Abstract
The current study aimed to explore whether the efficiency of the standard International Prognostic Index (S-IPI), revised-IPI (R-IPI) and enhanced-IPI (NCCN-IPI) in evaluating the prognosis of patients with diffuse large B-cell lymphoma (DLBCL) may be improved by interim 18F-FDG PET/CT. A total of 185 patients with newly diagnosed DLBCL were enrolled in the current study. All patients underwent interim PET/CT following the 4th cycle of chemotherapy. Patients were divided into different risk groups using S-IPI, R-IPI and NCCN-IPI and further subdivided into risk groups using interim PET/CT. Interpretations were evaluated for 2-year progression-free survival (PFS) and overall survival (OS). With a median follow-up time of 44 months, the 2-year PFS and OS were 60% [95% confidence interval (CI) 53–67%] and 81% (95% CI 74–86%), respectively. Analysis of S-IPI and NCCN-IPI identified no significant difference in PFS and OS between high intermediate and high risk groups. However, there were significant differences in the PFS and OS between the low and low intermediate risk groups (P<0.01). Interim PET/CT was used to redistribute patients in the higher risk group into PET negative and positive groups (P<0.01) and arallel results were observed in the lower risk group. In R-IPI, interim PET/CT identified a significant difference between PFS and OS in the good and poor risk groups but not in the very good risk group. Therefore, the results of the current study indicate that S-IPI, R-IPI and NCCN-IPI are three clinically useful prognostic indexes for patients with DLBCL. Interim PET/CT may improve the prognostic value of S-IPI, R-IPI and NCCN-IPI in predicting 2-year PFS and OS, particularly in patients with a high IPI score.
Keywords: diffuse large B-cell lymphoma, interim positron emission tomography/computed tomography, F-18 fluorodeoxyglucose, prognostic value
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the major histological subtype of non-Hodgkin lymphoma and accounts for 30–40% of all new diagnoses (1,2). It is an aggressive but potentially curable lymphoma. R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy regimens have been the primary treatment methods over the past decade (3). However, only ~60% of patients with DLBCL achieve durable remission following chemotherapy (4,5). Previous studies have demonstrated that the categorization of DLBCL phenotypes, particularly germinal centre B-cell-like (GCB) and non-GCB, may be used to determine patient prognosis (6,7). However, the results are controversial; it has been demonstrated that patients with the GCB phenotype have a better survival rate than those in the non-GCB group (8), however this has not been identified in other studies (9,10). Therefore, a reliable and reproducible prediction method is crucial to optimize patient care.
The standard International Prognostic Index (S-IPI) (4) is the most widely used and accepted prognostic tool for patients with aggressive lymphomas. Five individual risk factors, patient age, lactate dehydrogenase (LDH) concentration, Eastern Cooperative Oncology Group (ECOG) performance status (11), involvement of extra-nodal sites and Ann Arbor stage (12) are considered. S-IPI divides patients into four prognostic subgroups based on the number of risk factors present: A low risk group (0–1 risk factors), a low intermediate risk group (2 risk factors), a high intermediate risk group (3 risk factors) and a high-risk group (4–5 risk factors) (13). However, the addition of rituximab to CHOP-like regimens for patients with DLBCL resulted in a major improvement of patient outcome. Therefore, an updated version of IPI, the revised-IPI (R-IPI), was established by Sehn et al (14). Furthermore, an enhanced IPI (NCCN-IPI) was proposed by Zhou et al (15) which incorporates the same five variables as the S-IPI, but assigns different weights to age [>40–60, 1 point (pt); >60–75, 2 pts; >75, 3 pts] and elevated LDH [>1–3 (upper limit of normal; ULN), 1 pt; ≥3 ULN, 2 pts] and identifies the presence of extranodal involvement in the bone marrow, central nervous system (CNS), liver, gastrointestinal tract or lung as a positive parameter. These methods of predicting patient prognosis (S-IPI, R-IPI and NCCN-IPI) are based solely on the clinical features of patients prior to chemotherapy. Owing to the clinical and biological heterogeneity of DLBCL (7,16), it would be beneficial to add the assessment of responses to treatment.
F-18 fluorodeoxyglucose positive emission tomography/computed tomography (18F-FDGPET/CT) may be a powerful tool for monitoring the response to therapy in aggressive lymphomas (17,18). It was demonstrated that PET/CT could be used to evaluate the response in FDG-avid tissues using the 5-point scale (5-PS) (19) at the 11th International Conference, which was held in Lugano, Switzerland. Studies have suggested that the use of FDG PET/CT to monitor early responses may guide therapeutic strategies for patients with DLBCL (20–22). Therefore, interim 18F-FDG PET/CT following the 4th cycle of R-CHOP-like regimens was used to monitor the response of patients with DLBCL to treatment in the current study.
