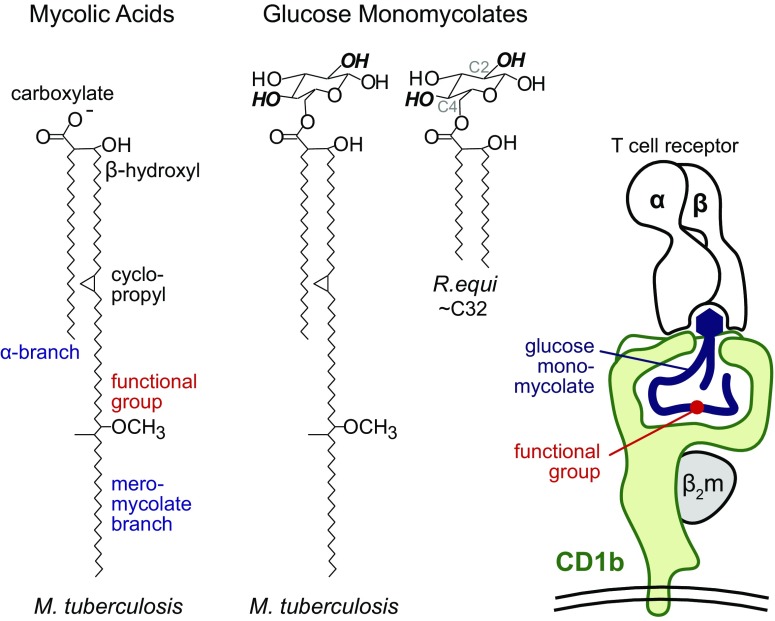
Solving the mechanism by which MHC I and II proteins capture protein fragments represented a triumph for T cell biologists. This discovery highlighted the peptidic nature of antigens for T cells. Investigation of MHC-peptide–T cell receptor (TCR) interactions provides the basis for epitope mapping, which is the process of identifying the particular chemical elements of an antigen that contact the TCR and determine immune response. Accordingly, for decades, nearly every aspect of T cell-related technology development in autoimmunity, infection, antitumor response, and vaccination has focused on peptide epitopes. However, the known universe of antigens for human T cells widened considerably based on work in the early 1990s by Porcelli, Brenner, and colleagues (1, 2). They identified lipid antigens for T cells, including mycolic acid, a membrane lipid from Mycobacterium tuberculosis (Fig. 1). This discovery forced a collision of two previously unrelated fields: lipid chemistry and antigen presentation. Mycolic acids and other lipids are now well established to bind within CD1b antigen-presenting molecules, and the resulting CD1b–lipid complexes are recognized by TCRs (3). In PNAS, Salah Mansour’s laboratory returns to the question of epitope mapping of mycobacterial mycolic acid antigens (4). Their work not only solves certain questions, but also highlights new controversies regarding how clonotypic TCRs can specifically grasp and recognize lipids.
Fig. 1.
Mycolic acid and glucose monomycolate antigens possess the same kinds of mycolyl groups, yet function differently in activation of T cells. T cell response to GMM absolutely requires the glucose head group, including the indicated hydroxyl groups at positions C2 and C4. T cell response to GMM proceeds normally when the lipid is truncated from C80 to C32 removing functional groups from its meromycolate chain (5). In contrast, the functional groups on mycolic acid lipid moiety strongly control T cell response (4, 12, 13). The CD1b–GMM–TCR image derives from solved crystal structures (7, 14), which suggest that the distal meromycolate chain carrying the functional groups might lie deep within CD1b. The positioning of free mycolic acid is modeled here (4) with molecular dynamics simulations, but its actual position within CD1b based on crystal structures is unknown.
