Significance
Neonatal jaundice, a condition caused by the accumulation of bilirubin in the bloodstream, affects approximately half of all newborns. In high-resource settings, babies with elevated serum bilirubin levels are identified through routine hospital laboratory testing. When identified, jaundice is easily treated using blue-light phototherapy. Low-cost, rugged phototherapy lights have been developed and shown to be effective in low-resource settings. However, jaundice regularly goes undetected in these settings due to a lack of diagnostic tools to measure bilirubin levels. Left untreated, jaundice can lead to permanent neurological damage and mortality, the vast majority of which currently occurs in low-resource settings. In this paper, we present a low-cost method to measure total bilirubin at the point of care in low-resource settings.
Keywords: neonatal jaundice, point-of-care, lateral flow, low-resource setting
Abstract
Newborns are at increased risk of jaundice, a condition in which excess bilirubin accumulates in blood. Left untreated, jaundice can lead to neurological impairment and death. Jaundice resulting from unconjugated hyperbilirubinemia is easily treated with exposure to blue light, and phototherapy systems have been developed for low-resource settings; however, there are no appropriate solutions to diagnose and monitor jaundice in these settings. To address this need we present BiliSpec, a low-cost reader and disposable lateral flow card designed to measure the concentration of total bilirubin from several drops of blood at the point of care. We evaluated the performance of BiliSpec, using blood from normal volunteers spiked with varying amounts of bilirubin; results measured using BiliSpec correlated well with a reference laboratory bilirubinometer (r = 0.996). We then performed a pilot clinical study using BiliSpec to measure total bilirubin in neonates at risk for jaundice at Queen Elizabeth Central Hospital in Blantyre, Malawi. Concentrations measured using BiliSpec correlated well with those measured using a laboratory reference standard in 94 patient samples ranging from 1.1 mg/dL to 23.0 mg/dL in concentration (r = 0.973). The mean difference between bilirubin levels measured with BiliSpec and the reference standard was 0.3 mg/dL (95 CI: −1.7–2.2 mg/dL).
Every year, 24 million newborns develop jaundice. Jaundice resulting from hyperbilirubinemia is especially common in preterm babies, who lack sufficient liver function to excrete excess bilirubin. Neonatal jaundice is easily treated using blue-light phototherapy, exploiting the strong optical absorbance of bilirubin at 460 nm to photodecompose bilirubin to a form that can be excreted. In extreme cases, exchange transfusions can be performed to quickly lower the concentration of bilirubin in the blood. In high-resource settings, morbidity and mortality from jaundice are extremely rare due to the widespread availability of tools to measure serum bilirubin levels and treat newborns suffering from jaundice. In contrast, jaundice remains a significant source of neonatal morbidity and mortality in low-resource settings. Globally, 120,000 babies still die each year from jaundice and many more suffer permanent neurological damage (kernicterus). The vast majority of these deaths occur in low-income countries in sub-Saharan Africa and South Asia (1).
Severe jaundice may not present until several days after birth, and thus early monitoring of bilirubin is critical, particularly in premature babies who are at greater risk of death and disability due to jaundice (2). World Health Organization guidelines for managing hyperbilirubinemia using serum bilirubin concentration shift based on age and prematurity (Table S1) (3). While several low-cost phototherapy systems have recently been developed (D-rev; Brilliance, Design that Matters; Firefly) for use in low-resource settings, there is still a lack of low-cost diagnostic tools to measure bilirubin levels to identify babies who require treatment and monitor their response to therapy. In high-resource settings a number of laboratory devices and methods are available to measure total serum bilirubin (TSB); these tools have proved too expensive and complex to implement in under-resourced settings (2). For example, spectrophotometric methods to measure TSB require a centrifuge to separate plasma from whole blood and prevent hemoglobin interference, as well as a spectrophotometer—tools that can cost thousands of dollars and are often not available in district hospital or health center laboratories (4). Bilirubin levels can also be measured using chemical approaches such as the diazo method, enzymatic determination, or high-performance liquid chromatography. The expensive reagents and laboratory analyzers needed to perform these tests are not available in the majority of low-resource settings (5, 6). Alternatively, transcutaneous measurement of TSB does not require blood collection and has been proposed as an appropriate solution for low-resource settings. However, transcutaneous readers are expensive and some require a costly disposable calibration standard. Even if cost could be reduced, studies have shown the accuracy of transcutaneous assessment of TSB is lower in babies with darker skin or who are premature. Moreover, transcutaneous measurement can only be used for initial measurement of TSB and does not accurately monitor response to blue-light phototherapy (7–10). As a result, jaundice is diagnosed clinically in most low-resource settings based on visual assessment of yellowing of the skin or sclera. Unfortunately, visual assessment is subjective and accuracy is poor compared with laboratory measurement of TSB (11). Thus, there is an important need for improved methods to measure TSB in low-resource settings.