The present study aimed to explore the value of S-IPI, R-IPI and NCCN-IPI in predicting the prognosis of patients with DLBCL and to determine whether the prognostic value may be improved by interim 18F-FDG PET/CT response.
Patients and methods
Patients
Between January 2004 and January 2014, a total of 185 patients with newly diagnosed DLBCL were enrolled from Nanfang Hospital (Guangzhou, China) in the retrospective study, which was approved by the institutional ethics review board of Nanfang Hospital. Informed consent was obtained from all individual participants included in the study. The patients' clinical and biological characteristics are summarized in Table I.
Table I.
Patient characteristics.
| Characteristic | n (%) |
|---|---|
| Male/female ratio | 116/69 |
| Median age, years (range) | 49 (16–82) |
| ≤40 | 54 (29.2) |
| 40–60 | 87 (47.0) |
| 60–75 | 38 (20.6) |
| >75 | 6 (3.2) |
| Ann Arbor stage | |
| I | 23 (12.4) |
| II | 38 (20.6) |
| III | 27 (14.6) |
| IV | 97(52.4) |
| LDH, normalized | |
| ≤1 | 108 (58.4) |
| 1–3 | 57 (30.8) |
| >3 | 20 (10.8) |
| ECOG performance status | |
| 0–1 | 142 (76.8) |
| ≥2 | 43 (23.2) |
| Extranodal disease | |
| Bone marrow | 18 (9.7) |
| CNS | 11 (5.9) |
| Liver | 17 (9.2) |
| GI tract | 41 (22.2) |
| Lung | 14 (7.6) |
| Others | 103 (55.7) |
| Standard IPI score | |
| 0–1 | 74 (40.0) |
| 2 | 40 (21.6) |
| 3 | 40 (21.6) |
| 4–5 | 31 (16.8) |
| Revised IPI score | |
| 0 | 35 (18.9) |
| 1–2 | 79 (42.7) |
| 3–5 | 71 (38.4) |
| NCCN-IPI score | |
| 0–1 | 38 (20.6) |
| 2–3 | 90 (48.6) |
| 4–5 | 45 (24.3) |
| 6–8 | 12 (6.5) |
All values are presented as n (%). ECOG, Eastern Cooperative Oncology Group; CNS, central nervous system; NCCN-IPI, enhanced International Prognostic Index; LDH, lactate dehydrogenase.
Inclusion criteria were: i) ≥16 years of age at diagnosis, ii) histologically proven DLBCL, iii) treatment with R-CHOP-like regimens with curative intent and iv) an interim FDG PET/CT scan following the 4th cycle of chemotherapy. Patients were excluded if they were treated with surgery or exhibited evidence of a secondary malignant tumor. All patients were reviewed on pathological diagnosis by two hematopathologists.
PET/CT scanning protocol
All patients underwent whole body 18F-FDG PET/CT scan using a Discovery LS PET/CT scanner (GE Healthcare, Chicago, IL, USA). Following 6 h fasting, 185–370 MBq 18F-FDG (5.18 MBq/kg) was administered intravenously to each patient. Blood glucose levels were monitored prior to the scan to ensure that blood glucose levels were normal (<7 mmol/l). Approximately 60 min after the injection of FDG, whole-body PET/CT (from the vertex of the skull to the mid-thigh) was performed following the guidelines for tumor imaging with PET/CT (23). A spiral CT scan was performed with a scout view using an 0.8 sec rotation time, 80 mA, 140 kVp, 5-mm slice thickness and a 4.25-mm interval in high-speed mode. A whole-body PET/CT scan was acquired in the 2-dimensional acquisition mode with 3 min per bed position. Acquired PET and CT images were sent to the Xeleris workstation (version 2.1; GE Healthcare) for registration and fusion.
PET/CT analysis
The interim PET scan was performed following the 4th cycle of chemotherapy, with a median interval of 16 days after the first day of the second or third cycle (range, 14–21 days). All PET/CT images were interpreted by two experienced nuclear physicians in consensus using the Xeleris (version 2.1; GE Healthcare) workstation. A visual interpretation was performed using the Deauville five-point scale (24): 1, no residual uptake; 2, uptake ≤mediastinum; 3, uptake >mediastinum but ≤liver; 4, uptake moderately >liver; and 5, uptake markedly increased compared with the liver and/or progression of new lesions (25). Interim PET/CT images were reclassified into negative and positive groups; scores of 1–3 were considered negative and scores of 4–5 were considered positive (24). These criteria were used to determine extranodal disease and Ann Arbor stage.
