Abstract
Chidamide, a histone deacetylase (HDAC) inhibitor, has been applied in clinical trials for various types of hematological and solid tumors. Although acquired resistance is common in chemotherapy, the mechanism of resistance to chidamide is poorly characterized. The goal of the present study was to explore, in detail, the mechanism for the induced resistance to chidamide, and investigate a potential cross-resistance to other chemotherapeutic drugs. A549 cells were exposed to gradually increasing chidamide concentrations to establish a chidamide-resistant non-small cell lung cancer cell line (A549-CHI-R). The IC50 for chidamide, the proliferation inhibition rate, the total HDAC activity and the HDAC protein level were determined by an MTT assay, colony formation, a fluorometric HDAC activity assay and western blotting, respectively. Overexpression of the HDAC1 gene and HDAC1 gene-knockdown were achieved via plasmid transfection. A549-CHI-R cells demonstrated increased resistance to chidamide (8.6-fold). HDAC1 protein degradation was inhibited and HDAC activity was significantly higher in the A549-CHI-R cells relative to the parental A549 cells. A549-CHI-R cells demonstrated cross-resistance to paclitaxel, vinorelbine and gemcitabine, but not to cisplatin (CDDP) or 5-fluorouracil (5-FU). These results indicated that HDAC1 may be associated with resistance to chidamide, and HDAC1 may therefore be a predictive marker for chidamide sensitivity in cancer. In addition, A549-CHI-R cells remained sensitive to 5-FU and CDDP, indicating a potential strategy for cancer therapy.
Keywords: chidamide, acquired resistance, histone deacetylase 1 degradation, cross-resistant, G2 cell cycle arrest
Introduction
Histone deacetylases (HDACs) are enzymes that remove acetyl groups from histones and a number of non-histone proteins, leading to chromatin condensation and transcription repression (1). To date, 18 HDAC enzymes have been identified in humans, which have been categorized into four classes (2). Class I HDACs including HDAC1, 2, 3 and 8 have been reported to be overexpressed in several cancers, including gastric (3), esophageal (4), colorectal (5), prostate (6) and lung (7) cancer. Aberrant HDAC activity has been detected in a number of types of human cancer, thereby contributing to tumor initiation and progression (2,8). Targeting HDACs is a novel strategy in the development of anticancer drugs (2).
Small molecular HDAC inhibitors (HDACis) are a promising new class of anticancer drugs (9). The USA Food and Drug Administration (FDA) has approved three HDACis: Vorinostat and romidepsin, for the treatment of cutaneous T cell lymphoma, and belinostat, for the treatment of relapsed or refractory peripheral T cell lymphoma (10–12). Over 20 chemically distinct HDACis are currently in clinical trials for the treatment of various types of hematological malignancy and solid tumor (13).
Chidamide, a new HDACi, was approved for the treatment of recurrent or refractory peripheral T cell lymphoma in December 2014 by the Chinese FDA (14). Chidamide selectively inhibits HDAC1, 2, 3 and 10, the HDAC isotypes documented to be associated with malignant phenotypes (15). Chidamide has been applied in clinical trials for various types of hematological malignancy and solid tumor (14,16). Several in vitro studies reported that chidamide alone induced apoptosis, and a combination of chidamide with other chemotherapeutic drugs enhanced cell apoptosis in cancer cells (17,18). In addition, chidamide was demonstrated to induce cell apoptosis, cell cycle arrest and cell growth inhibition (17–19).
Acquired resistance to anticancer agents is common in cancer therapy. Previous studies have revealed that the acquired resistance to the HDACi vorinostat is associated with a lack of G2 checkpoint activation and a lack of HDAC6 expression, with an increased level of HDAC1, 2 and 4 expression (20,21). Another HDACi, romidepsin, may cause the reversible induction of multidrug resistance protein expression in tumor cells, leading to transient resistance (22,23). Resistance following chronic treatment with the HDACi valproic acid is associated with elevated Akt activation in renal cell carcinoma in vivo (24).