To meet this need, we developed BiliSpec, a low-cost, battery-powered reader designed to rapidly quantify serum bilirubin levels from small drops of whole blood applied to a lateral flow card. BiliSpec consists of two components: (i) a lateral-flow separation card to collect blood from a heel prick, separate plasma, and stabilize the sample and (ii) a reader to measure light transmitted through the plasma on the card and display a digital report of the concentration of TSB. Here, we describe laboratory experiments to optimize the design of the lateral flow card and to characterize accuracy of the system compared with a laboratory reference standard using blood from normal volunteers spiked with varying levels of bilirubin. We then describe results from a pilot study to evaluate the accuracy of BiliSpec to measure TSB in neonates at risk for jaundice at Queen Elizabeth Central Hospital in Blantyre, Malawi.
Results
Design of Lateral Flow Cards and Reader.
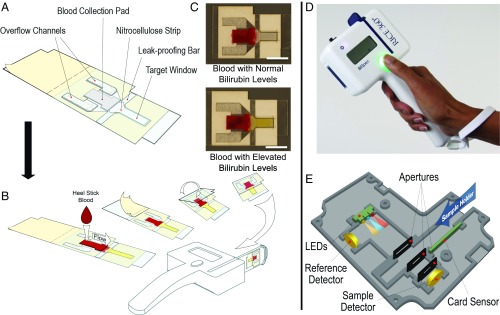
The lateral flow cards are designed to accept drops of whole blood obtained directly from a heel or finger prick, separate plasma from whole blood within 1–2 min, and preserve the sample so that bilirubin concentration remains constant over time (Fig. 1A). The blood collection pad is a glass fiber plasma separation membrane which traps red blood cells, allowing plasma to flow out along the nitrocellulose strip. The hemoglobin containing red blood cells must be separated from the plasma, because hemoglobin has a strong Soret band absorption peak at 420 nm which overlaps with the bilirubin absorption peak at 460 nm. The lateral flow cards are designed to be operated by visual cues alone. Users are instructed to visually fill the blood collection pad, seal the device, and then insert the device into the reader once the plasma has reached the end of the nitrocellulose strip. The blood collection pad is sized to appear visually full following application of 40–50 L of blood (typically two to three drops of blood). The target window (Fig. 1A) consists of a clear piece of acetate covering the area illuminated in the reader to measure plasma absorbance, preventing exposure to the air and reducing drying after sample collection. The target window is made from acetate not coated with adhesive to avoid interference of glue with flow of plasma along the strip. The leak-proofing bar and overflow channels are designed to prevent excess blood from leaking into the target area from a saturated collection pad or out of the lateral flow card if the card is squeezed or overfilled. After the blood collection pad has been visibly filled, the device can be immediately sealed. Separation of plasma takes ∼1–2 min (Movie S1). When the plasma reaches the end of the nitrocellulose strip, the card can be inserted into the reader and analyzed (Fig. 1B). Representative photographs of lateral flow cards spotted with blood containing normal or elevated TSB levels ready to be measured are shown in Fig. 1C. The reader is a hand-held battery-powered device that measures absorbance of the separated plasma samples on the lateral flow cards (Fig. 1D). Within the reader, three LEDs with center peak wavelengths of 470 nm (blue), 590 nm (amber), and 660 nm (red) are used in sequence to measure absorbance of bilirubin, free hemoglobin, and background absorbance of the card, respectively (Fig. 1E). Light intensity values are recorded by the sample and reference photodiode detectors. The reference detector, placed near the LEDs, is used to record the amount of incident light on the sample. The sample detector measures the amount of light transmitted through the sample. The device is calibrated by measuring signal from three neutral density filters, allowing conversion of the raw intensity values into an optical density. Total bilirubin concentration is then calculated by subtracting the optical density measured in the hemoglobin (amber) and background (red) channels from that measured in the bilirubin (blue) channel.
Fig. 1.
BiliSpec lateral flow card and reader. (A) Diagram of the lateral flow card components. To use the card, blood is applied to the collection pad, protective paper is removed from the adhesive-coated acetate, and the card is folded to seal blood inside. (B) Plasma flows down to the distal tip of the card before being inserted into the reader for measurement. (C) Representative photographs of sealed lateral flow cards following application of blood with normal and elevated TSB levels. (Scale bar, 1 cm.) (D) Photograph of the hand-held reader. (E) Diagram illustrating the internal optical and electronic layout of the reader.
Laboratory Validation of Lateral Flow Cards.