S-IPI, R-IPI and NCCN-IPI
S-IPI, R-IPI and NCCN-IPI were examined in this cohort of 18F-FDG PET/CT staged patients with DLBCL. The risk factors identified by S-IPI were: Age >60 years, ECOG performance score ≥2, elevated LDH (>ULN), involvement of >1 extranodal site and Ann Arbor stage III/IV. S-IPI divided the patients into four prognostic subgroups, based on the number of risk factors present: A low risk group (0–1 risk factors), a low intermediate risk group (2 risk factors), a high intermediate risk group (3 risk factors) and a high-risk group (4–5 risk factors) (13). The R-IPI involves the same individual factors, but with only three risk groups: Very good (0 risk factors), good (1–2 risk factors) and poor (>2 risk factors) (14). NCCN-IPI also uses the same five risk factors as the IPI but further refines the categorization of age and normalized LDH and specific sites of involvement (bone marrow, CNS, liver, gastrointestinal tract or lung). Four risk groups were formed: A low risk group (0–1), a low intermediate group (2–3), a high intermediate group (4–5) and a high-risk group (6–8) (15). All risk factors of patients were available in the present study.
Statistical analysis
Descriptive statistics of clinical characteristics were generated as proportions. Fisher's exact test was analyzed to compare the differences between groups of categorical values. End points were 2-year progression free survival (PFS; defined as time from diagnosis to progression, relapse or mortality from any cause) and 2-year overall survival (OS; defined as time from diagnosis to mortality from any cause). PFS and OS were determined by Kaplan-Meier analysis and differences across groups were analyzed using a log-rank test. Prognostic factors were tested using a Cox proportional hazard model. All tests were considered significant when P<0.05 and were not adjusted for multiple comparisons. Statistical analyses were performed by GraphPad Prism version 5.0 (GraphPad software, Inc., La Jolla, CA, USA).
Results
Patient outcomes
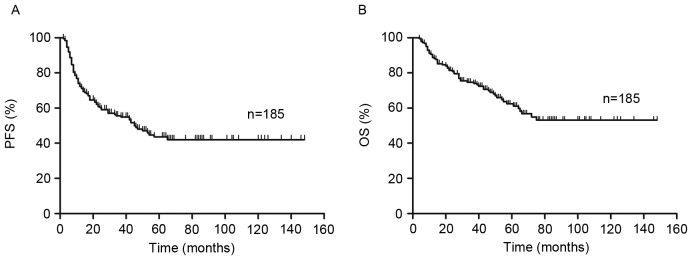
The median follow-up time of patients was 44 months (range 4–148 months). Of all 185 patients, 88 patients exhibited no progression (PFS 47.6%) and the median time prior to relapse was 34 months (range 2–148 months). By the end of follow-up (148 months was the longest follow-up time for one patient), OS was 67% (124/185 patients).
Outcomes according to S-IPI and interim PET/CT
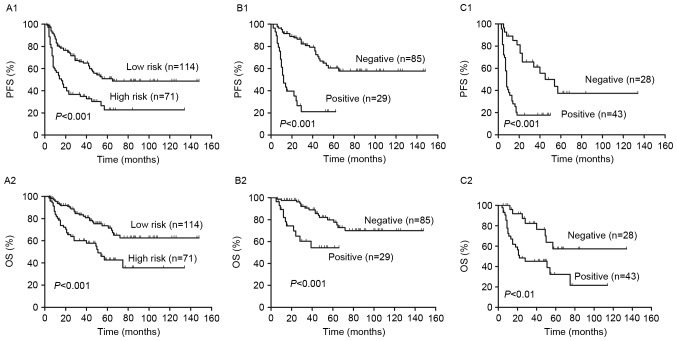
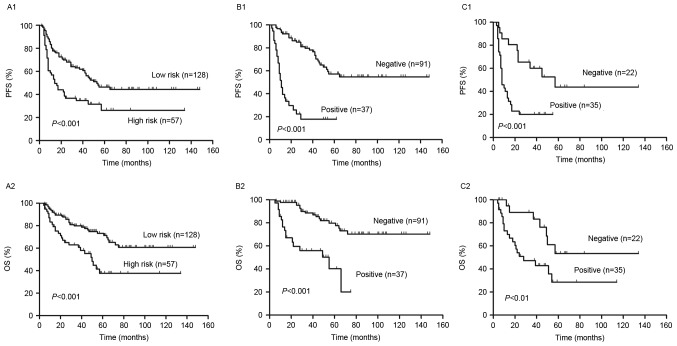
PFS and OS curves present the outcome of patients treated with R-CHOP like regimens (Fig. 1). The 2-year PFS and OS of all risk subgroups are presented in Table II. Of the entire cohort, 2-year PFS and OS were 60% [95% confidence interval (CI), 53–67%] and 81% (95% CI, 74–86%), respectively (Fig. 1). As demonstrated in Fig. 2, analysis of S-IPI results identified statistically significant differences in PFS and OS between patients in the lower and higher risk groups (P<0.001; Fig. 2A). The results also identified statistically significant differences in the lower risk group between score 0–1 and score 2 in PFS (P=0.01) and OS (P<0.01). However, in the higher risk group, it exhibited no statistically significant difference in PFS (P=0.47) and OS (P=0.16) between score 3 and score 4–5.