In the present study, an acquired chidamide-resistant A549-CHI-R cell line was established, with the aim of characterizing in detail the mechanism of chidamide resistance. In addition, the possible cross-resistance to other chemotherapeutic drugs was investigated.
Materials and methods
Chemicals and reagents
Chidamide was supplied by Shenzhen ChipScreen Biosciences, Ltd., (Shenzhen, China), and was dissolved in dimethyl sulfoxide (DMSO). Cisplatin (CDDP) was obtained from Qilu Pharmaceutical Co. Ltd., (Jinan, China). Vinorelbine (VNR) and gemcitabine (GEM) were purchased from Jiangsu Hansoh Pharmaceutical Co. Ltd., (Jiangsu, China). Paclitaxel (TAX) was obtained from Bristol-Myers Squibb (New York, NY, USA). 5-fluorouracil (5-FU) was obtained from Tianjin Jinyao Amino Acid Co. Ltd., (Tianjin, China). Cycloheximide (CHX) was obtained from Beyotime Institute of Biotechnology (Jiangsu, China). MTT was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). RPMI-1640 medium was purchased from Beijing Xigong Biotechnology Co. Ltd., (Being, China). Fetal bovine serum was obtained from Shanghai Ex Cell Biology Inc., (Shanghai, China).
Cell culture and establishment of chidamide-resistant cell lines
The human non-small cell lung cancer (NSCLC) A549 cell line was purchased from the Cell Bank of the Cancer Institute, Chinese Academy of Medical Science (Beijing, China). Cells were cultured in RPMI-1640 medium containing 10% (v/v) fetal bovine serum at 37°C in a humidified atmosphere with 5% (v/v) CO2. A549 cells were exposed to gradually increasing chidamide concentrations of 4, 8, 16, 32 and 64 µM for ~6 months, and a chidamide-resistant lung cancer cell line was established, designated A549-CHI-R.
Growth inhibition
Cell viability was evaluated using an MTT assay. Growing cells (5×103 cells/well) were seeded on 96-well plates with 100 µl medium. To assess cell viability, 100 µl medium containing serial dilutions (Table I) of chidamide, 5-FU, cisplatin, GEM, VNR or TAX was added, and the cultures were incubated at 37°C. At 72 h, the medium was discarded, 20 µl saline containing 100 µg MTT was added to each well and the cells were incubated at 37°C for 4 h. The supernatant was removed and 150 µl DMSO was added to each well. The absorbance was measured at 570 nm using a plate reader.
Table I.
Drug dilutions in growth inhibition assay.
| Items | Chidamid (µM) | 5-FU (µM) | CDDP (µM) | GEM (µM) | VNR (nM) | TAX (nM) |
|---|---|---|---|---|---|---|
| A549 | 0/2.5/5/10/20 | 0/2.5/5/7.5/10 | 0/2.5/5/10/20 | 0/0.01/0.1/0.5/1 | 0/1/10/100/500 | 0/0.01/0.1/1/5 |
| A549-CHI-R | 0/25/50/75/100 | 0/2.5/5/7.5/10 | 0/2.5/5/10/20 | 0/0.01/0.1/1/10 | 0/1/10/100/1000 | 0/1/10/100/500 |
5-FU, 5-fluorouracil; CDDP, cis-platinum; GEM, gemcitabine; VNR, vinorelbine; TAX, paclitaxel; A549-CHI-R, chidamide-resistant A549 cells.
The surviving cell fraction was calculated using the following formula: [(Mean absorbance of test cells-mean absorbance of background)/(mean absorbance of control cells-mean absorbance of background)] ×100%. The IC50 was determined by plotting the logarithm of the drug concentration vs. the percentage of surviving cells. Each assay was performed in quadruplicate at least three times, and the mean and standard deviation were calculated. Percentage inhibition values of compounds were calculated by comparison with DMSO-treated control wells.
