Significance
It is crucial to understand what governs the growth and spread of populations colonizing novel environments to better predict species responses to global change, including range shifts in response to warming and biological invasions. Evolutionary processes can be rapid enough to influence colonizing populations; however, it is unclear whether evolution governs the course of colonization events or if it is an outcome that arises gradually after successful establishment. We either allowed or restricted evolution in replicate populations released in a novel environment, and found that populations that were allowed to evolve grew three times larger and expanded their ranges 46% faster compared with nonevolving populations. Thus, evolution facilitates colonization from the outset and should be considered in management decisions.
Keywords: eco-evolutionary dynamics, range expansion, dispersal evolution, rapid evolution, adaptation
Abstract
Colonization and expansion into novel landscapes determine the distribution and abundance of species in our rapidly changing ecosystems worldwide. Colonization events are crucibles for rapid evolution, but it is not known whether evolutionary changes arise mainly after successful colonization has occurred, or if evolution plays an immediate role, governing the growth and expansion speed of colonizing populations. There is evidence that spatial evolutionary processes can speed range expansion within a few generations because dispersal tendencies may evolve upwards at range edges. Additionally, rapid adaptation to a novel environment can increase population growth rates, which also promotes spread. However, the role of adaptive evolution and the relative contributions of spatial evolution and adaptation to expansion are unclear. Using a model system, red flour beetles (Tribolium castaneum), we either allowed or constrained evolution of populations colonizing a novel environment and measured population growth and spread. At the end of the experiment we assessed the fitness and dispersal tendency of individuals originating either from the core or edge of evolving populations or from nonevolving populations in a common garden. Within six generations, evolving populations grew three times larger and spread 46% faster than populations in which evolution was constrained. Increased size and expansion speed were strongly driven by adaptation, whereas spatial evolutionary processes acting on edge subpopulations contributed less. This experimental evidence demonstrates that rapid evolution drives both population growth and expansion speed and is thus crucial to consider for managing biological invasions and successfully introducing or reintroducing species for management and conservation.
Evolution can proceed so rapidly that it shapes ecological dynamics (1–4). However, the role of rapid evolution in colonization and expansion into novel landscapes is unresolved, despite its potential importance in predicting and managing biological invasions (5) and conducting successful translocations, introductions, and reintroductions of species (6–8). Many forms of rapid evolution have been demonstrated in colonizing populations, including local adaptation to new habitats (5), the evolution of geographic clines along climatic gradients (9, 10), and the evolution of dispersal ability (11, 12). However, it is generally unknown whether evolved differences in colonizing populations are outcomes that arise after those populations have successfully established and spread (13) or if evolution acts as an architect of colonization, determining the growth of colonizing populations and the speed of expansion.
Population growth rate and dispersal are traits theoretically predicted to jointly determine the speed of range expansion (14–16). Ecological theory treats population growth rate and dispersal as fixed characteristics of species (14–16). If there is genetic variation for these traits, they may instead rapidly evolve as organisms colonize and disperse through novel environments. If populations adapt to novel aspects of the environment, population growth rates could increase across the entire range (17), driving an increase in the speed of range expansion (14, 16, 18).
Concurrently, spatial evolutionary processes can increase dispersal tendency via spatial sorting, and either increase or decrease growth rate of individuals residing at the edge of expansions relative to the range core (19, 20). Spatial sorting occurs when individuals exhibiting traits that enhance dispersal accumulate at range edges and mate with each other (19, 20). Theory predicts that this sorting will increase the frequency of dispersive genotypes at the range edges in subsequent generations compared with individuals residing in the population core (19, 20). Population growth in the expanding edge can evolve relative to the core to either increase or decrease. Increased growth rates in low-density edge populations can evolve via reductions in Allee effects (21) or via increases in reproduction (essentially favoring r-selected individuals) (19, 22, 23). Decreased growth rates also can evolve in two ways. First, in a process called gene surfing, serial founding events occurring at the expanding edge can lead to an increase in the frequency of deleterious alleles in a spatial analog to genetic drift (24–26). The reduction in fitness associated with the buildup of deleterious alleles at the expanding edge is called expansion load (24–26). Second, growth rates may also decrease in edge populations if there is a tradeoff between dispersal and reproduction (27, 28).
The individual, additive, or antagonistic effects of the above spatial evolutionary processes acting on dispersal tendencies and population growth rates at range edges, combined with the potential effects of adaptation on overall population growth rates, will thus jointly determine the population dynamics and speed of range expansion of colonizing populations. Because previous experiments have largely been restricted to evaluating rapid evolution in populations spreading in natal environments to which they are already adapted (29–33), the roles and interactions of multiple evolutionary processes, including adaptation to a novel environment, remain unknown.
We evaluated the role of rapid evolution during colonization by founding red flour beetle (Tribolium castaneum) populations in a novel environment that posed an adaptive challenge but in which persistence and population growth were possible even without adaptation. We imposed two treatments: normally evolving populations, and populations in which we constrained evolution. In this latter no-evolution treatment, we replaced beetles one-for-one each generation with offspring of individuals drawn randomly from a large source population. This prevented adaptation, and is an approach used in parasite–host studies (3, 34, 35). Because we were interested in spatial expansion, this control also prevented spatial evolutionary processes while maintaining demographic processes. We incorporated dispersal into our design by using a linear series of habitat patches connected by holes as experimental landscapes that allowed populations to expand their range. We tracked population growth and expansion for six generations. Using a common garden experiment, we then evaluated adaptation to the novel environment, and whether dispersal tendencies evolved.