Figure 1.
Overall outcome. (A) PFS and (B) OS in the 185 patients with DLBCL treated with R-CHOP-like regimens. PFS, progression free survival; OS, overall survival; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; DLBCL, diffuse large B-cell lymphoma.
Table II.
Comparison of S-IPI, R-IPI to NCCN-IPI for risk stratification and outcomes of 2-year PFS and OS in these risk groups redistributed by interim PET/CT into PET negative (−) and positive (+) groups.
| S-IPI | R-IPI | NCCN-IPI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Low risk (0–2) | High risk (3–5) | Very good (0) | Good (1–2) | Poor (3–5) | Low risk (0–3) | High risk (4–8) | |||||||
| 2-year | 74 (64–81) | 37 (26–48) | 97 (80–100) | 63 (51–73) | 37 (25–48) | 70 (61–77) | 37 (24–49) | |||||||
| PFS | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) |
| 88 (78–94) | 31 (15–49) | 66 (44–80) | 18 (8–31) | 100 | 97 (80–100) | 82 (67–92) | 26 (11–44) | 66 (44–81) | 18 (8–31) | 85 (76–91) | 22 (10–38) | 65 (41–82) | 20 (9–34) | |
| 2-year | 90 (82–94) | 66 (53–76) | 97 (80–100) | 85 (75–91) | 60 (47–71) | 88 (80–93) | 65 (51–76) | |||||||
| OS | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) | PET(−) | PET(+) |
| 98 (90–99) | 65 (43–80) | 82 (59–93) | 45 (29–60) | 100 | 97 (80–100) | 94 (82–98) | 63 (40–79) | 92 (71–98) | 48 (32–63) | 95 (88–98) | 60 (41–74) | 89 (63–97) | 51 (33–66) | |
All results are presented as the % (95% CI). OS, overall survival; PFS, progression free survival; S-IPI, standard International Prognostic Index; R-IPI, revised International Prognostic Index; NCCN-IPI, enhanced International Prognostic Index; PET, positron emission tomography; CT, computed tomography.
Figure 2.
Outcomes according to the S-IPI and interim PET/CT. (A) PFS and OS in the low and high risk groups, as determined by S-IPI. Redistribution of the (B) low and (C) high risk groups into PET negative and positive groups. Differences between the 2-year PFS and OS between the PET negative and positive groups were significant. S-IPI, standard International Prognostic Index; PET, positron emission tomography; CT, computed tomography; PFS, progression free survival; OS, overall survival.
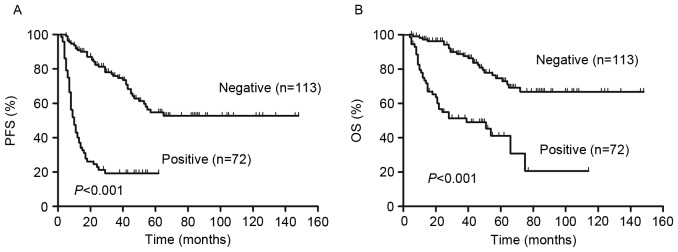
Analysis of visual 5-PS results demonstrated that there were significant differences in the PFS and OS of patients with a positive interim PET scan compared with patients that had a negative interim PET scan (P<0.01; Fig. 3). The 2-year PFS and OS were 82% (95% CI, 76–88%) and 96% (95% CI, 90–99%), respectively, in patients with negative PET results. By contrast, in patients with positive PET results, 2-year PFS and OS were 23% (95% CI 14–34%) and 55% (95% CI 42–66%), respectively.
Figure 3.
(A) PFS and (B) OS according to interim PET/CT (negative and positive) in 185 patients with DLBCL treated with R-CHOP-like regimens. PFS, progression free survival; OS, overall survival; PET/CT, positron emission tomography/computed tomography; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; DLBCL, diffuse large B-cell lymphoma.
In the higher risk group, 2-year PFS and OS were 37% (95% CI, 26–48%) and 66% (95% CI, 53–76%), respectively (Table II). Patients in the low risk group were reclassified into PET negative and positive groups by interim PET/CT and it was demonstrated that patients in the PET positive group had a significantly lower PFS and OS than those in the PET negative group (P<0.001; Fig. 2B). Furthermore, patients in the low and low intermediate risk groups were reclassified into PET negative and positive groups using interim PET/CT (Fig. 4). It was demonstrated that the differences in PFS and OS between the PET negative and positive groups were significant in the low risk group (both P<0.05; Fig. 4A). However, in the low intermediate risk group, the difference in PFS between PET negative and positive patients was significant (P=0.0001); however, the difference in OS was not (Fig. 4B).