Clone formation assay
A total of 800 cells were plated on 6-well plates. At 24 h, 5 µM chidamide was added, and the cells were allowed to proliferate. The cell culture medium was replaced when necessary. At ~10 days, when the differences in the growth of colonies had appeared, the cells were washed with saline, fixed with 100% methanol at room temperature for approximately 5 min and dyed with 0.005% crystal violet (Sigma-Aldrich; Merck KGaA), a chromatin-binding stain, at room temperature for approximately 20 min. The colony formation rates were calculated using the following formula: Number of clones formed/number of seeding cells ×100%.
HDAC1 gene transfection and knockout
Prior to transfection, 2×106 cells were seeded into 6-well plates. When cells reached ~70% confluence, they were transiently transfected with a 2 µg human HDAC1 plasmid (Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA, USA) or crisper/cas9 HDAC1-knockout plasmid (Viewsolid Biotech Co. Ltd, Beijing, China). Lipofectamine® 3000 transfection reagent (Thermo Fisher Scientific Inc.) was used for cell transfection, according to the manufacturer's protocol. At 48 h post-transfection, total protein or nuclear protein was extracted as described in the subsequent sections, or the cells were seeded at 5,000 per well in 96-well tissue culture plates, and several concentrations (0 and 5 µM for A549 or 0 and 50 µM for A549-CHI-R) of chidamide were added to assess cell viability.
Western blot analysis
The cells were plated on 6-well plates, allowed to attach for 24 h and then treated with 5 µM chidamide for 72 h, or 10 µg/ml CHX for 0, 4, 8, 12 or 24 h. The cells were washed with saline and lysed with radioimmunoprecipitation assay lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin and 1X protease inhibitor cocktail). A total of 50 µg protein samples (the protein was quantified using the BSA method) were separated on 10% SDS-PAGE, and were transferred electrophoretically to a nitrocellulose membrane (GE Healthcare, Chicago, IL, USA). Blocking was performed using skimmed milk at room temperature for 2 h. Membranes were then incubated with the following primary antibodies: β-actin (cat. no. A5441; Sigma-Aldrich; Merck KGaA; 1:5,000), HDAC1, 2 and 3 (HDAC antibody sampler kit, cat. no. 9928; dilutions 1:1,000, 1:1,000 and 1:500, respectively; Cell Signaling Technology, Inc., Danvers, MA, USA) and HDAC10 (1:500; cat. no. BS1161; Bioworld Technology Inc., St. Louis Park, MN, USA) at 4°C overnight. Following incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (OriGene Technologies Inc., Rockville, MD, USA), including Anti-Mouse IgG/HRP (cat. no. TA130004; 1:3,000) or Anti-Rabbit IgG/HRP (cat. no. TA140003; 1:3,000) at room temperature for 2 h, the membranes were developed using a luminol chemiluminescence detection kit (Santa Cruz Biotechnology Inc., Dallas, TX, USA) according to the manufacturer's protocol.
HDAC activity assay
HDAC activity was measured with the fluorometric HDAC Activity Assay kit (cat. no., ab156064; Abcam, Cambridge, UK) according to the manufacturer's protocol. Briefly, HDAC assay buffer containing a substrate peptide was incubated with HeLa nuclear extract (supplied with the kit) as a positive control, or A549 or A549-CHI-R nuclear extract, in a microtiter plate. Trichostatin A (supplied with the kit) was then added to the inhibitor control assay wells. At 20 min, 20 µl developing solution was added to each well for a further 20 min. Finally, 5 µl stop buffer was added to every well and incubated for 30 min at room temperature. At the end of the treatment, plates were detected using fluorescence filters (excitation, 355 nm; emission, 480 nm).