Reasoning from first principles, the chemical structures of mycolic acids invite certain questions about how they can be recognized by T cells (Fig. 1). Mycolic acids are defined as α-branched, β-hydroxy fatty acids. These unusual chemical features, along with their long chain length (C32–C100), distinguish mycolic acids from short-chain fatty acids present in mammalian cells. Unlike peptides, lipids are not gene products that can mutate to escape from recognition, and mycolic acids are essential for mycobacterial survival. Therefore, mycolic acids are foreign molecules that could plausibly alert the immune system to infection by intracellular mycobacteria. Less satisfyingly, their extreme hydrophobicity and unconstrained chemical bonds beg the question of how such floppy and hydrophobic molecules could specifically interact with TCRs. Clonotypic receptors on B cells and T cells typically act in an aqueous environment to bind chemically rigid and hydrophilic epitopes.
This objection was answered, in part, through the subsequent discovery of a chemically defined glycolipid antigen, glucose monomycolate (GMM) (5). GMM antigens are related to mycolic acid in structure, but carry a 6-linked hexose sugar (Fig. 1). GMM epitopes were mapped in experiments in which T cells failed to recognize analogs in which a single hydroxyl group at C2 or C4 of the hexose sugar was altered. However, T cells responded strongly to GMM from Rhodococcus equi, which has shorter (C32) and chemically simplified meromycolate chains that lack functional groups (Fig. 1). This result rules out functional groups as necessary for this T cell response. Together with the structural insight that CD1 proteins fold to form a large hydrophobic cleft (6), a head group recognition hypothesis emerged (5). This model predicted that the carbohydrate head group functioned as the TCR epitope and that key aspects of the alkyl chains were dispensable for recognition.
Last year, Gras et al. (7) solved the CD1b–GMM–TCR structure, showing that the TCR α- and β-chains act like tweezers to surround and bind the glucose moiety of GMM, including interactions with the C2 and C4 hydroxyl units (Fig. 1, Right). This mechanism is similar to that of TCR contact with α-galactosyl ceramide by TCRs on NKT cells (8), and as shown earlier this month, also describes TCR contact with phosphoglycerol head groups on phospholipid antigens presented by CD1b (9). In these crystal structures, the aliphatic hydrocarbon chains in antigens were largely tucked inside CD1b, where they formed relatively nonspecific nonpolar interactions with the inner surface of CD1 proteins. Thus, the riddle of how TCRs grasp floppy lipids seemed to be answered: they do not see hydrocarbon chains buried within CD1b, but simply bind the particular rigid and hydrophilic epitopes, like sugars and phosphate esters, that emerge from the cleft (Fig. 1).
However, this model cannot account for recognition of free mycolic acid and other lipids, like free fatty acids, squalene (10), and cholesterol esters (11), which do not carry any large hydrophilic or charged head group. Furthermore, studies of CD1b and mycolic acids by Grant et al. and Van Rhijn et al. (12, 13), as well as new work by Chancellor et al. (4), show that the oxygen-containing functional groups on the distal portion of free mycolic acid can strongly control whether T cells respond. Whereas certain TCRs favor activation by α-mycolic acids that lack oxygen on the meromycolate chain, others preferentially recognize mycolic acids with keto or methoxy functional groups (Fig. 1). These experiments were carried out with synthetic molecules prepared by Baird (4) and others (13), which makes them particularly reliable because contamination of with copurifying immunogens from mycobacteria is ruled out.
The discovery of mycolic acid subclass-specific recognition raises new questions about the molecular mechanism. One possible means of discrimination of functional groups on the meromycolate chain is that the mycolic acids fold within CD1b such that the critical methoxy or keto groups protrude outside CD1b and are presented for direct TCR contact. However, if mycolic acids bind in the same manner by which the mycolyl unit of GMM sits within CD1b (7, 14), then the keto and methoxy functional groups would reside near the bottom of the cleft, where direct TCR contact cannot occur (Fig. 1, red). Chancellor et al.’s (4) work in PNAS favors the latter model and reports simulations in which the functional groups act indirectly to regulate movement or position of the mycolic acid within the groove. While molecular dynamics simulations require validation through solved crystal structures of CD1b-mycolic acid, they predict that the role of the functional groups is to interact with the interior sidewall of CD1b to position the mycolic acid at the right height within the cleft. In this view, the actual epitope is the carboxylate group of the mycolic acid, and the meromycolate variations play an indirect yet crucial role in stabilizing that epitope (Fig. 1).