Results and Discussion
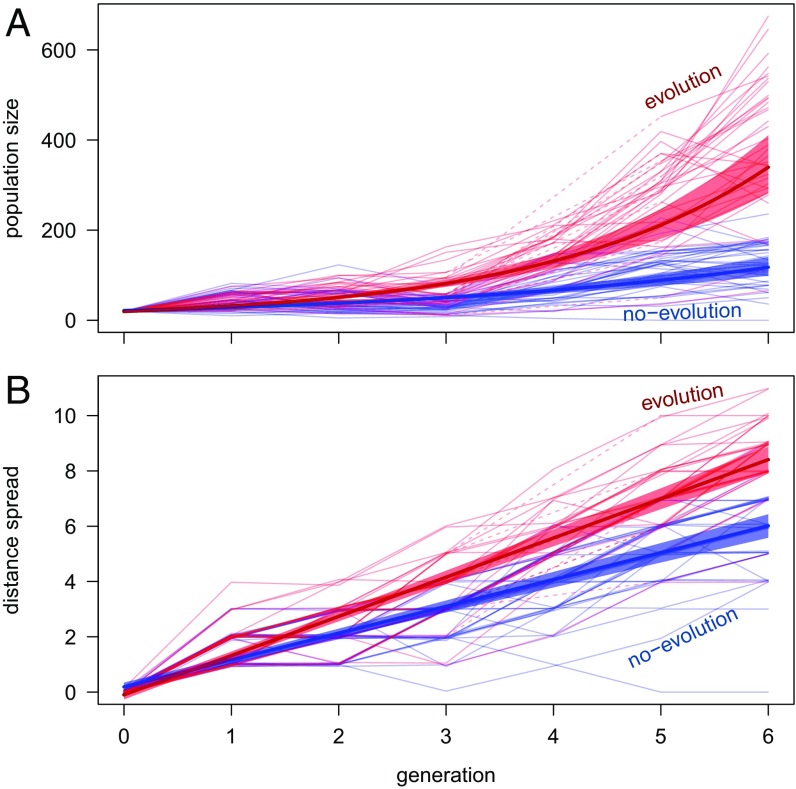
We found that evolving populations grew faster and 2.89 (95% CI 2.24–3.73) times larger than nonevolving populations (parametric bootstrap, treatment by generation interaction: P = 0.0001) within the first six generations (Figs. 1 and 2A). Evolving populations also expanded their range 46.0% faster than populations that were restricted from evolving (CI 32.2–62.0%, parametric bootstrap, treatment by generation interaction: P = 0.0001; Figs. 1 and 2B). The magnitude of the effect of evolution increased with time, and was evident within only three generations (Figs. 1 and 2B). The large differences between the evolution and no-evolution treatment suggest that evolution strongly drives range expansions, even within the first few generations. There was also substantial variation among evolving populations, particularly in population size (Fig. 2A). This variation among evolving populations could be linked to the amount of genetic variation the founders harbored by chance at the start (36). In this system, the amount of genetic variation can strongly influence population sizes in these experimental arenas (17).
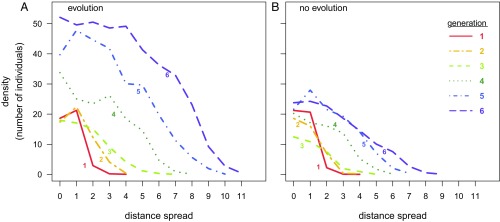
Fig. 1.
Density (mean number of individuals per habitat patch) of T. castaneum populations through space and time, showing population growth and range expansion. Populations were founded with 20 individuals (generation 0) in patch 1 (distance spread = 0) in a novel environment and were either allowed to evolve (A) or prevented from evolving (B) for six generations.
Fig. 2.
(A) Total population size (number of individuals) and (B) distance spread of T. castaneum populations founded with 20 individuals that were either allowed to evolve (red) or prevented from evolving (blue) in a novel environment for six generations. Populations were initiated in the first patch of linear landscapes. The distance spread indicates the distance between the farthest habitat patch into which adults dispersed in any given generation and the core patch within a landscape of linearly connected habitat patches. Model means are shown as bolded lines and shading represents bootstrapped 95% CIs. Individual replicate raw data are shown as thin red lines (evolution treatment) or thin blue lines (no-evolution treatment). In B, thin lines are offset to reveal overlapping values. Dashed lines between generations 3 and 5 for 10 replicates of evolving populations represent linear interpolations of spread for generation 4 for which data were missing.
