Abstract
Introduction
Currently, the detection of pathogens or mutations associated with intraocular lymphomas heavily relies on prespecified, directed PCRs. With metagenomic deep sequencing (MDS), an unbiased high-throughput sequencing approach, all pathogens as well as all mutations present in the host’s genome can be detected in the same small amount of ocular fluid.
Methods
In this cross-sectional case series, aqueous fluid samples from two patients were submitted to MDS to identify pathogens as well as common and rare cancer mutations.
Results
MDS of aqueous fluid from the first patient with vitreal lymphoma revealed the presence of both Epstein-Barr virus (HHV-4/EBV) and human herpes virus 8 (HHV-8) RNA. Aqueous fluid from the second patient with intraocular B-cell lymphoma demonstrated a less common mutation in the MYD88 gene associated with B-cell lymphoma.
Conclusion
MDS detects pathogens that, in some instances, may drive the development of intraocular lymphomas. Moreover, MDS is able to identify both common and rare mutations associated with lymphomas.
Keywords: aqueous humour, immunology, infection, inflammation
Introduction
Molecular diagnostics such as pathogen-directed PCRs previously revolutionised the field of ophthalmology by accurately providing diagnoses for ocular infections using minute amounts of ocular samples.1 2 A major limitation with these assays is that only prespecified pathogens can be detected leading to potential missed pathogens. Additionally, directed PCR or DNA microarray may overlook less common mutations associated with lymphoproliferative disorders. Metagenomic deep sequencing (MDS) is an unbiased high-throughput sequencing approach that can theoretically detect all pathogens in a clinical sample. Further, it simultaneously provides genetic information that may be useful for evaluating the host’s immune system. Distinguishing uveitis as infectious or non-infectious is vital as such knowledge guides appropriate treatment. It is also important to identify uveitic masqueraders such as intraocular malignancies as the treatment is likewise impacted by this knowledge. Herein, we present two cases of intraocular lymphoproliferative disorders that were identified using MDS.
Methods
Study design
Cross-sectional case studies in which patients’ aqueous fluid samples were submitted to MDS to identify infectious pathogens as well as host genome sequencing to identify mutations associated with lymphoproliferative disorders. Our research adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants.
Sequencing library preparation
Samples were prepared for MDS as previously described.3 4 Briefly, RNA was extracted from 20 to 50 µL of aqueous fluid with the Direct-zol RNA Microprep (Zymo Research, California, USA) per manufacturer’s recommendations and eluted in 20 µL nuclease-free water. Five microlitres of the extracted RNA were used to prepare libraries using the New England Biolabs’ (NEB) NEBNext RNA First Strand Synthesis Module (E7525) and NEBNext Ultra Directional RNA Second Strand Synthesis Module (E7550) to generate double-stranded cDNA. The cDNA was converted to Illumina libraries using the NEBNext Ultra II DNA Library Prep Kit (E7645) according to the manufacturer’s recommendation and then amplified with 14 PCR cycles. Samples were sequenced on the Illumina HiSeq 4000 using 125-nucleotide (nt) paired-end sequencing.
Bioinformatics
Analysis of sequenced data was made using a rapid computational pipeline developed in-house to classify sequencing reads and identify potential pathogens against the entire National Center for Biotechnology Information (NCBI) nt reference database.3 4 To identify the somatic mutations in the myeloid differentiation primary response 88 (MYD88) gene (NC_000003.12), we aligned the filtered human sequences against the MYD88 gene using HISAT2 (V.2.0.5).5
Results
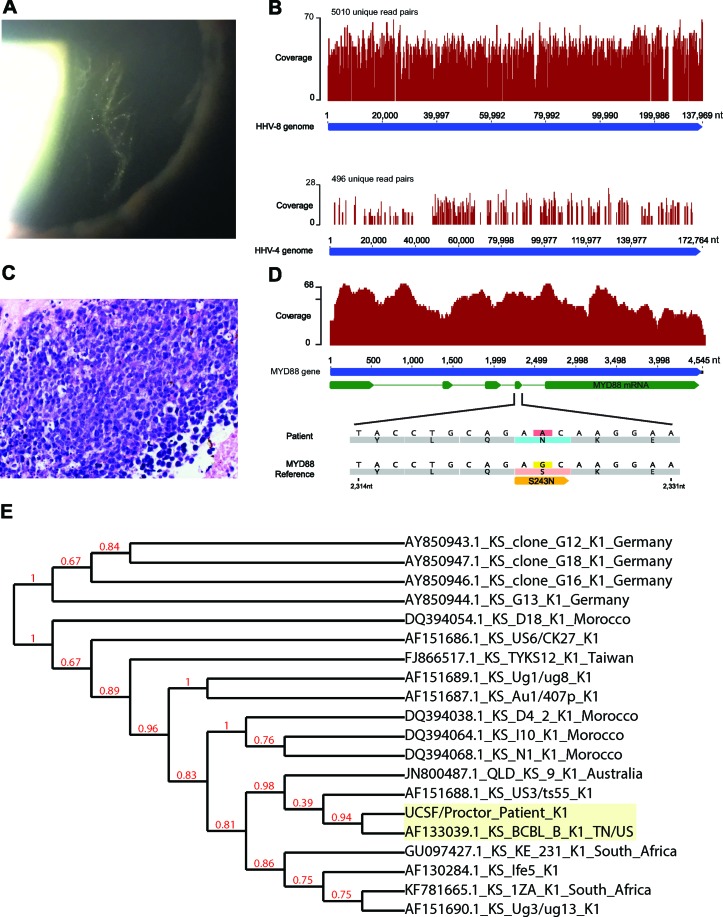
