmed that neurons in both PFC and MTL are strongly and specifically engaged in the encoding of temporal-order information. However, neurons in these two areas signaled the task-related information in different ways. First, while both PFC and MTL neurons signaled the conjunction of information about item and temporal order, their representation manners differed. This conjunctive information developed earlier in MTL relative to PFC. Second, during the delay interval between the two cue stimuli, while many cells in PFC exhibited cue-selective delay activity, MTL neurons did not convey the cue-item information. Instead, HPC neurons provided a prominent signal of incremental timing. While incremental-timing information was also present in PFC, it was substantially weaker than that observed in HPC. These findings suggest that these two brain areas may be coding this prominent task-related information for different purposes.
Perhaps the most striking difference seen between PFC and MTL is the distinct ways in which neurons across these two brain areas integrated information about item and temporal order. In v-PFC, one group of item cells showed stimulus selectivity only for cue 1, while the other group of item cells did so only for cue 2 (Fig. S6), providing highly selective signals for particular items presented during either cue 1 or cue 2, but not both cue periods. By contrast, PRC and ERC cells tended to show stimulus selectivity for both cues and signaled the conjunction of item and temporal order information by modulating their stimulus-selective activity as a function of time (i.e., cue 1 vs. cue 2) (26) (Fig. S6). It should be noted that the independence of stimulus selectivity between two cue-stimulus presentations in v-PFC could not be explained by its relatively smaller number of item cells since the proportion of item cells in v-PFC (31%) was larger than that in ERC (23%) (Fig. S1) and item cells in ERC tended to show stimulus selectivity to both cue 1 and cue 2, similar to those cells in PRC (Fig. 3A). This pattern is consistent with the modulation of stimulus-selective responses previously described in the perirhinal (36, 37) and entorhinal cortices (38) during tasks of recognition memory.
v-PFC exhibited independence of the presence/absence of stimulus selectivity not only across the two cue stimulus periods but also between the cue period and the choice period of the task (Fig. S5). By contrast, neurons in PRC and TE that showed stimulus selectivity to the cue stimuli (i.e., item cells) also exhibited stimulus selectivity during the choice period with a significantly larger frequency compared with nonitem cells (Fig. S5). This distinctive pattern of v-PFC activity is consistent with what Rigotti et al. (39) have called the “mixed selectivity” of PFC neurons. This group argued that PFC cells represent high-dimensional information by integrating multiple variables related to task requirements (e.g., item, order, and task type) in nonlinear ways, which could provide a computational advantage to solving various tasks. By contrast, MTL cortical areas conveyed consistent information about stimulus identity across task periods along with information about relative timing that may be more useful for keeping track of information in an ongoing episode.
The second major difference between MTL and PFC signals was seen in the predominant patterns of neural activity during the delay interval between the two cue presentations. We previously described a prominent incremental timing signal in the monkey HPC during the delay intervals of two different memory tasks (26, 27). Similarly, a growing body of work describes the presence of “time cells” in rodent HPC that represented the temporal organization during fixed intervals (∼10 s) by showing selective firings within particular time periods (i.e., “time field”) rather than the incremental signal during the intervals (23, 24, 40, 41). These different response properties of time cells between our present study and previous rodent studies may come from the different lengths of delay periods (i.e., ∼1 vs. ∼10 s) although we cannot deny the possibility that the different response patterns might reflect a species difference between primates and rodents. Salz et al. (41) showed that the time cells in HPC exhibited their temporal coding even in situations that did not demand working memory. These results suggest that the generation of a timing signal in HPC may be independent of the requirement of active maintenance of temporal information and instead may be a more automatic process, consistent with the nontask-related coding of HPC place cells. It will be of great interest to know whether the incremental timing signal contributes to the integrated representation of item and temporal order in PRC and v-PFC during the encoding of item sequences (26). While HPC cells provide a prominent incremental timing signal during the delay period of the task, it is not surprising that prominent signals seen in v-PFC cells sustained item-identity signal for a particular cue 1 stimulus, consistent with its prominent role in working memory (42–45).