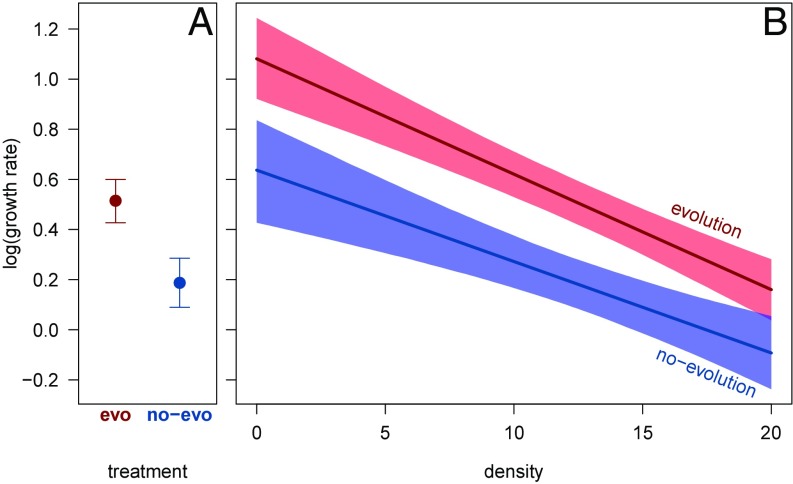
Population growth rates, measured in the common garden, more than doubled in evolving populations compared with nonevolving populations, confirming adaptation to the novel environment [parametric bootstrap, treatment: P = 0.0001; multiplicative increase in growth rate averaged across densities on log scale: 2.73 (CI 1.72–5.77); Fig. 3A, intercept in Fig. 3B indicates density-independent growth rates, which differ by 0.44 on log scale (CI 0.188–0.708)]. Such adaptive increases in growth rates can directly increase expansion speed (14, 16).
Fig. 3.
(A) Average growth rate across densities, and (B) density-dependent growth rates of T. castaneum populations measured in a common garden using descendants from evolving populations or individuals from the source population representing nonevolving populations. The density-independent (intrinsic) growth rate is the intercept at density = 0 in B. Growth rates for the core and edge subpopulations of evolving landscapes were pooled for analyses since they were similar. Error bars (A) and shading (B) represent bootstrapped 95% CIs.
In theory, at the range edge lower density can select for individuals with high reproductive rates, whereas expansion load or tradeoffs between dispersal and reproduction may lower population growth rate. Population growth rates in our experiment remained similar between the core and edge of evolving populations (difference at average density = 0.049 on log scale, CI −0.066–0.166; parametric bootstrap, location: P = 0.409). It is possible that the above-described spatial evolutionary processes increasing or decreasing population growth rates at the edge acted together in opposing directions to balance growth rates between the core and edge of evolving populations. However, it is probable that the increase in growth rates across the entire population due to adaptation was so high (Fig. 3) that it overwhelmed the more subtle effects of spatial selection acting on life histories at the expanding edge (37).
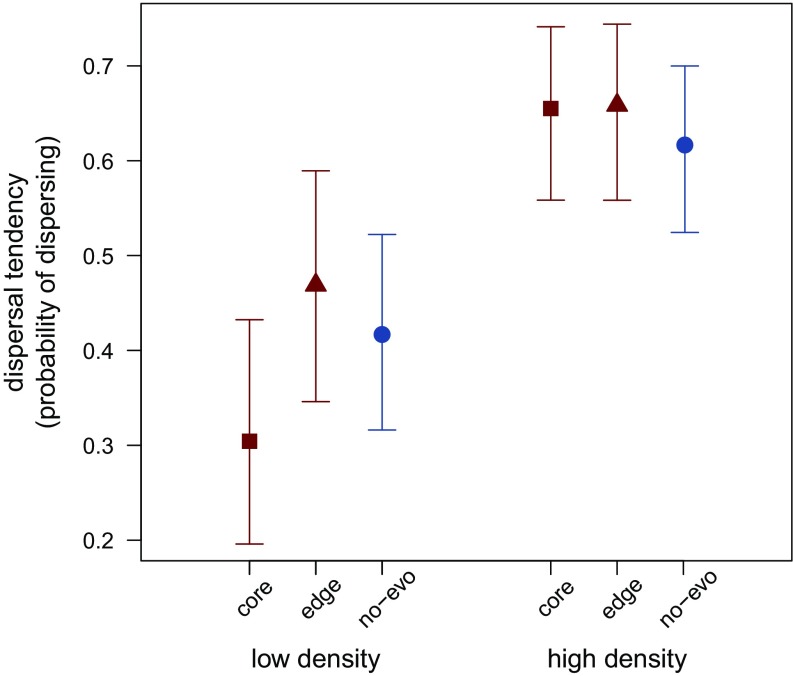
If spatial evolution of dispersive genotypes contributed to the observed rapid expansion speed of evolving populations then individuals at range edges would show increased dispersal tendencies compared with individuals from the core or from nonevolving populations. We did not find a statistically significant effect of evolution on dispersal tendency at the edge or in the core for either low (parametric bootstrap, source at low density: P = 0.269) or high densities (parametric bootstrap, source at high density: P = 0.776) due to high variability in dispersal tendency. However, lack of significance does not allow us to conclude that dispersal evolution was negligible compared with adaptation to the novel environment. Indeed, our estimates for dispersal tendency are in line with theoretical predictions (19, 20), and evidence from other experimental evolution studies (29–32). We found that dispersal tendency of descendants of individuals from range edges (last two to three patches) was 12.3% (CI −22.3–61.7%) higher than that of individuals from nonevolving populations, and 54.1% (CI −1.6–150.3%) higher than that of descendants of the core of evolving populations, when dispersing at low densities (Fig. 4). This increase is consistent with evidence from range expansions in nature, where highly dispersive phenotypes were found in edge populations in a number of butterfly and cricket species (38–41), invasive plants (42), cane toads (11), and invasive ladybird beetles (12). We also found that descendants of individuals from the core were 27.1% (CI −13.7–55.6) less likely to disperse out of low-density patches than individuals from nonevolving populations (Fig. 4). This is consistent with an evolved response of core individuals to take advantage of low-density conditions that they do not normally experience. Individuals from the core may have perceived the low-density environment as high quality and therefore exhibited lower dispersal tendencies in that environment, favoring reproduction over dispersal. Finally, our results show striking accord with the findings of Weiss-Lehman et al. (31) who used the same system to infer spatial evolution in a natal environment, both in terms of increased dispersal of individuals from the edge and decreased dispersal of those from the core when at low density (Supporting Information). Thus, on balance, it is likely that spatial evolution played some role in increasing expansion speeds and thus should be considered relative to adaptive evolution of population growth rates.