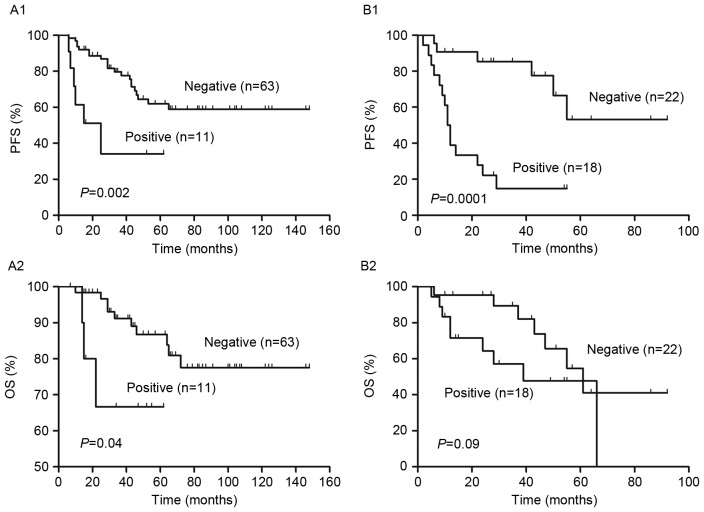
Figure 4.
Analysis of the (A) low (score=0–1) and (B) low intermediate (score=2) risk group in the S-IPI. 2-year PFS and OS were redistributed by interim PET/CT into PET positive and negative groups. Differences between PFS and OS were significant between PET positive and negative patients, apart from the difference in 2-year OS in the low intermediate risk group (P=0.09). PFS, progression free survival; OS, overall survival; S-IPI, standard International Prognostic Index; PET, positron emission tomography; CT, computed tomography.
Outcomes according to R-IPI and interim PET/CT
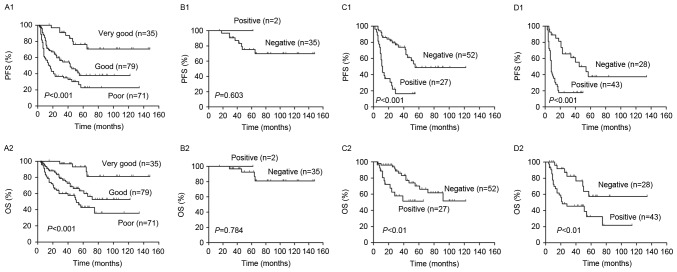
R-IPI is a valid predictor of outcomes of patients with DLBCL treated with R-CHOP like regimens. Fig. 5 demonstrates that R-IPI identified 3 distinct prognostic groups with a very good, good and poor outcome, respectively, with significant differences between all groups in PFS and OS (P<0.001; Fig. 5A). The 2-year PFS and OS of all risk subgroups are presented in Table II. Analysis of PET results identified significant differences in the PFS and OS between PET positive and negative patients in the good and poor risk groups (P<0.01; Fig. 5C and D). However, no significant differences in PFS (P=0.60) or OS (P=0.07) between PET negative and positive patients were identified in the very good risk group (Fig. 5B). In all groups, 2-year PFS and OS were decreased in PET positive patients compared with PET negative patients (P<0.01; Fig. 5 and Table II).
Figure 5.
Outcomes according to the R-IPI and interim PET/CT. (A) PFS and OS according to the very good, good and poor risk patient groups, as determined by R-IPI. Redistribution of the (B) very good, (C) good and (D) poor risk groups into PET negative and positive groups. There were significant differences in the 2-year PFS and OS between PET-negative and positive patients in the good and poor risk group, but not in the very good risk group. PFS, progression free survival; OS, overall survival; R-IPI, revised International Prognostic Index; PET, positron emission tomography; CT, computed tomography.
Outcomes according to NCCN-IPI and interim PET/CT
Patients were classified into high and low risk groups, according to NCCN-IPI and it was determined that the PFS and OS were significantly lower in high risk patients compared with low risk patients (P<0.001, Fig. 6A). The 2-year PFS and OS of the risk subgroups are presented in Table II. In the higher risk group, there was no significant difference in PFS (P=0.84) and OS (P=0.51) between scores 4–5 and 6–8. However, there were significant differences in the PFS (P<0.01) and OS (P<0.01) of patients with scores 0–1 and 2–3.
Figure 6.
Outcomes according to the NCCN-IPI and interim PET/CT. (A) PFS and OS according to the low and high risk group of NCCN-IPI. Redistribution of the (B) low and (C) high risk groups into PET negative and positive. There were significant differences in the 2-year PFS and OS between PET negative and positive patients in all groups. PFS, progression free survival; OS, overall survival; NCCN-IPI, enhanced International Prognostic Index; PET, positron emission tomography; CT, computed tomography.