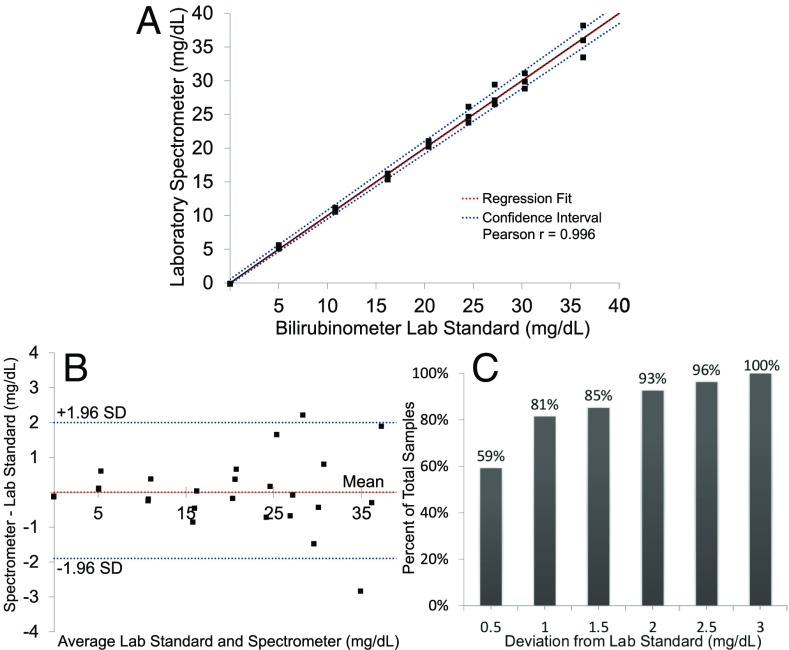
To simulate hyperbilirubinemia in the laboratory, whole blood from normal adult volunteers was spiked with varying concentrations of bilirubin (Materials and Methods). Prepared samples were gently mixed and blood was pipetted onto the collection pads until visually filled. The remaining sample was centrifuged to separate plasma, and the TSB was measured by a laboratory reference standard using the direct spectrophotometric method for measuring total bilirubin (UNISTAT; Reichert Technologies). The UNISTAT has been shown to have strong correlation with diazo measurement of TSB with a mean difference of −0.37 mg/dL and mean absolute difference of 0.60 mg/dL (12). After spotting blood on the collection pad, the lateral flow cards were allowed to flow for 2 min and then absorbance was measured using a laboratory spectrometer (Cary 5000 UV-VIS; Agilient). Absorption values at 460 nm, 532 nm, and 656 nm were recorded for each concentration corresponding to bilirubin, hemoglobin, and background, respectively. Each concentration of total bilirubin was run in triplicate, and concentrations ranged from 0.0 mg/dL to 36.3 mg/dL. As bilirubin concentration increased, measured spectra showed increasing absorbance in the blue with little to no change in absorption due to hemoglobin (Fig. S1). The correlation between absorbance and concentration was found by fitting a curve to describe the relationship between concentration and absorbance, using leave-one-out cross-validation analysis. Good agreement (Pearson’s coefficient r = 0.996) was observed between the laboratory standard bilirubinometer and concentration calculated by measuring absorbance using the spectrometer (Fig. 2A). Passing–Bablok regression analysis (n = 27) showed a slope value of 1.0013 with a confidence interval (CI) of 0.9664–1.0238 and an intercept of 0.0397 with a 95 CI of −0.169–0.5522. There was no significant deviation from linearity (P = 0.86), using a cusum test for linearity. Bland–Altman analysis showed no significant mean bias with a SD of 1.0 mg/dL from the laboratory reference and a 95 CI of −1.9–2.0 mg/dL (Fig. 2B). All samples measured using the lateral flow cards and spectrometer were within 3.0 mg/dL of the reference measurement (Fig. 2C).
Fig. 2.
Laboratory validation of lateral flow card plasma separation. (A) Correlation between TSB concentration measured using the lateral flow card with a laboratory spectrophotometer and that measured with a reference bilirubinometer using leave-one-out cross-validation. (B) Bland–Altman plot showing the difference between TSB measurements using the lateral flow card and reference bilirubinometer. Confidence interval (95) is −1.9–2.0 mg/dL. (C) Percentage of TSB measurements within a given deviation from the reference standard. In total, 100 were within 3.0 mg/dL of the laboratory standard.
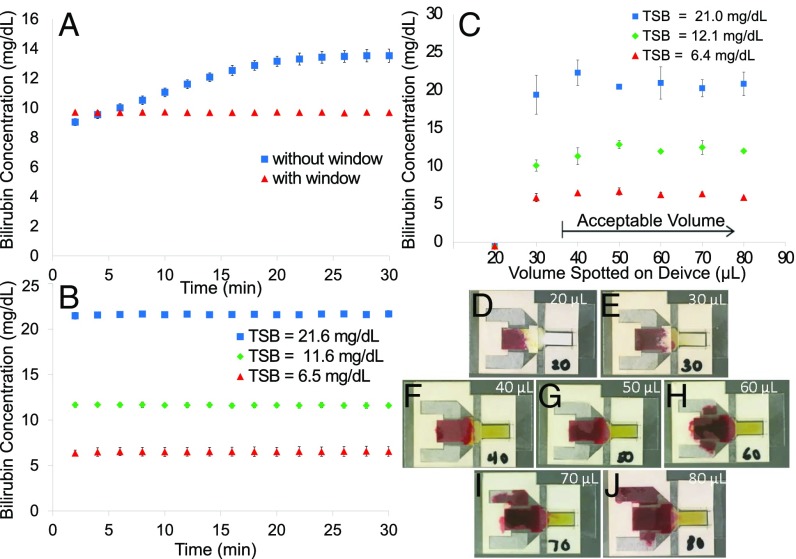
We next evaluated temporal variations in absorbance measurements of the lateral flow cards over time, comparing temporal stability of measurements made from cards with the window removed and the strip exposed directly to air to ones with an intact window (Fig. 3A). Blood was applied to the card and each sample was allowed to separate for 2 min and then measured every 2 min for 30 min. The data shown in Fig. 2 were used as a training set to calculate the concentration of bilirubin from absorbance measured over time. Each concentration was measured in triplicate. Over the 30-min period, the apparent concentration of TSB measured in cards without a window changed by 4.5 0.3 mg/dL (9.0–13.5 mg/dL). In contrast, TSB levels measured in cards with intact windows remained stable at 9.7 0.1 mg/dL over the 30-min period. In cards without a protective window, the measured absorbance spectra changed dramatically over time, while remaining constant in cards with intact windows (Fig. S2 A and B). At longer intervals in normally constructed cards with intact windows, apparent concentration changed by 0.3 0.1 mg/dL (12.0–12.3 mg/dL) at 1 h and 1.2 0.1 mg/dL (12.0–13.2 mg/dL) at 2 h (Fig. S2C).