Fig. 4.
Dispersal tendency (probabilities of dispersing) of first-generation descendants of T. castaneum individuals sampled either from the core or edge of populations evolving and spreading in a novel environment for six generations or of individuals originating from the source population of the no-evolution treatment. Dispersal tendency was calculated using the proportion of individuals that dispersed out of the first patch of a landscape that was founded with 10 (low density) or 33 (high density) individuals in a common garden experiment. Bars represent bootstrapped 95% CIs.
To estimate the relative contributions of spatial evolution and adaptive evolution to expansion speed, we used results for population growth rates and dispersal tendency from the common garden to calculate the linear approximation of the expansion speed, , where r is the density independent population growth rate and D is the diffusion coefficient (Methods). Evolving populations were estimated to expand 39% faster than nonevolving ones, a value similar to the observed 46% difference in expansion speed. We estimated that most of the increase in expansion speed stemmed from differences in growth rate associated with adaptation (29 of the 39%). Spatial evolutionary processes increasing dispersal at the edge played a smaller role (accounting for at most 10 of the 39%), which was of comparable magnitude to the effect found in a previous study (6%) where populations were not challenged to adapt to a novel environment (31). These results suggest that adaptation to the novel environment and the accompanying increase in growth rates were the main drivers of increased expansion speed with spatial evolutionary processes playing a relatively smaller, although potentially important, role.
Furthermore, the interaction of an increased carrying capacity with positive density-dependent dispersal is not accounted for in these linearized calculations but it could have contributed to a further increase in expansion speed in the evolving populations. Evolution more than doubled carrying capacity (parametric bootstrap, treatment: P = 0.0001), increasing population densities in the core (first patch) of evolving populations to an average 46.5 individuals (CI 39.5–54.9), relative to 21.6 individuals (CI 18.6–25.1) in nonevolving populations (Fig. S1). In this experimental system, carrying capacity is jointly determined by population growth rate and intraspecific interaction strength (ref. 43 and Supporting Information). Because intraspecific interaction strength was little influenced by evolution (similar slopes in Fig. 3B), the increase in carrying capacity was largely due to evolution of increased population growth rate. Dispersal was strongly density dependent, but the degree of density dependence was little affected by evolution (Fig. 4). In the common garden, beetles were more likely to move out of the first patch in higher-density than lower-density dispersal arenas (parametric bootstrap, density: P = 0.001), regardless of whether individuals originated from the core or edge of evolving populations or nonevolving populations (parametric bootstrap, density by location interaction: P = 0.282; Fig. 4). The linearized calculations above describe a classic “pulled” traveling wave where the density-independent rates of growth and dispersal at the expansion front determine the speed, whereas density-dependent dispersal changes the traveling wave to a “pushed” type (44–46). In pushed fronts, the speed is determined by a larger part of the wave, which can speed the expansion relative to pulled fronts (44–46). Thus, the steeper wave front of the evolving populations (Fig. 1) combined with density-dependent dispersal (Fig. 4) likely increased the velocity of the expansion (Supporting Information). We expect that the possibility of evolution increasing carrying capacity, which in turn alters invasion speed, may be general, because positive density-dependent dispersal is a common response to competition in insects (47), birds, and mammals (48). To conclude, consideration of density-dependent dispersal reinforces that evolution of growth rate was the main driver of expansion speed, because an effect of density-dependent dispersal is an indirect effect of growth rate.
Our experimental design simulated a sudden, large, environmental change that invasive species or biological control agents would experience when introduced into a novel environment. Native populations face similar environmental shifts following disturbance events such as deforestation. By implementing a control for evolutionary processes, we show clearly that rapid evolution drives both increases in population densities and expansion speed. Thus, evolution can be an architect of successful colonization and range expansion from the outset. Overall, our findings suggest that promoting demographic and genetic processes that increase evolutionary potential should be an important consideration in planning introductions of biological control agents or translocations or rereleases of threatened or declining species. For example, increasing the number of individuals released or augmenting earlier releases with more individuals may provide a buffer against the demographic cost of selection, increasing the time available for adaptation to occur (21, 49). Further, rapid adaptation can be facilitated by ensuring sufficient genetic variation in founders or by enabling admixture between isolated populations (17, 49–52). Conversely, preventing admixture and multiple introductions should be a priority for invasive species, because rapid evolution can drive invasions by increasing population growth rates and densities, and thus the rate of range expansion.