The first patient was a middle-aged Caucasian man who presented with ‘foggy vision’ in the left eye. His medical history included Kaposi sarcoma of the chest and thigh 30 years ago and now in remission, and a diagnosis of HIV 15 years prior, currently on antiretroviral treatment. His most recent absolute CD4 count was 800×106/L. Examination of the right eye was unremarkable. Notably, the left eye exhibited ½+ anterior chamber cells and 1+ flare, 3+ vitreous cells and ½+ vitreous haze (figure 1A). There were no retinal or choroidal lesions. Anterior chamber paracentesis was performed as well as a diagnostic and therapeutic vitrectomy of the left eye. Pathogen-directed PCR analysis of the aqueous fluid was negative for herpes simplex virus (HSV), varicella zoster virus (VZV) and cytomegalovirus (CMV). MDS of the aqueous fluid identified the presence of both Epstein-Barr virus (HHV-4/EBV) and human herpes virus 8 (HHV-8) RNA (figure 1B). The vitrectomy fluid yielded rare, small lymphocytes on cytopathology, no B-cells on flow cytometry, but Ig-kappa gene rearrangement suggestive of lymphoma. In the context of a patient with HIV, the finding of lymphoma with a coinfection with HHV-8 and EBV can be associated with a phenotype most similar to primary effusion lymphoma (PEL).6 Interestingly, phylogenetic analysis of the patient’s HHV-8 strain indicated that it was most similar to a PEL-associated strain isolated from a patient in the USA (NCBI GenBank AF1330391.1, figure 1E).
Figure 1.
Clinical, histopathology and metagenomic deep sequencing (MDS) findings of patient #1 and #2. (A) Anterior vitreous cells on slit lamp examination, and (B) HHV-8 and HHV-4 (also known as EBV) genomes were detected in the aqueous fluid by MDS for patient #1; (C) photomicrograph of H&E-stained specimen from the anterior chamber biopsied material disclosing the presence of numerous intermediate to large cells with irregular nuclei. Immunohistochemistry of these cells showed diffusely positive staining with B-lymphocyte antigen CD20 and most of the large cells stained positively with BCL-2 (not shown), and (D) analysis of the MYD88 gene indicated the presence of a serine to asparagine mutational change at amino acid position 243 for patient #2. (E) Phylogenetic analysis of the HHV-8 sequence obtained from MDS for patient #1 using the ORF K1 hypervariable region with HHV-8 isolates from different diseases and geographic origins using multiple sequence comparison by log-expectation (MUSCLE).12 HHV-8, human herpes virus-8; HHV-4, human herpes virus-4; EBV, Epstein-Barr virus; BCL-2, B-cell lymphoma 2; MYD88, myeloid differentiation primary response 88; ORF, open reading frame; BCBL, body cavity-based lymphoma.
The second patient was a mid-30s Caucasian man with bilateral intermediate uveitis of 5 years’ duration who presented for initiation of systemic immunomodulatory therapy. A vitrectomy performed 1 year after onset of disease showed mixed lymphocytes, but was read as negative for lymphoma. On presentation in our clinic, the patient exhibited 1+ anterior chamber cells, 3+ anterior vitreous cells and 1+ vitreous haze in the right eye. The left eye had no anterior chamber cell or flare, but with 3+ anterior vitreous cells and no vitreous haze. There were no retinal or choroidal lesions in either eye. An anterior chamber paracentesis was negative on directed PCR for HSV, VZV, CMV and Toxoplasma gondii. Likewise, MDS was negative for infectious agents. Given that MDS captures mostly host sequence, we were able to use the same data set to interrogate the MYD88 gene for both common and rare mutations associated with lymphoma. Indeed, the patient exhibited the S243N mutation (figure 1D), which has been associated with the activated B-cell (ABC) subtype of diffuse large B-cell lymphoma. S243N is a recognised gain of function mutant that can contribute to constitutive NF-kB expression.7 This finding was intriguing as 50%–70% of primary vitreoretinal lymphomas exhibit the L265P mutation.8 Thus, routine screening using directed PCR for the most common mutation would not have identified this patient’s lymphoma. A definitive diagnosis of lymphoma was provided when biopsy of material in the anterior chamber revealed large B-cell lymphoma on histopathology (figure 1C).