Fig. 3.
Evaluation of BiliSpec performance vs. time between sample collection and measurement and for different sample volumes. (A) Measured TSB concentration as a function of time between sample collection and measurement for cards with and without the protective target window in place. (B) Measured bilirubin concentration vs. time at three different concentrations of TSB measured using the card with a window. (C) Measured TSB concentration vs. input blood volume. (D–J) Photographs of lateral flow cards with different volumes applied to the blood collection pad: 20 L (D), 30 L (E), 40 L (F), 50 L (G), 60 L (H), 70 L (I), and 80 L (J).
We further assessed temporal variations in measured TSB concentration, using spiked samples corresponding to clinically low, medium, and high concentrations of bilirubin in normally constructed cards with a target window. The spectra again showed minimal change over 30 min (Fig. S2D). Over 30 min, the lowest concentration averaged 6.5 0.5 mg/dL, the middle concentration averaged 11.6 0.3 mg/dL, and the highest averaged 21.6 0.2 mg/dL (Fig. 3B).
We then assessed whether variations in the volume of blood applied to the card affected accuracy of TSB measurement (Fig. 3C). Lateral flow cards were measured using the spectrometer in triplicate at three different concentrations measured by the laboratory reference of TSB: 6.0 0.1 mg/dL, 11.9 0.2 mg/dL, and 21.3 1.0 mg/dL at input volumes of blood ranging from 20 L to 80 L. The smallest volumes (20 L and 30 L) are below the threshold to visibly fill the blood collection pad. The higher volumes of blood visibly fill the collection pad while excess blood begins to fill the overflow channels (Fig. 3 D–J). At 20 L, well below the threshold required to visually fill the collection pads, plasma did not reach the target area, so no absorbance was measured due to bilirubin. At 30 L, pads were underfilled, but plasma still reached the target area. The mean absolute deviation from the laboratory standard at 30 L of blood volume over the three concentrations was 1.4 1.7 mg/dL. At volumes where the pad appears visually filled (40 L), the mean absolute deviation was lower at 0.8 0.8 mg/dL.
Clinical Results.
To evaluate the performance and usability of the BiliSpec system in a low-resource setting, we measured bilirubin levels in neonates at risk for jaundice at Queen Elizabeth Central Hospital. The study was approved by the IRB at Rice University and by the University of Malawi College of Medicine Research and Ethics Committee. Heel-prick blood samples were collected from 68 patients (Table 1). One patient was removed from the study due to a congenital condition which can cause hemolysis. In total 94 samples were collected. A trained nurse performed heel pricks and collected blood directly onto the blood collection pads until they were visually filled. Cards were then measured by the BiliSpec reader. After the collection pad was filled, the nurse collected additional drops of blood into a tube. Collected blood was centrifuged (2,000 g, 5 min) Galaxy MiniStar; VWR) and plasma was then pipetted out of the sample and measured using the UNISTAT bilirubinometer laboratory reference standard described earlier (12).
Table 1.