Methods
Experimental Design.
Source populations of T. castaneum used for the experiment were reared on a standard medium consisting of 95% wheat flour and 5% brewer’s yeast, and maintained as described in Szűcs et al. (53). To evaluate the effects of evolution on population dynamics and spread into a novel habitat, we founded populations in a novel environment that consisted of 98.8% corn flour, 1.14% wheat flour, and 0.06% brewer’s yeast. This environment represents a novel carbohydrate source and a nutritional challenge for T. castaneum, and causes an initial reduction of the growth rate (R = Nt/Nt−1) of the SF strain used as the source population, to just above 1 (53) (Fig. S2). Thus, the environment presents an evolutionary challenge, but populations would not be expected to decline to extinction deterministically and can grow even without adaptation.
Populations were founded (generation 0) by placing 20 adults in the first patch of a linear landscape with novel medium consisting initially of five habitat patches connected by 2-mm-diameter holes on the sides, following Melbourne and Hastings (54). Maternal environment can influence fecundity strongly in T. castaneum (49, 55, 56), and thus beetles from the source population spent a single generation in the novel medium before the start of the experiment to reduce maternal carryover effects from the natal environment. Adults were allowed to lay eggs for 24 h and were then removed, leaving the eggs to develop to adults. Thirty-four days later, barriers between habitat patches were removed to allow dispersal for 24 h. Dispersal was then halted by replacing the barriers, and adult beetles were censused by patch and provided with fresh medium in which to lay eggs for 24 h, reinitiating the cycle. These procedures were repeated for six generations, with extra patches added to the end of the landscapes each generation to ensure dispersal was not limited by the size of the landscape.
Two treatments were implemented: one allowed T. castaneum populations to evolve normally and one constrained evolution. The procedures for evolving populations followed the protocol described above, with censused beetles used to found the next generation. In contrast, for populations in which we constrained evolution, censused adults were discarded each generation and replaced one-for-one with individuals from the stock colony. These replacement individuals had spent a single generation in the novel environment to minimize maternal environmental carryover effects while also minimizing the opportunity for adaptation. The stock population was of course still able to evolve via drift, as well as via natural selection. However, stock populations were maintained at over 2,000 reproductive individuals, a size at which drift should have been negligible over the course of six generations. Further, populations had been maintained in our standard laboratory environment for over 15 generations at the start of this experiment, and thus should have been close to the adaptive peak for their natal environment. Initially, there were 40 replicate populations, in two temporal blocks of 20 replicates each, for both the evolution and no-evolution treatments, but five evolving populations were inadvertently dropped and one no-evolution population went extinct in the fifth generation, leaving 35 evolving and 39 nonevolving populations. Temporal blocks were started 1 wk apart.
Common Garden.
At the end of the colonization experiment, we measured growth rate and dispersal tendency at standardized densities to evaluate adaptive evolution to the novel environment and dispersal evolution in edge subpopulations. We sampled 40 individuals randomly both from the core (first patch) and leading edge (last 2–3 patches) of evolving populations at the end of generation 6. For the no-evolution treatment, 39 replicate samples of 40 individuals each were established with beetles drawn from the stock population (which, maintained on resource-rich medium, had a high growth rate and thus could easily provide this many surplus individuals each generation). Sampled individuals from evolving populations and the stock colony were reared for one generation in the novel environment to standardize maternal environment effects. The adults that emerged from this generation that standardized maternal environmental effects were used to assess adaptation to the novel environment. Adaptation was assayed by placing 5, 10, 15, or 20 of these beetles to lay eggs for 24 h into individual habitat patches and measuring population growth rate across these densities over a single generation. Due to insufficient numbers of beetles emerging from the experimental environment, ∼4% of replicates were initiated with beetle numbers that were close to (within two individuals), but not equal to, the standard densities of 5, 10, 15, or 20 beetles. We used descendants of these same beetles to assay dispersal tendencies. We measured movement of beetles out of the first patch of a linear landscape at two different densities (low, n = 10; high, n = 33). These densities were selected based on the number of beetles available for the core and edge of each evolving population and to represent a relatively low and a higher population density. Beetles spent 24 h in the first patch to allow for tunneling and equilibrating, and then barriers between patches were removed to allow for dispersal. After a 24-h dispersal period, the number of individuals that left the first patch was recorded.
Statistical Analyses.
Population sizes and expansion speed (the slope of distance spread over time) of evolving and nonevolving populations across generations were compared using linear mixed models. Population sizes were natural logarithm-transformed to meet the assumption of normality. In both models, treatment (evolution or no-evolution), generation (continuous value from 0 to 6), their interaction, and temporal block (n = 2) were fixed effects. Individual landscapes were included as random slopes across generations. At generation 0, both treatments had the same population size (N0 = 20) and distance spread (first patch); therefore, the effect of interest is the treatment-by-generation interaction, which reveals whether population size and expansion diverged through time between treatments. Data were missing for 10 replicates in the evolution treatment for generation 4 due to a lost data sheet; however, that should have minimal impact on the results.