The interim PET results divided patients in the high and low risk groups into PET negative and positive groups (Fig. 6). The 2-year PFS and OS were significantly higher in the PET negative group compared with the PET positive group in the low and high risk groups (P<0.01, Fig. 6B and C). Patients in the low risk were further subdivided into a low risk group and a low intermediate risk group. In the low intermediate risk group, PFS and OS were significantly lower in PET negative patients compared with PET positive patients (P<0.001; Fig. 7B). However, in the low risk group, there were no significant differences in PFS and OS between the PET negative and positive groups (P>0.05, Fig. 7A).
Figure 7.
Analysis of the (A) low (score=0–1) and (B) low intermediate (score=2–3) risk group of the NCCN-IPI. Interim PET/CT was used to redistribute patients into PET positive and negative groups. There were significant differences in 2-year PFS and OS in the low intermediate risk group (P<0.001) but not the in the low risk group (P>0.05). PFS, progression free survival; OS, overall survival; NCCN-IPI, enhanced-IPI; PET, positron emission tomography; CT, computed tomography.
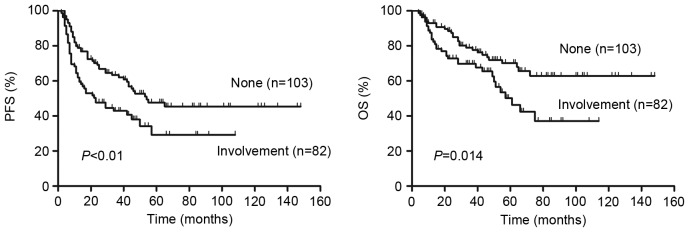
Patients that had DLBCL with involvement of bone marrow, CNS, liver, gastrointestinal tract or lung exhibited a significantly lower PFS (P=0.005, Fig. 8A) and OS (P=0.014, Fig. 8B) than patients without involvement of these organs. No significant difference in survival was observed between these involved organs for patients with DLBCL.
Figure 8.
Outcomes according to the involvement of bone marrow, central nervous system, liver, gastrointestinal tract or lung. The 2-year PFS and OS differed significantly between patients that exhibited involvement of these sites and those that did not exhibit involvement of these sites. PFS, progression free survival; OS, overall survival.
Discussion
In the present study, the prognostic value of S-IPI, R-IPI and NCCN-IPI in patients with DLBCL treated with R-CHOP-like regimens was assessed. Interim PET following 4 cycles induction chemotherapy was used to predict the 2-year PFS and OS of patients with DLBCL. The results of the current study demonstrated that S-IPI, R-IPI and NCCN-IPI are three clinically useful prognostic indexes that may guide the treatment planning of patients with DLBCL. Furthermore, interim PET/CT improves the prognostic value of S-IPI, R-IPI and NCCN-IPI in predicting the 2-year PFS and OS of patients with DLBCL, particularly in patients with high IPI scores.
The use of R-CHOP-like regimens has resulted in a major improvement of survival in patients with DLBCL across all risk groups (3,26). Consequently, the usefulness of S-IPI in discriminating between patients in different risk groups has declined, particularly in higher risk groups (4,14). In the current study, the S-IPI revealed no significant differences in PFS and OS between patients in the high intermediate (score 3) and high risk groups (score 4–5). NCCN-IPI is more powerful than S-IPI at predicting the survival of low- and high-risk patients (15). However, the results of the current study demonstrated that differences in PFS and OS between the high intermediate (score 4–5) and high-risk groups (score 6–8) of the NCCN-IPI were not significant. R-IPI identified 3 distinct prognostic groups with very good (score 0), good (score 1–2) and poor (score 3–5) outcomes (P<0.001). From this, the R-IPI integrated the high intermediate risk group (score 3) and the high risk group (score 4–5) in S-IPI into one group: The poor risk group (score 3–5). However, R-IPI was not able to differentiate between the high intermediate and the high risk groups. Therefore, the ability of S-IPI, NCCN-IPI and R-IPI to differentiate between the high and high intermediate risk groups is limited.
Alizadeh et al (16) demonstrated that DLBCL is a heterogeneous group of malignancies rather than a single clinical or pathological entity. The World Health Organization acknowledged the biological heterogeneity of DLBCL in the version of lymphoid malignancies in 2008 (27). To date, the Hans algorithm has been the most widely used method of reclassifying DLBCL patients into GCB and non-GCB groups, based on the presence of three immunohistochemical markers [cluster of differentiation 10, B-Cell lymphoma 6 and melanoma associated antigen (mutated) 1] (28). However, the results of previous studies with regard to the prognostic value of GCB and non-GCB phenotypes for patients with DLBCL are conflicting (8–10). S-IPI, R-IPI and NCCN-IPI are all based solely on the clinical features of patients prior to chemotherapy. Considering the clinical and biological heterogeneity of DLBCL, improving the prediction of patient responses to treatment based on the IPI score is imperative.