Conclusion
The present results help explain the evidence, emerging from imaging studies, that both PFC and MTL contribute to tasks of temporal-order memory (12–22), despite the fact that lesions of these two areas result in such different patterns of impairment (1–6, 10, 11). Specifically, the largely differential patterns of neural activity seen in the PFC and MTL suggest that these areas may be solving temporal-order memory tasks in differential ways or for differential purposes (46). PFC neurons are tuned for planning actions or decisions by manipulating the contents of working memory (47, 48), while MTL neurons organize incoming information in a continuous way to help encode ongoing information across multidimensional domains that include item, time, space, and associative context (49).
Methods
Subjects.
Two male rhesus monkeys (8.1 kg, monkey B; 10.3 kg, monkey G) were used for the experiments. All procedures and treatments were done in accordance with NIH guidelines and approved by the New York University Animal Welfare Committee.
Behavioral Task.
We trained the two animals on a temporal-order task with visual objects (Fig. 1B). The procedure for the temporal-order task has been described in detail previously (26). A trial of the temporal-order task consists of an encoding phase and a response phase. In the encoding phase, two cue stimuli (0.32 s) were presented sequentially with a delay interval (0.92 s). Cue stimuli were pseudorandomly chosen from a pool of eight well-learned visual items, resulting in 56 (8 × 7) different two-stimulus sequences. The encoding phase was followed by a blank interphase delay interval of 0.7–1.5 s where no fixation was required. The response phase started with a preresponse delay period of 1.02 s with fixation required. Then, three choice stimuli were presented simultaneously on the screen: two of them were the items that had been presented as cue stimuli in the encoding phase, and the third was a distracter stimulus chosen from the pool of the remaining six possible items. The animal was required to touch the two cue items in the same temporal order as they were presented in the encoding phase.
Electrophysiological Recording.
Following initial behavioral training, the animals were implanted with a head post and recording chambers under aseptic conditions using isoflurane anesthesia. The procedure for the recording of MTL has been described in detail previously (26). After we had almost finished the recording from MTL, we implanted recording chambers for the lateral PFC. The chambers were implanted so that the insertion of the microelectrodes would be most vertical to the brain surface. To avoid an interaction to a manipulator for MTL (Flex Alfaomega), we used an ultramicro manipulator for lateral PFC (Narishige). We set one to four ultramicromanipulators to the recording chamber for PFC, which allowed us to record single-unit activities from one to four independent glass-coated single-wire tungsten microelectrodes (0.5–1.0 MΩ; Alfaomega). Placement of microelectrodes into the target areas was guided by the individual brain atlases from MRI scans (3T). To confirm recording sites in PFC, we left four to five electrodes in the brain through the grid system in each recording chamber as references after all of the recording sessions. The animals were perfused transcardinally with saline followed by formalin with the reference electrodes in their brains. We localized the positions of reference electrodes relative to the principal sulcus and reconstructed the recording sites. The recording sites covered dorsal and ventral parts along the posterior half to two-thirds of the principal sulcus (Fig. 1A).
Data Analysis.
All neuronal data were analyzed with custom-written Matlab programs including the Statistics Toolbox. We examined the effect of item identity on a response triggered by a cue stimulus presentation as the firing rate during the period extending from 80 to 840 ms after the cue onset. We used this analysis time window (760 ms) that was larger than the duration of the cue stimulus presentation (320 ms) to better characterize the heterogeneous nature of the signals seen across PFC, MTL, and TE. We tested the differential stimulus selectivity of each neuron for cue 1 and cue 2 separately using a one-way ANOVA with the eight stimuli as a main factor. We examined the temporal dynamics of stimulus-selective activity for each item cell using an R2 statistic from the ANOVA table; we calculated how much of the variance in the factor of stimuli could account for time-averaged firing rates during a 100-ms window centered at the given time point t (33, 50). This time window was stepped in 5-ms increments. The R2 statistics at the time point t were defined as STI(t) for both cue 1 and cue 2.