Because dispersal is often density dependent in insects and other organisms, we evaluated whether the carrying capacity, and thus equilibrium population densities, differed between evolution treatments over time. We focused on densities in the core (first patch) of the landscapes, because these were likely closest to equilibrium and least influenced by expansion at the range edge. We compared densities in the cores of evolving and nonevolving populations using a linear mixed model and assessed whether these densities had stabilized (the carrying capacity had been reached) or were increasing (the carrying capacity had not yet been reached) during the last three generations of the experiment. Fixed effects were evolution treatment, generation, their interaction, and block. Individual landscapes were included as random intercepts and slopes across generations. Densities were natural logarithm-transformed to meet the assumption of homogeneity of variance.
Growth rates measured in the common garden experiment were compared between offspring of individuals sampled either from the core or edge of evolving populations to evaluate spatial evolutionary processes, and between the evolution and no-evolution treatments to evaluate adaptation to the novel environment. Growth rates were calculated as Nt/Nt−1 with each initial density, Nt−1. One replicate in the common garden experiment went extinct and was removed from the analysis. Fixed effects in the model assessing the effects of spatial evolution were location (core or edge), initial density (continuous), their interaction, and block. Individual landscapes were included as random intercepts. To assess adaptation, growth rates were pooled between core and edge subpopulations of the evolution treatment because they did not differ. The same model was used as above by replacing location as an effect with treatment (evolution or no-evolution). Growth rates were natural logarithm-transformed to meet the assumption of homogeneity of variance.
Dispersal tendencies from low- and high-density patches were compared between evolving and nonevolving populations by evaluating the proportion of individuals that moved out of the first patch in the common garden experiment using a generalized linear mixed model with a logit link function and binomial distribution. Fixed effects in the full model included source of beetles (nonevolving, core of evolving landscapes, or edge of evolving landscapes), initial density (categorical: 10 or 33 individuals), their interaction, and block. Individual landscapes were random effects, and an observation-level random effect was added to account for overdispersion.
Statistical analyses were performed using the package lme4 (version 1.1.12) in R version 3.2.3 (57). For all models, parametric bootstraps were used with 10,000 iterations to evaluate significance of model terms, and we estimated CIs using the adjusted bootstrap percentile method with 10,000 iterations using packages pbkrtest (version 0.4.6) and boot (version 1.3.18). Data are deposited in Dryad.
Relative Contribution of Adaptation and Dispersal Evolution to Expected Change in Expansion Speed.
We estimated the change in expansion speed due to evolution using the theoretically and empirically supported linear approximation of the expansion speed, , where r is the density-independent growth rate and D is the diffusion coefficient, where and p is the probability of a beetle dispersing from a patch (31). We used estimates of r and p from evolving edge populations and nonevolving populations from the common garden experiment (Figs. 3 and 4), where p is the probability of dispersing from the first patch in the low-density treatment (i.e., the low density expected at range edges). We then calculated the proportional change in speed, , where is the proportional change in density-independent growth rate and is the proportional change in diffusion coefficient between edge and nonevolving populations. We estimated the contribution of change in growth rate (i.e., adaptation) to the total expected change in expansion speed as . We estimated the maximum contribution of change in diffusion coefficient (i.e., dispersal evolution) as .
Supplementary Material
Acknowledgments
We thank Madeline Morris and Consuelo Reyes for help with data collection and Andrew Norton for useful discussions. This manuscript was improved by thoughtful comments from the editor and two anonymous reviewers. Funding was provided by US National Science Foundation Grants DEB-0949619 (to R.A.H.) and DEB-0949595 (to B.A.M.), and Graduate Research Fellowship 1144083 (to C.W.-L.), with additional support from the US Department of Agriculture National Institute of Food and Agriculture, the Colorado Agricultural Experiment Station via Hatch Projects 0231900 and 0229555, Agropolis Fondation (CfP 2015-02, Labex Agro:ANR-10-LABX-0001-01), and LabEX-CeMEB.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data are available from the Dryad digital repository (https://doi.org/10.5061/dryad.6tc61).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712934114/-/DCSupplemental.