Discussion
Intraocular lymphomas are challenging to diagnose given the small amounts of tissue that can be obtained on biopsy. Lymphomatous cells are fragile and may appear as non-diagnostic ‘smudge cells’ on cytopathological inspection.9 10 Further, the time of disease onset to diagnosis is often prolonged because its clinical signs may be attributed to undifferentiated uveitis.11 The cases described here demonstrated that MDS can accurately detect pathogen-related causes of inflammation that drive intraocular lymphomas as well as common and rare cancer-associated mutations in as little as 20 to 50 µL of aqueous fluid. MDS, therefore, has the potential to improve the diagnostic rates of intraocular lymphomas as the diagnosis may be possible with a single outpatient anterior chamber paracentesis as the initial work-up.
Acknowledgments
We thank Derek Bogdanoff and Eric Chow in the UCSF Center for Advanced Technology for their expertise and assistance operating the Illumina sequencer and Armin Hinterwirth for his assistance with bioinformatics. Lastly, we thank our patients for their participation in this research program.
Footnotes
Contributors: Conception and design of study: JAG, TD, MRW, JLD, NA. Acquisition of data: JAG, TD, JGS, MB, NRA. Analysis and/or interpretation of data: TD, JAG, MB, MRW, JLD, NRA. Drafting the manuscript: JAG, TD, JGS, MB, MRW, JLD, NRA. Revising the manuscript critically for important intellectual content: JAG, TD, MRW, JLD, NRA. Approval of the version of the manuscript to be published: JAG, TD, JGS, MB, MRW, JLD, NRA.
Funding: Research reported in this publication was supported by the UCSF Resource Allocation Program for Junior Investigators in Basic and Clinical/Translational Science (TD); Research to Prevent Blindness Career Development Award (TD); the National Eye Institute of the National Institutes of Health (NIH) under Award Number K08EY026986 (TD); Silicon Valley Community Foundation/Huang Pacific Foundation (TD), and the Chan Zuckerberg Biohub (JLD).
Competing interests: None declared.
Ethics approval: The Institutional Review Board of the University of California, San Francisco approved the study (16–19151).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Taravati P, Lam D, Van Gelder RN. Role of molecular diagnostics in ocular microbiology. Curr Ophthalmol Rep 2013;1:181–9. 10.1007/s40135-013-0025-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Groot-Mijnes JD, de Visser L, Zuurveen S, et al. Identification of new pathogens in the intraocular fluid of patients with uveitis. Am J Ophthalmol 2010;150:628–36. 10.1016/j.ajo.2010.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doan T, Wilson MR, Crawford ED, et al. Illuminating uveitis: metagenomic deep sequencing identifies common and rare pathogens. Genome Med 2016;8:90 10.1186/s13073-016-0344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson MR, Shanbhag NM, Reid MJ, et al. Diagnosing Balamuthia mandrillaris Encephalitis With Metagenomic Deep Sequencing. Ann Neurol 2015;78:722–30. 10.1002/ana.24499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 2015;12:357–60. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horenstein MG, Nador RG, Chadburn A, et al. Epstein-Barr virus latent gene expression in primary effusion lymphomas containing Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8. Blood 1997;90:1186–91. [PubMed] [Google Scholar]
- 7. Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011;470:115–9. 10.1038/nature09671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonzheim I, Giese S, Deuter C, et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood 2015;126:76–9. 10.1182/blood-2015-01-620518 [DOI] [PubMed] [Google Scholar]
- 9. Davis JL, Solomon D, Nussenblatt RB, et al. Immunocytochemical staining of vitreous cells. Indications, techniques, and results. Ophthalmology 1992;99:250–6. [DOI] [PubMed] [Google Scholar]
- 10. Gonzales JA, Chan CC. Biopsy techniques and yields in diagnosing primary intraocular lymphoma. Int Ophthalmol 2007;27:241–50. 10.1007/s10792-007-9065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan CC, Wallace DJ. Intraocular lymphoma: update on diagnosis and management. Cancer Control 2004;11:285–95. 10.1177/107327480401100502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dereeper A, Guignon V, Blanc G, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 2008;36:W465–W469. 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]