Are the naturally occurring meromycolate functional groups irrelevant for T cell response, as suggested by prior GMM studies, or crucial, as suggested by the new mycolic acid studies? All good data are true, and the seemingly contradictory outcomes can be reconciled based on the concept of “head group positioning” (7, 13). The glucose moiety, which is present in GMM but not mycolic acid antigens, binds to the outer surface of CD1b (7). This interaction can pin the 6-linked mycolyl moiety of GMM in place so any secondary effects of alkane chains within the cleft play no role in perturbing the TCR epitope (Fig. 1, Right). Indeed, GMM antigens can undergo extreme truncation from C80 to C32 lipids without loss of recognition (5), whereas short-chain C32 mycolic acids have never been shown to activate T cells (13). The difference likely results from the fact that all mycolic acids lack the pinning effect of glucose, and Chancellor et al.’s (4) new model invokes the weaker effects of meromycolate functional groups as a kind of secondary or back-up mechanism to position the lipid within the cleft. Thus, working from differing perspectives, a unifying model is emerging whereby the hydrophilic head group typically dominates both TCR contact and antigen positioning within CD1. However, lipid fine structure can influence the final position of lipid ligands when head group positioning is weak.
In PNAS, Salah Mansour’s laboratory returns to the question of epitope mapping of mycobacterial mycolic acid antigens. Their work not only solves certain questions, but also highlights new controversies regarding how clonotypic TCRs can specifically grasp and recognize lipids.
Unlike MHC proteins, CD1b proteins are nearly nonpolymorphic in human populations. Whereas designing universal peptide epitopes that can be presented to T cells from all people is difficult or infeasible, lipid antigens are predicted to be displayed in nearly the same way by CD1b in all humans. This observation provides the premise for lipid antigen-based technology development, including efforts to produce mycolic acid bound to CD1b as a recall antigen test for tuberculosis (15) and lipid vaccination. Prior work on GMM antigens points to a strategy for synthesizing nonnatural ligands with short and simplified meromycolate chains that approximate those present in C32 R. equi GMM (Fig. 1). Such antigens load readily onto CD1b and stimulate many human T cells (5, 7). However, a key implication of new work on mycolic acids is that the mixed forms of mycolic acids present in mycobacteria are not one kind antigen (4, 12, 13). Instead the keto, methoxy, and other functional groups control T cell response differently, so each molecular variant of mycolic acid should be conceived of as a distinct antigen that is separately synthesized and tested for immunogenicity. Furthermore, these results imply that lipid-based vaccines or recall antigen tests must use mycolic acids that are chemically matched to the particular type of mycolic acid present in the bacterium of interest.
Finally, Chancellor et al.’s (4) work helps to answer a controversy about CD1b expression in tuberculosis disease. Among human MHC and CD1 antigen-presenting molecules, CD1b shows the most restricted pattern of expression in the periphery. Most tissues lack CD1b, but it appears in an activation-induced process on myeloid dendritic cells and macrophages. This pattern contrasts strongly with the ubiquitous expression of MHC I and suggests that the timing and location of CD1b expression could control whether T cell response occurs. Indeed, M. tuberculosis, acting through Toll-like receptors and cytokine response, can induce CD1b expression (16). However, one study of human cell infection by M. tuberculosis demonstrated loss of CD1b, which might represent immune evasion or simply macrophage death in culture (17). Resolving whether mycobacteria induce or block CD1b expression is important, because CD1b induction by the pathogens is a tidy mechanism that could account for local T cell responses at the site of infection, while limiting CD1b expression and function in other situations that could lead to autoimmunity.