References
- 1.Yoshida T, Jones LE, Ellner SP, Fussmann GF, Hairston NG., Jr Rapid evolution drives ecological dynamics in a predator-prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. [DOI] [PubMed] [Google Scholar]
- 2.Hendry AP. Eco-Evolutionary Dynamics. Princeton Univ Press; Princeton, NJ: 2016. [Google Scholar]
- 3.Fussmann GF, Loreau M, Abrams PA. Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol. 2007;21:465–477. [Google Scholar]
- 4.Schoener TW. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331:426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- 5.Colautti RI, Lau JA. Contemporary evolution during invasion: Evidence for differentiation, natural selection, and local adaptation. Mol Ecol. 2015;24:1999–2017. doi: 10.1111/mec.13162. [DOI] [PubMed] [Google Scholar]
- 6.Fauvergue X, Vercken E, Malausa T, Hufbauer RA. The biology of small, introduced populations, with special reference to biological control. Evol Appl. 2012;5:424–443. doi: 10.1111/j.1752-4571.2012.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: Contemporary evolution and the dynamics of persistence. Funct Ecol. 2007;21:444–454. [Google Scholar]
- 8.LaRue EA, Chambers SM, Emery NC. Eco-evolutionary dynamics in restored communities and ecosystems. Restor Ecol. 2017;25:19–26. [Google Scholar]
- 9.Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L. Rapid evolution of a geographic cline in size in an introduced fly. Science. 2000;287:308–309. doi: 10.1126/science.287.5451.308. [DOI] [PubMed] [Google Scholar]
- 10.Colautti RI, Barrett SCH. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science. 2013;342:364–366. doi: 10.1126/science.1242121. [DOI] [PubMed] [Google Scholar]
- 11.Phillips BL, Brown GP, Webb JK, Shine R. Invasion and the evolution of speed in toads. Nature. 2006;439:803. doi: 10.1038/439803a. [DOI] [PubMed] [Google Scholar]
- 12.Lombaert E, et al. Rapid increase in dispersal during range expansion in the invasive ladybird Harmonia axyridis. J Evol Biol. 2014;27:508–517. doi: 10.1111/jeb.12316. [DOI] [PubMed] [Google Scholar]
- 13.Dlugosch KM, Anderson SR, Braasch J, Cang FA, Gillette HD. The devil is in the details: Genetic variation in introduced populations and its contributions to invasion. Mol Ecol. 2015;24:2095–2111. doi: 10.1111/mec.13183. [DOI] [PubMed] [Google Scholar]
- 14.Fisher RA. The wave of advance of advantageous genes. Ann Eugen. 1937;7:355–369. [Google Scholar]
- 15.Lockwood JL, Hoopes MF, Marchetti MP, editors. Modeling the Geographic Spread of Invasive Species. Blackwell; Oxford: 2007. pp. 132–157. [Google Scholar]
- 16.Skellam JG. Random dispersal in theoretical populations. Biometrika. 1951;38:196–218. [PubMed] [Google Scholar]
- 17.Szűcs M, Melbourne BA, Tuff T, Weiss-Lehman C, Hufbauer RA. Genetic and demographic founder effects have long-term fitness consequences for colonising populations. Ecol Lett. 2017;20:436–444. doi: 10.1111/ele.12743. [DOI] [PubMed] [Google Scholar]
- 18.García-Ramos G, Rodríguez D. Evolutionary speed of species invasions. Evolution. 2002;56:661–668. doi: 10.1554/0014-3820(2002)056[0661:esosi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Phillips BL, Brown GP, Shine R. Life-history evolution in range-shifting populations. Ecology. 2010;91:1617–1627. doi: 10.1890/09-0910.1. [DOI] [PubMed] [Google Scholar]
- 20.Shine R, Brown GP, Phillips BL. An evolutionary process that assembles phenotypes through space rather than through time. Proc Natl Acad Sci USA. 2011;108:5708–5711. doi: 10.1073/pnas.1018989108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanarek AR, Webb CT, Barfield M, Holt RD. Overcoming Allee effects through evolutionary, genetic, and demographic rescue. J Biol Dyn. 2015;9:15–33. doi: 10.1080/17513758.2014.978399. [DOI] [PubMed] [Google Scholar]
- 22.Burton OJ, Phillips BL, Travis JMJ. Trade-offs and the evolution of life-histories during range expansion. Ecol Lett. 2010;13:1210–1220. doi: 10.1111/j.1461-0248.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- 23.MacArthur R, Wilson E. The Theory of Island Biogeography. Princeton Univ Press; Princeton, NJ: 1967. [Google Scholar]
- 24.Hallatschek O, Nelson DR. Gene surfing in expanding populations. Theor Popul Biol. 2008;73:158–170. doi: 10.1016/j.tpb.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Peischl S, Dupanloup I, Kirkpatrick M, Excoffier L. On the accumulation of deleterious mutations during range expansions. Mol Ecol. 2013;22:5972–5982. doi: 10.1111/mec.12524. [DOI] [PubMed] [Google Scholar]
- 26.Peischl S, Kirkpatrick M, Excoffier L. Expansion load and the evolutionary dynamics of a species range. Am Nat. 2015;185:E81–E93. doi: 10.1086/680220. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson B, Johansson A. Seasonal polyphenism and developmental trade-offs between flight ability and egg laying in a pierid butterfly. Proc Biol Sci. 2008;275:2131–2136. doi: 10.1098/rspb.2008.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zera AJ, Denno RF. Physiology and ecology of dispersal polymorphism in insects. Annu Rev Entomol. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. [DOI] [PubMed] [Google Scholar]