Pilot clinical study at Queen Elizabeth Central Hospital
| Profile of Queen Elizabeth Central Hospital Bilispec study | Patient metrics | Laboratory standard, mg/dL | BiliSpec, mg/dL |
| No. of patients | 68 | ||
| No. of samples, n | 94 | ||
| Median age at sample, d | 3.5 (range: 0–24) | ||
| Median birth weight, kg | 2.3 (range: 0.9–3.8) | ||
| % male | 58 | ||
| Bilirubin measurements | |||
| Average ±SD | 10.6 ± 4 | 10.8 ± 3.7 | |
| Minimum recorded TSB | 1.1 | 1.2 | |
| Maximum recorded TSB | 23.0 | 21.9 |
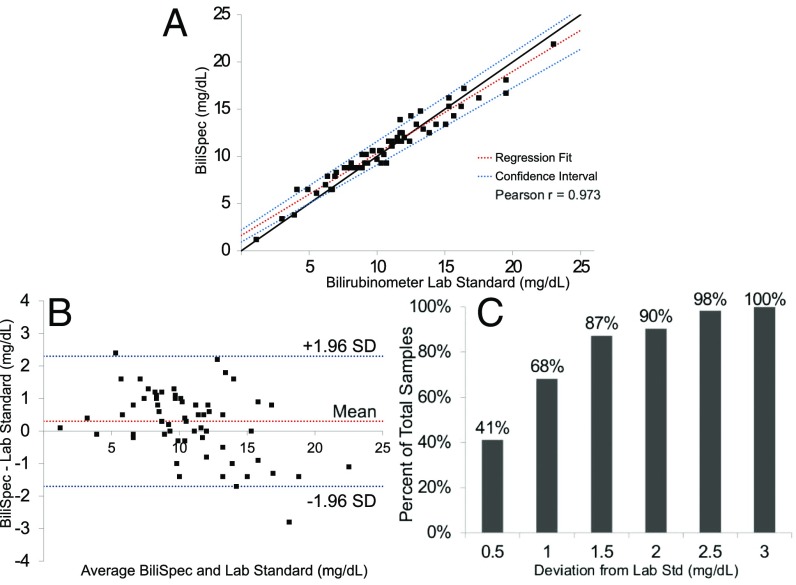
The average TSB of the 94 collected samples was 10.6 4.0 mg/dL as measured by the reference standard (range 1.1–23.0 mg/dL). The data measured using the BiliSpec device were divided into a training and a test set. To create the training set, samples were ordered from lowest to highest value of TSB, as measured by the reference standard, and every third sample was selected to be in the training set (n = 31); this process ensured that data in the training set spanned the entire range of bilirubin levels while avoiding possible selection bias. The remaining samples (n = 63) were used as a test set. Excellent agreement was observed in the test set between TSB levels measured with BiliSpec and the reference standard, with a Pearson’s correlation coefficient of r = 0.973 (Fig. 4A). Passing–Bablok regression analysis showed a slope value of 0.8684 with a CI of 0.8166–0.9385 and an intercept of 1.6355 with a 95 CI of 0.9338–2.2012. There was no significant deviation from linearity (P = 0.8). Bland–Altman analysis showed a mean bias of 0.3 mg/dL with a SD of 1.0 mg/dL with a 95 CI of −1.7–2.2 mg/dL (Fig. 2B). All samples measured by BiliSpec were within 3.0 mg/dL of the laboratory standard (Fig. 4C).
Fig. 4.
Results from clinical pilot study at Queen Elizabeth Central Hospital. (A) Correlation between TSB levels measured in the test set using BiliSpec and a laboratory reference standard bilirubinometer. (B) Bland–Altman plot showing the difference between TSB levels measured using the reference standard and BiliSpec. (C) Plot showing the percentage of samples vs. deviation between TSB measured using BiliSpec and the reference standard. In total, 100 were within 3.0 mg/dL of the laboratory standard.
Discussion
This study evaluated the ability of BiliSpec, a system consisting of a disposable lateral flow card and a hand-held reader, to measure and display TSB concentration at the bedside in a low-resource setting. BiliSpec was designed to be used with minimal training, to be affordable, and to be accurate compared with currently available methods for measuring TSB.
The system is designed to be used at the bedside by collecting drops of blood from a heel prick directly onto the disposable card. To optimize the disposable card, we evaluated its performance using 50 L volume of blood; this value was chosen for several reasons. First, 50L is a common target volume used in dried-blood spot cards for HIV testing, a blood collection method familiar to many healthcare workers in low-resource settings (13). Additionally, previous work suggests that using smaller volumes of blood can lead to drop-to-drop variations in analyte concentration (14). Using this larger volume of blood helps reduce potential sample variability. However, it is impractical for users to precisely control the volume of blood applied directly from a heel prick at the bedside. To allow collection in this way, we designed the cards so that a consistent amount of plasma containing bilirubin is delivered to the target area for measurement, independent of the input volume. To ensure that samples did not have to be measured at a precise time, the lateral flow cards were designed to maintain temporal stability for at least 30 min, ample time for a clinician to measure the sample after collection at the bedside. These properties allow the device to be operated based solely on visual cues without the need for precise volume measurement or timing. The clinician needs only to visibly fill the pad and wait for the plasma to reach the end of the nitrocellulose before measuring.
The reader was also designed to require as little user interaction as possible. The reader detects automatically if a calibration card or sample has been inserted and responds appropriately. The reader requires only a power on/off switch and a single button for operation. The calibration cards are housed inside the reader. The calibration process requires only 2–3 min to complete and was performed once per day during the clinical study.
The BiliSpec lateral flow cards and reader are designed to facilitate an extremely low per-test cost. The lateral flow cards have a $0.05 material cost when manufactured at low volumes in our laboratory and do not appear to require individual packaging for short-term storage. The cards and reader were stored in a room without air conditioning and out of direct sunlight during the humid and hot rainy season in Malawi for 5 wk without notable degradation in performance. Materials for the lateral flow card were cut using a laser cutter and then assembled by hand (Fig. S3) and could be produced and assembled locally in a low-resource setting. For reference, lancets and alcohol pads for performing heel pricks cost ∼$0.10 and $0.08, respectively (https://supply.unicef.org/). The molded plastic housing and printed circuit boards (PCBs) were the most expensive pieces of the reader for a single prototype. Costs for housing and PCBs are greatly reduced when scaling to larger production runs by using injection molding and bulk PCB orders. We estimate that the total material cost of the reader will be ∼$150 at low production volumes.