Most in vivo evidence, including analysis of skin from tuberculoid leprosy patients and lung lavage from tuberculosis patients, points to CD1b up-regulation (18, 19). However, the key question of whether CD1b appears in lung granulomas of human tuberculosis patients has not been clearly answered. (The fixation treatments needed to safely analyze M. tuberculosis-infected tissue distort epitopes normally needed to detect CD1b with monoclonal antibodies.) Using an antigen recovery technique, Chancellor et al. (4) demonstrate abundant CD1b expression on myeloid cells in human tuberculous granulomata. These data cinch the case for CD1b up-regulation in vivo, placing CD1b at the right place and time for its proposed function in mediating host response to natural M. tuberculosis infection.
Supplementary Material
Acknowledgments
I acknowledge Rachel Cotton, Ildiko Van Rhijn, and Tan-Yun Cheng for contributing graphics and for critical reading of the manuscript. D.B.M. is supported by the Bill and Melinda Gates Foundation and NIH Grants AI111224 and AI049313.
Footnotes
The author declares no conflict of interest.
See companion article on page E10956.
References
- 1.Beckman EM, et al. Recognition of a lipid antigen by CD1-restricted α β+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 2.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 3.Grant EP, et al. Molecular recognition of lipid antigens by T cell receptors. J Exp Med. 1999;189:195–205. doi: 10.1084/jem.189.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chancellor A, et al. CD1b-restricted GEM T cell responses are modulated by Mycobacterium tuberculosis mycolic acid meromycolate chains. Proc Natl Acad Sci USA. 2017;114:E10956–E10964. doi: 10.1073/pnas.1708252114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moody DB, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 6.Zeng Z, et al. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 7.Gras S, et al. T cell receptor recognition of CD1b presenting a mycobacterial glycolipid. Nat Commun. 2016;7:13257. doi: 10.1038/ncomms13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borg NA, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 9.Shahine A, et al. A molecular basis of human T cell receptor autoreactivity toward self-phospholipids. Sci Immunol. 2017;2:eaao1384. doi: 10.1126/sciimmunol.aao1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong A, et al. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat Immunol. 2010;11:1102–1109. doi: 10.1038/ni.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour S, et al. Cholesteryl esters stabilize human CD1c conformations for recognition by self-reactive T cells. Proc Natl Acad Sci USA. 2016;113:E1266–E1275. doi: 10.1073/pnas.1519246113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant EP, et al. Fine specificity of TCR complementarity-determining region residues and lipid antigen hydrophilic moieties in the recognition of a CD1-lipid complex. J Immunol. 2002;168:3933–3940. doi: 10.4049/jimmunol.168.8.3933. [DOI] [PubMed] [Google Scholar]
- 13.Van Rhijn I, et al. CD1b-mycolic acid tetramers demonstrate T-cell fine specificity for mycobacterial lipid tails. Eur J Immunol. 2017;47:1525–1534. doi: 10.1002/eji.201747062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batuwangala T, et al. The crystal structure of human CD1b with a bound bacterial glycolipid. J Immunol. 2004;172:2382–2388. doi: 10.4049/jimmunol.172.4.2382. [DOI] [PubMed] [Google Scholar]
- 15.Montamat-Sicotte DJ, et al. A mycolic acid-specific CD1-restricted T cell population contributes to acute and memory immune responses in human tuberculosis infection. J Clin Invest. 2011;121:2493–2503. doi: 10.1172/JCI46216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roura-Mir C, et al. Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR-2. J Immunol. 2005;175:1758–1766. doi: 10.4049/jimmunol.175.3.1758. [DOI] [PubMed] [Google Scholar]
- 17.Stenger S, Niazi KR, Modlin RL. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J Immunol. 1998;161:3582–3588. [PubMed] [Google Scholar]
- 18.Sieling PA, et al. CD1 expression by dendritic cells in human leprosy lesions: Correlation with effective host immunity. J Immunol. 1999;162:1851–1858. [PubMed] [Google Scholar]
- 19.Buettner M, et al. Inverse correlation of maturity and antibacterial activity in human dendritic cells. J Immunol. 2005;174:4203–4209. doi: 10.4049/jimmunol.174.7.4203. [DOI] [PubMed] [Google Scholar]