18F-FDG PET/CT is a powerful method of evaluating the response to chemotherapy in patients with DLBCL (29). It has been demonstrated that the use of interim PET/CT to assess the response to treatment may induce chemosensitivity and may help to guide therapeutic strategies for patients with DLBCL (21,30). Itti et al (31) reported that the accuracy at four cycles was better than at two cycles when visual interpretation was used. Patients who were PET2-positive (PET imaging following two cycles of chemotherapy) became PET4-negative (PET imaging following four cycles of chemotherapy), of whom only a small number experienced an event, whereas patients who were PET2-positve remained PET4-positive, of whom most rapidly had an event. By comparison, all PET2-negative patients who underwent PET-4 remained negative and few experienced an event (31). Therefore, in the current study, interim PET following four cycles of chemotherapy was used to monitor the treatment response of patients with DLBCL.
The results of the current study confirmed that the redistribution of high risk patients into PET negative and positive groups provided a more clinically relevant prediction of patient outcome. Compared with the PET negative group, PET positive patients had a significantly lower PFS and OS. Therefore, an investigational approach involving clinical trials on PET positive patients in high or poor risk groups should be considered to ensure potential curative therapy. Although S-IPI and NCCN-IPI were able to discriminate between low and low intermediate risk groups in predicting PFS and OS, the results indicated that PFS and OS were higher in patients in the lower risk group that were PET negative than those that were evaluated using IPI scores alone. However, no significant differences were observed between PET negative and PET positive groups in patients determined to be in the low intermediate risk group by S-IPI. In the low risk NCCN-IPI group, there were also no significant differences in 2-year PFS and OS between PET negative and positive patients. This may have been due to the fact that the number of patients in these groups was too small. Furthermore, the number of patients that were PET positive in the very good risk group of R-IPI was too small to obtain a valid statistical interpretation.
Zhou et al (15) suggested that the involvement of the bone marrow, CNS, liver, gastrointestinal tract or lung in lymphoma may be a better predictor of survival in patients with DLBCL than simply the number of extranodal sites involved. The results of the current study demonstrated that patients with involvement of these organs had a significantly lower 2-year PFS and OS than those without involvement. No significant difference of survival was observed among these involved organs for patients with DLBCL. This may be due to the fact that the involvement of the organs is small and often overlapping, thus it is difficult to clearly identify the effects of organ involvement alone on PFS and OS.
The present study used three different meaningful prognostic tools, S-IPI, R-IPI and NCCN-IPI, to evaluate the prognosis of patients with DLBCL. All of them identified significant differences in the PFS and OS between low and high risk groups. The results of the current study demonstrated that low and high risk groups can be further reclassified into PET positive and negative groups using interim PET/CT at the 4th cycle of treatment with an R-CHOP like regimen. Patients that were PET negative in the low or high risk groups had a higher PFS and OS than those that were PET positive. However, the current study was a retrospective analysis and there were low numbers of patients in the low intermediate risk group in S-IPI, the low risk group in NCCN-IPI and in the very good risk group in R-IPI that were PET positive. Therefore, the results should be validated prospectively in a larger population of patients with DLBCL.
In conclusion, the present study demonstrated that the S-IPI, R-IPI and NCCN-IPI are three clinically useful prognostic indexes that may guide the treatment planning of patients with DLBCL. The results suggest that interim PET/CT improves the risk stratifications of S-IPI, R-IPI and NCCN-IPI in predicting 2-year PFS and OS, particularly in patients with high IPI scores.
Acknowledgements
The study was funded by the National Natural Science Foundation of China (grant no. 81271641) and the projects of medical and health technology program in Zhejiang province (grant no. 2018KY676).