Our pilot clinical study was performed at Queen Elizabeth Central Hospital. We brought the same laboratory standard used during our laboratory validation to Blantyre to analyze clinical samples from neonates at risk for jaundice. A nurse collected the heel-prick samples which were then analyzed by the reader and the laboratory standard. The instructions for operating BiliSpec were simply to visually fill the blood collection pad, wait for the plasma to reach the end of the lateral flow strip, seal, and measure with the reader. The sample-to-answer time was ∼2 min.
The total bilirubin concentration measured by BiliSpec during the clinical study correlated well with that measured using the reference standard; 90 of samples evaluated were within 2 mg/dL of the reference standard. Additionally, 95 of measured samples fell within the Clinical Laboratory Improvement Amendments (CLIA) guidelines (20 or 0.4 mg/dL, whichever is greater), suggesting that with some small improvements BiliSpec could meet CLIA regulations (Fig. S4). Results show that BiliSpec outperforms transcutaneous measurement of bilirubin in comparison with other studies conducted in African neonates. In our study, a mean bias of 0.3 mg/dL was observed, meaning BiliSpec tended to slightly overestimate bilirubin concentration compared with the laboratory reference. In contrast, a recent study evaluating the performance of two transcutaneous systems in 1,553 African neonates found a mean bias of 3.0 mg/dL and 1.3 mg/dL. Furthermore, the 95 CI for BiliSpec was −1.7–2.2 mg/dL; whereas the transcutaneous study found a 95 CI of −0.7–6.7 mg/dL and −2.2–4.8 mg/dL (7). More recently, an alternative low-cost device has been described to measure TSB. However, evaluation shows the device has a 95 CI of −5.8–3.3 mg/dL relative to a laboratory reference standard, less accurate than many studies of transcutaneous measurement of TSB (15). Moreover, this system requires metered blood collection, measurement of the sample immediately, and recalibration between each sample (ref. 16; Bilimetrix; Bilistick). Based on our preliminary clinical results, BiliSpec has improved accuracy while requiring fewer user steps and disposables.
While results are promising, our study has some limitations. In this pilot study, we did not encounter any cases of extremely severe jaundice (TSB 25 mg/dL). Therefore, further clinical testing is needed to assess clinical accuracy at high levels of total bilirubin. The clinical pilot study was performed over the course of 5 wk and the reader and lateral flow cards functioned properly over the course of the study. However, further evaluation is needed to assess long-term storage needs. Calibration during our clinical study was performed once per day. Calibration over the clinical study remained stable (Fig. S5) and we believe that in the future we can decrease the frequency at which calibration must be performed. However, more testing is still needed in a variety of environmental conditions. We accounted for background absorbance of the lateral flow card by measuring absorbance at 660 nm. If the lateral flow card material is changed in the future we would need to reevaluate what wavelength could be used as a background measurement. Additionally, BiliSpec, like spectrophotometric and transcutaneous determination, is limited to measuring only total bilirubin and not direct, indirect, and delta bilirubin fractions due to overlapping absorbance spectra. Future work could include exploring a device to measure fractions in addition to TSB.
In conclusion, BiliSpec performed well compared with laboratory measurement of total bilirubin with all samples being within 3.0 mg/dL of the laboratory reference standard. We believe our system offers a more affordable and appropriately designed alternative to currently available techniques to measure TSB in low-resource settings. The device is designed to require minimal user interaction and integrate easily into a low-resource clinical setting. During the pilot study in Malawi, clinicians could consistently visually fill the pads, seal the strips, and insert them into the reader. BiliSpec offers a simple and accurate method to measure TSB to improve the diagnosis and monitoring of neonatal jaundice in low-resource settings.
Materials and Methods
Lateral Flow Card Construction.
Lateral flow card construction requires six elements (Fig. S3A): the card base, leak-proofing bar, target window, nitrocellulose strip, blood collection pad, and two overflow collection pads. All components were cut using a laser cutter. The card base and leak-proofing bar are cut from a sheet of Grafix Dura Lar clear adhesive backed film consisting of an acetate sheet with a paper protecting the adhesive backing. The target window is made from a Grafix Dura Lar acetate alternative sheet (0.010 inch thick) and the nitrocellulose strip is from a Hi-Flow Plus HF090 nitrocellulose sheet with plastic backing. The blood collection pad is cut from a Whatman Blood Separator MF1 glass fiber reel. The overflow collection pads are cut from a sheet of Ahlstrom Grade 8951 glass fiber. The overflow channels are used to prevent any blood from leaking out of the card or into the target area with the help of the leak-proofing bar.
The lateral flow cards (Fig. S3B) were assembled as follows: (i) The leak-proofing bar was separated from the card base and the acetate backing covering the target window hole, the edge of the target window, the nitrocellulose strip, the collection pad, and the area for the leak-proofing bar was removed. (ii) The nitrocellulose strip was placed, with plastic backing side down, onto the main adhesive surface, so the strip did not touch the edges of the paper backing. The overflow pads were placed in the backflow channels and the target window was placed in the target area. (iii) The paper backing of the bar was removed and placed, adhesive side down, over the nitrocellulose strip. The spotting pad was placed onto the main adhesive surface with its winglet side over the strip. (iv) The paper backing around the target window was removed and folded over onto the rest of the card base, leaving only the blood collection pad exposed. Once cards were assembled they were ready for use (Fig 1A).