- 29.Fronhofer EA, Altermatt F. Eco-evolutionary feedbacks during experimental range expansions. Nat Commun. 2015;6:6844. doi: 10.1038/ncomms7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochocki BM, Miller TE. Rapid evolution of dispersal ability makes biological invasions faster and more variable. Nat Commun. 2017;8:14315. doi: 10.1038/ncomms14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss-Lehman C, Hufbauer RA, Melbourne BA. Rapid trait evolution drives increased speed and variance in experimental range expansions. Nat Commun. 2017;8:14303. doi: 10.1038/ncomms14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JL, Kendall BE, Levine JM. Rapid evolution accelerates plant population spread in fragmented experimental landscapes. Science. 2016;353:482–485. doi: 10.1126/science.aaf6268. [DOI] [PubMed] [Google Scholar]
- 33.Wagner NK, Ochocki BM, Crawford KM, Compagnoni A, Miller TEX. Genetic mixture of multiple source populations accelerates invasive range expansion. J Anim Ecol. 2017;86:21–34. doi: 10.1111/1365-2656.12567. [DOI] [PubMed] [Google Scholar]
- 34.Pimentel D, al-Hafidh R. Ecological control of a parasite population by genetic evolution in the parasite-host system. Ann Entomol Soc Am. 1965;58:1–6. doi: 10.1093/aesa/58.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Pimentel D, Nagel WP, Madden JL. Space-time structure of environment and survival of parasite–host systems. Am Nat. 1963;97:141–167. [Google Scholar]
- 36.Holt R, Barfield M, Gomulkiewicz R. Theories of niche conservatism and evolution: Could exotic species be potential tests? In: Sax D, Stachowicz J, Gaines S, editors. Species Invasions: Insights into Ecology, Evolution, and Biogeography. Sinauer; Sunderland, MA: 2005. pp. 259–290. [Google Scholar]
- 37.Gilbert KJ, et al. Local adaptation interacts with expansion load during range expansion: Maladaptation reduces expansion load. Am Nat. 2017;189:368–380. doi: 10.1086/690673. [DOI] [PubMed] [Google Scholar]
- 38.Hanski I, Breuker CJ, Schops K, Setchfield R, Nieminen M. Population history and life history influence the migration rate of female Glanville fritillary butterflies. Oikos. 2002;98:87–97. [Google Scholar]
- 39.Hughes CL, Dytham C, Hill JK. Modelling and analysing evolution of dispersal in populations at expanding range boundaries. Ecol Entomol. 2007;32:437–445. [Google Scholar]
- 40.Simmons AD, Thomas CD. Changes in dispersal during species’ range expansions. Am Nat. 2004;164:378–395. doi: 10.1086/423430. [DOI] [PubMed] [Google Scholar]
- 41.Thomas CD, et al. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- 42.Monty A, Mahy G. Evolution of dispersal traits along an invasion route in the wind-dispersed Senecio inaequidens (Asteraceae) Oikos. 2010;119:1563–1570. [Google Scholar]
- 43.Melbourne BA, Hastings A. Extinction risk depends strongly on factors contributing to stochasticity. Nature. 2008;454:100–103. doi: 10.1038/nature06922. [DOI] [PubMed] [Google Scholar]
- 44.Newman WI. Some exact solutions to a non-linear diffusion problem in population genetics and combustion. J Theor Biol. 1980;85:325–334. doi: 10.1016/0022-5193(80)90024-7. [DOI] [PubMed] [Google Scholar]
- 45.Sherratt J, Marchant B. Nonsharp travelling wave fronts in the Fisher equation with degenerate nonlinear diffusion. Appl Math Lett. 1996;9:33–38. [Google Scholar]
- 46.Stokes A. On two types of moving front in quasilinear diffusion. Math Biosci. 1976;31:307–315. [Google Scholar]
- 47.Denno R, Peterson M, editors. Density-Dependent Dispersal and Its Consequences for Population Dynamics. Academic; San Diego: 1995. pp. 113–130. [Google Scholar]
- 48.Matthysen E. Density-dependent dispersal in birds and mammals. Ecography. 2005;28:403–416. [Google Scholar]
- 49.Hufbauer RA, et al. Three types of rescue can avert extinction in a changing environment. Proc Natl Acad Sci USA. 2015;112:10557–10562. doi: 10.1073/pnas.1504732112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tallmon DA, Luikart G, Waples RS. The alluring simplicity and complex reality of genetic rescue. Trends Ecol Evol. 2004;19:489–496. doi: 10.1016/j.tree.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Frankham R. Genetic rescue of small inbred populations: Meta-analysis reveals large and consistent benefits of gene flow. Mol Ecol. 2015;24:2610–2618. doi: 10.1111/mec.13139. [DOI] [PubMed] [Google Scholar]
- 52.Whiteley AR, Fitzpatrick SW, Funk WC, Tallmon DA. Genetic rescue to the rescue. Trends Ecol Evol. 2015;30:42–49. doi: 10.1016/j.tree.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Szűcs M, Melbourne BA, Tuff T, Hufbauer RA. The roles of demography and genetics in the early stages of colonization. Proc Biol Sci. 2014 doi: 10.1098/rspb.2014.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melbourne BA, Hastings A. Highly variable spread rates in replicated biological invasions: Fundamental limits to predictability. Science. 2009;325:1536–1539. doi: 10.1126/science.1176138. [DOI] [PubMed] [Google Scholar]
- 55.Van Allen BG, Bhavsar P. Natal habitat effects drive density-dependent scaling of dispersal decisions. Oikos. 2014;123:699–704. [Google Scholar]
- 56.Van Allen BG, Rudolf VHW. Ghosts of habitats past: Environmental carry-over effects drive population dynamics in novel habitat. Am Nat. 2013;181:596–608. doi: 10.1086/670127. [DOI] [PubMed] [Google Scholar]
- 57.R Core Team 2016. R: A Language and Environment for Statistical Computing, version 3.2.3 (R Foundation for Statistical Computing, Vienna)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.