References
- 1.No authors listed: A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 2.Coiffier B. Diffuse large cell lymphoma. Curr Opin Oncol. 2001;13:325–334. doi: 10.1097/00001622-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, Loeffler M. Standard international prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:2373–2380. doi: 10.1200/JCO.2009.26.2493. [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: A randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 6.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dybkær K, Bøgsted M, Falgreen S, Bødker JS, Kjeldsen MK, Schmitz A, Bilgrau AE, Xu-Monette ZY, Li L, Bergkvist KS, et al. Diffuse large B-cell lymphoma classification system that associates normal B-cell subset phenotypes with prognosis. J Clin Oncol. 2015;33:1379–1388. doi: 10.1200/JCO.2014.57.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrans SL, Carter I, Owen RG, Davies FE, Patmore RD, Haynes AP, Morgan GJ, Jack AS. Germinal center phenotype and bcl-2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood. 2002;99:1136–1143. doi: 10.1182/blood.V99.4.1136. [DOI] [PubMed] [Google Scholar]
- 9.Colomo L, López-Guillermo A, Perales M, Rives S, Martínez A, Bosch F, Colomer D, Falini B, Montserrat E, Campo E. Clinical impact of the differentiation profile assessed by immunophenotyping in patients with diffuse large B-cell lymphoma. Blood. 2003;101:78–84. doi: 10.1182/blood-2002-04-1286. [DOI] [PubMed] [Google Scholar]
- 10.Linderoth J, Jerkeman M, Cavallin-Ståhl E, Kvaløy S, Torlakovic E. Nordic Lymphoma Group Study: Immunohistochemical expression of CD23 and CD40 may identify prognostically favorable subgroups of diffuse large B-cell lymphoma: A nordic lymphoma group study. Clin Cancer Res. 2003;9:722–728. [PubMed] [Google Scholar]
- 11.Young J, Badgery-Parker T, Dobbins T, Jorgensen M, Gibbs P, Faragher I, Jones I, Currow D. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage. 2015;49:258–264. doi: 10.1016/j.jpainsymman.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL. The American Joint Committee on Cancer: Updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–15. [PubMed] [Google Scholar]
- 13.International Non-Hodgkin's Lymphoma Prognostic Factors Project: A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 14.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–1861. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 17.Terasawa T, Nihashi T, Hotta T, Nagai H. 18F-FDG PET for posttherapy assessment of Hodgkin's disease and aggressive Non-Hodgkin's lymphoma: A systematic review. J Nucl Med. 2008;49:13–21. doi: 10.2967/jnumed.107.039867. [DOI] [PubMed] [Google Scholar]
- 18.Terasawa T, Lau J, Bardet S, Couturier O, Hotta T, Hutchings M, Nihashi T, Nagai H. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin's lymphoma and diffuse large B-cell lymphoma: A systematic review. J Clin Oncol. 2009;27:1906–1914. doi: 10.1200/JCO.2008.16.0861. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA. Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium: Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casasnovas RO, Meignan M, Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, Vera P, Bologna S, Brière J, Jais JP, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood. 2011;118:37–43. doi: 10.1182/blood-2010-12-327767. [DOI] [PubMed] [Google Scholar]
- 21.Fuertes S, Setoain X, Lopez-Guillermo A, Carrasco JL, Rodríguez S, Rovira J, Pons F. Interim FDG PET/CT as a prognostic factor in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2013;40:496–504. doi: 10.1007/s00259-012-2320-8. [DOI] [PubMed] [Google Scholar]
- 22.Nols N, Mounier N, Bouazza S, Lhommel R, Costantini S, Vander Borght T, Vekemans MC, Sonet A, Bosly A, Michaux L, et al. Quantitative and qualitative analysis of metabolic response at interim positron emission tomography scan combined with International Prognostic Index is highly predictive of outcome in diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55:773–780. doi: 10.3109/10428194.2013.831848. [DOI] [PubMed] [Google Scholar]
- 23.Delbeke D, Coleman RE, Guiberteau MJ, Brown ML, Royal HD, Siegel BA, Townsend DW, Berland LL, Parker JA, Hubner K, et al. Procedure guideline for tumor imaging with 18F-FDG PET/CT 1.0. J Nucl Med. 2006;47:885–895. [PubMed] [Google Scholar]
- 24.Meignan M, Gallamini A, Haioun C, Polliack A. Report on the second international workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk Lymphoma. 2010;51:2171–2180. doi: 10.3109/10428194.2010.529208. [DOI] [PubMed] [Google Scholar]
- 25.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the first international workshop on interim-PET-scan in lymphoma. Leuk Lymphoma. 2009;50:1257–1260. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- 26.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 27.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 28.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 29.Kwon SH, Kang DR, Kim J, Yoon JK, Lee SJ, Jeong SH, Lee HW, An YS. Prognostic value of negative interim 2-[18F]-fluoro-2-deoxy-d-glucose PET/CT in diffuse large B-cell lymphoma. Clin Radiol. 2016;71:280–286. doi: 10.1016/j.crad.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Yang DH, Min JJ, Song HC, Jeong YY, Chung WK, Bae SY, Ahn JS, Kim YK, Bom HS, Chung IJ, et al. Prognostic significance of interim 18F-FDG PET/CT after three or four cycles of R-CHOP chemotherapy in the treatment of diffuse large B-cell lymphoma. Eur J Cancer. 2011;47:1312–1318. doi: 10.1016/j.ejca.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 31.Itti E, Lin C, Dupuis J, Paone G, Capacchione D, Rahmouni A, Haioun C, Meignan M. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-Cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med. 2009;50:527–533. doi: 10.2967/jnumed.108.057703. [DOI] [PubMed] [Google Scholar]