Laboratory Bilirubin Standard.
The method used for creating a laboratory bilirubin standard is described by Doumas et al. (5). In brief, bilirubin standards were prepared by diluting a concentrated stock with a standard blank, both of which were prepared using a 40-g/L BSA solution. The 40-g/L BSA solution was prepared in Tris buffer, pH adjusted to 7.3 0.1 with 1 M HCl. Once fully dissolved, the BSA solution was diluted with the remaining Tris buffer to final volume to achieve an approximate concentration of 40 g/L and was stored at 4 °C. A 60-mg/dL bilirubin stock was prepared under minimal lighting using 60 mg of SRM 916 bilirubin and washed with 2 mL of dimethyl sulfoxide. A total of 4 mL of 0.1 M Na2 CO3 (aq) was then added. The mixture was swirled until the bilirubin was fully dissolved and then diluted to volume with the 40-g/L BSA solution. This solution was stored short term (1 d or less) at 4 °C or long term at −20 °C and was protected from sources of light at all times. The standard blank solution was prepared by combining 2 mL of pure dimethyl sulfoxide and 4 mL of 0.1 M sodium carbonate (aq) in a 100-mL volumetric flask and diluting to volume with 40 g/L BSA solution. This was stored at 4 °C and was not light sensitive. Bilirubin standards were then prepared by diluting the bilirubin stock with standard blank to the desired concentration.
Laboratory Blood Sample Preparation.
We simulated clinically relevant elevated bilirubin levels in blood by replacing plasma from normal volunteers with dilutions of the bilirubin standard described above. Normal volunteers were recruited under a Rice University IRB approved study. Aliquots of 500 L of normal blood were centrifuged. A total of 250 L of plasma was removed and replaced with 250 L of diluted bilirubin stock. The samples were then mixed gently for 20–30 s, using a vortexer on the lowest speed setting. Then, 180 L of blood was drawn into a pipette and used to fill three different lateral flow cards until the blood collection pads appeared visibly filled. This was done to simulate variable input volume expected from heel pricks during our laboratory testing. Each strip was then measured in the spectrometer ∼2 min after spotting. The remaining blood sample was centrifuged again to separate the plasma for measurement by the laboratory standard bilirubinometer. Absorbance data from the spectrometer at 460 nm, 532 nm, and 656 nm were used to compute an optical density which was then correlated with the concentration of bilirubin measured using the laboratory bilirubinometer.
Volume and Time Variation Testing.
The volume variability test was conducted for three different bilirubin concentrations at volumes of 20 L, 30 L, 40 L, 50 L, 60 L, 70 L, and 80 L. Blood was spotted onto the collection pad at each concentration and volume. Separation was allowed to proceed for 2 min after which the absorbance spectrum of the strip was measured from 400 nm to 700 nm (Cary 5000 UV-Vis-NIR spectrophotometer). This was done in triplicate for each concentration–volume pair. The data shown in Fig. 2 were used as a training set to create a curve correlating absorbance to concentration. This algorithm was used to compute the concentration values shown in Fig. 3.
The time variation test was performed at three bilirubin concentrations where 50 L of blood was spotted onto the collection pad and allowed to separate for 2 min. At 2 min after spotting and every 2 min, the absorbance spectrum of the strip from 400 nm to 700 nm was measured (Cary 5000 UV-Vis-NIR). This was done in triplicate for each concentration and for up to 30 min or 2 h, depending on the experiment.
Reader Construction.
The prototype reader measures the absorbance of the target area at three wavelengths, using LEDs with peak wavelengths at 450 nm (LXML-PB01-0030), 580 nm (LXML-PL01-0040), and 660 nm (LXM3-PD01). These wavelengths were used to measure the absorbance of light due to bilirubin, hemoglobin, and background, respectively. Free hemoglobin in the plasma was measured to account for any hemolysis that might have occurred during blood collection. Two photodiodes (Thorlabs; FDS100) were used to measure incident and transmitted light through the sample (Fig. 1E). An algorithm described in Bond et al. (17) was used to compute the optical density of the sample. Apertures made of black cardstock were placed along the optical pathway to reduce the amount of scattered light entering the sample detector. To calibrate the reader, three calibration cards, containing neutral density filters (1.5 OD, 2.0 OD, and 2.5 OD), were inserted into the reader and measured. These cards were stored in the side of the reader. The reader automatically detects which calibration card has been inserted using the card sensor, so the cards can be inserted in any order. Once all three calibration cards have been measured, the user measures a dry lateral flow card to complete calibration. Calibration during our clinical study was done once per day and took 2–3 min to complete. The reader was controlled by a microcontroller (Microchip; ATmega3290A) located on a custom printed circuit board designed to the power electronics and display. The reader was powered by two rechargeable AA batteries. The system is operated by a power on/off switch and a button on the front of the reader.
Malawi Clinical Pilot Study.