To examine the effect of the temporal order of stimulus presentations on neuronal activities, we compared spike firing rates from 80 to 400 ms after the stimulus onset between cue 1 and cue 2 using the paired t test for each neuron. We referred to neurons with significantly (P < 0.05) different activities between two cue periods as “order-selective neurons.” Temporal dynamics of spike-firing rates during the delay period between cue 1 and cue 2 were examined for each neuron using a polynomial fitting method. We calculated the average response across trials for each 40 ms of time bin during the delay period (920 ms) from the cue 1 offset to the cue 2 onset. The firing rates at 23 time points were fit by a zero-order polynomial, a first-order polynomial, and a second-order polynomial curve. We compared the zero-order polynomial fitting with the first-order and the first-order polynomial fitting with the second order by F test (26, 27).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 MH086563 (to W.A.S. and Y.N.) and by National Natural Science Foundation of China Grant 31421003 (to Y.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712711114/-/DCSupplemental.
References
- 1.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 2.Milner B, Petrides M, Smith ML. Frontal lobes and the temporal organization of memory. Hum Neurobiol. 1985;4:137–142. [PubMed] [Google Scholar]
- 3.Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- 4.McAndrews MP, Milner B. The frontal cortex and memory for temporal order. Neuropsychologia. 1991;29:849–859. doi: 10.1016/0028-3932(91)90051-9. [DOI] [PubMed] [Google Scholar]
- 5.Kesner RP, Hopkins RO, Fineman B. Item and order dissociation in humans with prefrontal cortex damage. Neuropsychologia. 1994;32:881–891. doi: 10.1016/0028-3932(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 6.Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- 7.Kesner RP, Novak JM. Serial position curve in rats: Role of the dorsal hippocampus. Science. 1982;218:173–175. doi: 10.1126/science.7123228. [DOI] [PubMed] [Google Scholar]
- 8.Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. J Neurosci. 2002;22:5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayes AR, et al. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cogn Neuropsychol. 2001;18:97–123. doi: 10.1080/02643290125897. [DOI] [PubMed] [Google Scholar]
- 11.Downes JJ, Mayes AR, MacDonald C, Hunkin NM. Temporal order memory in patients with Korsakoff’s syndrome and medial temporal amnesia. Neuropsychologia. 2002;40:853–861. doi: 10.1016/s0028-3932(01)00172-5. [DOI] [PubMed] [Google Scholar]
- 12.Zorrilla LT, Aguirre GK, Zarahn E, Cannon TD, D’Esposito M. Activation of the prefrontal cortex during judgments of recency: A functional MRI study. Neuroreport. 1996;7:2803–2806. doi: 10.1097/00001756-199611040-00079. [DOI] [PubMed] [Google Scholar]
- 13.Cabeza R, et al. Brain regions differentially involved in remembering what and when: A PET study. Neuron. 1997;19:863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 14.Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: A positron emission tomography study. J Cogn Neurosci. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- 15.Konishi S, et al. Neural correlates of recency judgment. J Neurosci. 2002;22:9549–9555. doi: 10.1523/JNEUROSCI.22-21-09549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki M, et al. Neural basis of temporal context memory: A functional MRI study. Neuroimage. 2002;17:1790–1796. doi: 10.1006/nimg.2002.1303. [DOI] [PubMed] [Google Scholar]
- 17.Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins LJ, Ranganath C. Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. J Neurosci. 2010;30:15558–15565. doi: 10.1523/JNEUROSCI.1337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuBrow S, Davachi L. Temporal binding within and across events. Neurobiol Learn Mem. 2016;134:107–114. doi: 10.1016/j.nlm.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Jacques P, Rubin DC, LaBar KS, Cabeza R. The short and long of it: Neural correlates of temporal-order memory for autobiographical events. J Cogn Neurosci. 2008;20:1327–1341. doi: 10.1162/jocn.2008.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehn H, et al. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tubridy S, Davachi L. Medial temporal lobe contributions to episodic sequence encoding. Cereb Cortex. 2011;21:272–280. doi: 10.1093/cercor/bhq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichenbaum H. Time cells in the hippocampus: A new dimension for mapping memories. Nat Rev Neurosci. 2014;15:732–744. doi: 10.1038/nrn3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 27.Sakon JJ, Naya Y, Wirth S, Suzuki WA. Context-dependent incremental timing cells in the primate hippocampus. Proc Natl Acad Sci USA. 2014;111:18351–18356. doi: 10.1073/pnas.1417827111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ninokura Y, Mushiake H, Tanji J. Representation of the temporal order of visual objects in the primate lateral prefrontal cortex. J Neurophysiol. 2003;89:2868–2873. doi: 10.1152/jn.00647.2002. [DOI] [PubMed] [Google Scholar]
- 29.Warden MR, Miller EK. Task-dependent changes in short-term memory in the prefrontal cortex. J Neurosci. 2010;30:15801–15810. doi: 10.1523/JNEUROSCI.1569-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci USA. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoshi E, Tanji J. Area-selective neuronal activity in the dorsolateral prefrontal cortex for information retrieval and action planning. J Neurophysiol. 2004;91:2707–2722. doi: 10.1152/jn.00904.2003. [DOI] [PubMed] [Google Scholar]
- 32.Hoshi E. Functional specialization within the dorsolateral prefrontal cortex: A review of anatomical and physiological studies of non-human primates. Neurosci Res. 2006;54:73–84. doi: 10.1016/j.neures.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Naya Y, Yoshida M, Miyashita Y. Forward processing of long-term associative memory in monkey inferotemporal cortex. J Neurosci. 2003;23:2861–2871. doi: 10.1523/JNEUROSCI.23-07-02861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ninokura Y, Mushiake H, Tanji J. Integration of temporal order and object information in the monkey lateral prefrontal cortex. J Neurophysiol. 2004;91:555–560. doi: 10.1152/jn.00694.2003. [DOI] [PubMed] [Google Scholar]
- 35.Hirata Y, et al. Dorsal area 46 is a major target of disynaptic projections from the medial temporal lobe. Cereb Cortex. 2013;23:2965–2975. doi: 10.1093/cercor/bhs286. [DOI] [PubMed] [Google Scholar]
- 36.Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- 39.Rigotti M, et al. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–590. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howard MW, Eichenbaum H. Time and space in the hippocampus. Brain Res. 2015;1621:345–354. doi: 10.1016/j.brainres.2014.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salz DM, et al. Time cells in hippocampal area CA3. J Neurosci. 2016;36:7476–7484. doi: 10.1523/JNEUROSCI.0087-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 43.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 44.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 45.Bichot NP, Heard MT, DeGennaro EM, Desimone R. A source for feature-based attention in the prefrontal cortex. Neuron. 2015;88:832–844. doi: 10.1016/j.neuron.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eichenbaum H. Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci. 2017;18:547–558. doi: 10.1038/nrn.2017.74. [DOI] [PubMed] [Google Scholar]
- 47.D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baddeley A. Working memory: Theories, models, and controversies. Annu Rev Psychol. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- 49.Schiller D, et al. Memory and space: Towards an understanding of the cognitive map. J Neurosci. 2015;35:13904–13911. doi: 10.1523/JNEUROSCI.2618-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erickson CA, Desimone R. Responses of macaque perirhinal neurons during and after visual stimulus association learning. J Neurosci. 1999;19:10404–10416. doi: 10.1523/JNEUROSCI.19-23-10404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.