We evaluated the clinical accuracy of the BiliSpec device at Queen Elizabeth Central Hospital. The study was approved by Rice University IRB and the University of Malawi College of Medicine Research and Ethics Committee. A trained study nurse performed a heel prick on newborns (<28 d) at risk for jaundice. The nurse collected blood directly onto our lateral flow cards by visibly filling the blood collection pad which was then measured using the battery-powered reader. All remaining blood from the heel prick was collected directly into a tube and centrifuged, and the plasma was measured by a clinical laboratory spectrophotometric bilirubinometer (Reichert; UNISTAT). All instruments and lateral flow cards were stored in a room without air conditioning over the course of the study (5 wk). Passing–Bablok regression analysis (18) and Bland–Altman analysis (19) were performed using MedCalc v 17.5.5. Laboratory and deidentified clinical data are available by contacting the corresponding author.
Supplementary Material
Acknowledgments
The authors thank the incredible clinicians and healthcare providers at Queen Elizabeth Central Hospital for their contributions to this work. The authors especially thank B. Ballard and K. Payea for their contributions to the construction of the reader. This study is made possible by the generous support of the American people through the US Agency for International Development (USAID). Funding is provided under Award AID-GH-F-15-00012. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714020114/-/DCSupplemental.
References
- 1.Bhutani VK, et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: Incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res. 2013;74:86–100. doi: 10.1038/pr.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.March of Dimes, The Partnership for Maternal, Newborn & Child Health, Save the Children, World Health Organization . Born Too Soon: The Global Action Report on Preterm Birth. WHO; Geneva: 2012. [Google Scholar]
- 3.World Health Organization . Recommendations for Management of Common Childhood Conditions: Evidence for Technical Update of Pocket Book Recommendations: Newborn Conditions, Dysentery, Pneumonia, Oxygen Use and Delivery, Common Causes of Fever, Severe Acute Malnutrition and Supportive Care. WHO; Geneva: 2012. [PubMed] [Google Scholar]
- 4.Horecker BL. The absorption spectra of hemoglobin and its derivatives in the visible and near infra-red regions. J Biol Chem. 1943;148:173–183. [Google Scholar]
- 5.Doumas BT, Kwok-Cheung PP, Perry BW. Candidate reference method for determination of total bilirubin in serum: Development and validation. Clin Chem. 1985;31:1779–1789. [PubMed] [Google Scholar]
- 6.Blanckaert N, et al. Measurement of bilirubin and its monoconjugates and diconjugates in human serum by alkaline methanolysis and high-performance liquid chromatography. J Lab Clin Med. 1980;96:198–212. [PubMed] [Google Scholar]
- 7.Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/peds.2016-0907. [DOI] [PubMed] [Google Scholar]
- 8.Tan KL, Dong F. Transcutaneous bilirubinometry during and after phototherapy. Acta Paediatr. 2003;92:327–331. [PubMed] [Google Scholar]
- 9.Maisels MJ, et al. Evaluation of a new transcutaneous bilirubinometer. Pediatrics. 2004;113:1628–1635. doi: 10.1542/peds.113.6.1628. [DOI] [PubMed] [Google Scholar]
- 10.Olusanya BO, Emokpae AA. Use of transcutaneous bilirubin to determine the need for phototherapy in resource-limited settings. Neonatology. 2017;111:324–330. doi: 10.1159/000452788. [DOI] [PubMed] [Google Scholar]
- 11.Moyer va, Ahn C SS. Accuracy of clinical judgment in neonatal jaundice. Arch Pediatr Adolesc Med. 2000;154:391–394. doi: 10.1001/archpedi.154.4.391. [DOI] [PubMed] [Google Scholar]
- 12.Barko HA, Jackson GL, Engle W. Evaluation of a point-of-care direct spectrophotometric method for measurement of total serum bilirubin in term and near-term neonates. 2006;26:100–105. doi: 10.1038/sj.jp.7211436. [DOI] [PubMed] [Google Scholar]
- 13.Rutstein SE, et al. Measures of viral load using Abbott RealTime HIV-1 Assay on venous and fingerstick dried blood spots from provider-collected specimens in Malawian district hospitals. J Clin Virol. 2014;60:392–398. doi: 10.1016/j.jcv.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bond MM, Richards-Kortum RR. Drop-to-drop variation in the cellular components of fingerprick blood: Implications for point-of-care diagnostic development. Am J Clin Pathol. 2015;144:885–894. doi: 10.1309/AJCP1L7DKMPCHPEH. [DOI] [PubMed] [Google Scholar]
- 15.Greco C, et al. Comparison between Bilistick System and transcutaneous bilirubin in assessing total bilirubin serum concentration in jaundiced newborns. J Perinatol. 2017;37:1028–1031. doi: 10.1038/jp.2017.94. [DOI] [PubMed] [Google Scholar]
- 16.Coda Zabetta CD, et al. Bilistick: A low-cost point-of-care system to measure total plasma bilirubin. Neonatology. 2013;103:177–181. doi: 10.1159/000345425. [DOI] [PubMed] [Google Scholar]
- 17.Bond M, Mvula J, Molyneux E, Richards-Kortum R. 2014. Design and performance of a low-cost, handheld reader for diagnosing anemia in Blantyre, Malawi in 2014. IEEE Healthcare Innovation Conference (HIC) (IEEE), No. 0940902, pp 267–270. [DOI] [PMC free article] [PubMed]
- 18.Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part 1. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 19.Martin Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.