Cell cycle arrest
Cells were then plated onto 6-well plates, allowed to attach for 24 h and then treated with 1 nM TAX or 20 nM VNR. At 72 h, the cells were harvested and washed with saline then centrifuged at 335 × g for 5 min at 4°C. The pellets were re-suspended in 300 µl of saline and were fixed by adding 700 µl of cold absolute ethanol and incubating at −20°C overnight. The next day, the cells were centrifuged at 335 × g for 5 min at 4°C and the supernatant was removed. The cells were washed with cold saline twice, stained with propidium iodide on ice for 20 min and analyzed with a flow cytometer (BD LSR II system; BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Assays were performed in triplicate, and results are presented as the mean ± standard deviation. Statistical difference was assessed using Student's t-test (GraphPad_Prism V5.0; GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Establishment of a chidamide-resistant non-small cell lung cancer cell line
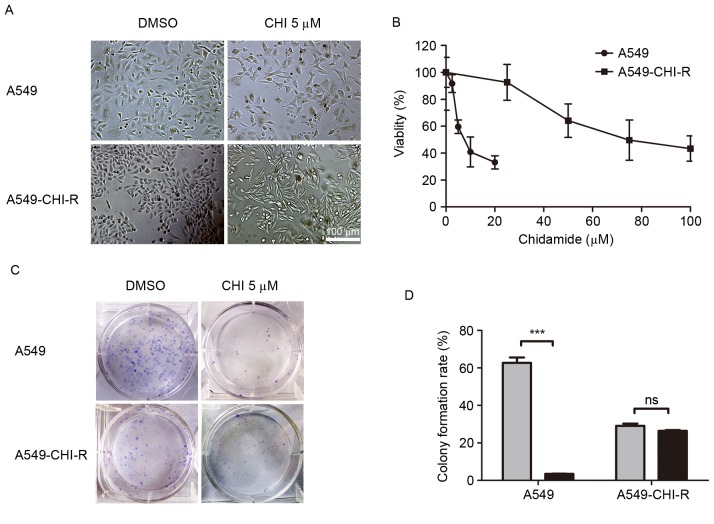
To investigate the acquired resistance to chidamide in cancer therapy, a chidamide-resistant lung cancer cell line, A549-CHI-R, was established (Fig. 1). When treated with 5 µM chidamide, the morphology of A549-CHI-R cells became elongated and thin (Fig. 1A). The IC50 values of chidamide for A549-CHI-R and parental A549 cells were 78.34 and 9.07, respectively. A549-CHI-R was ~9-fold more resistant to chidamide compared with the parental A549 cells (Fig. 1B). Following 10 days of chidamide treatment, parental A549 cells exhibited markedly decreased colony formation rates (P<0.001) compared with untreated controls, whereas colony formation inhibition was not observed in the A549-CHI-R cell line (Fig. 1C). The colony formation rates for A549/CHI 5 µM, A549/DMSO, A549-CHI-R/CHI 5 µM and A549-CHI-R/DMSO cells were 3.5, 56.9, 26.5 and 26.9%, respectively (Fig. 1D).
Figure 1.
Establishment of a chidamide-resistant cell line. A549 cells were continuously exposed to gradually increasing chidamide concentrations of 4, 8, 16, 32 and 64 µM for ~6 months, and a chidamide-resistant lung cancer cell line (A549-CHI-R) was established. (A) Representative bright field images of parental A549 and A549-CHI-R cells. Cells were treated with or without 5 µM chidamide for 40 h. (B) Cell survival curves of A549 and A549-CHI-R cells. Cells were treated with a range of concentrations of chidamide for 72 h. (C) Colony formation of parental A549 and A549-CHI-R cells. Parental A549 cells exhibited decreased colony formation following 5 µM chidamide treatment for 10 days compared with A549-CHI-R cells. (D) Quantified colony formation assay for parental A549 and A549-CHI-R cells. ***P<0.001 between control and chidamide-treated cells. A549-CHI-R, chidamide-resistant A549 cells; DMSO, dimethyl sulfoxide; CHI, chidamide; ns, no significance.
HDAC1 protein degradation is inhibited in chidamide-resistant lung cancer cells
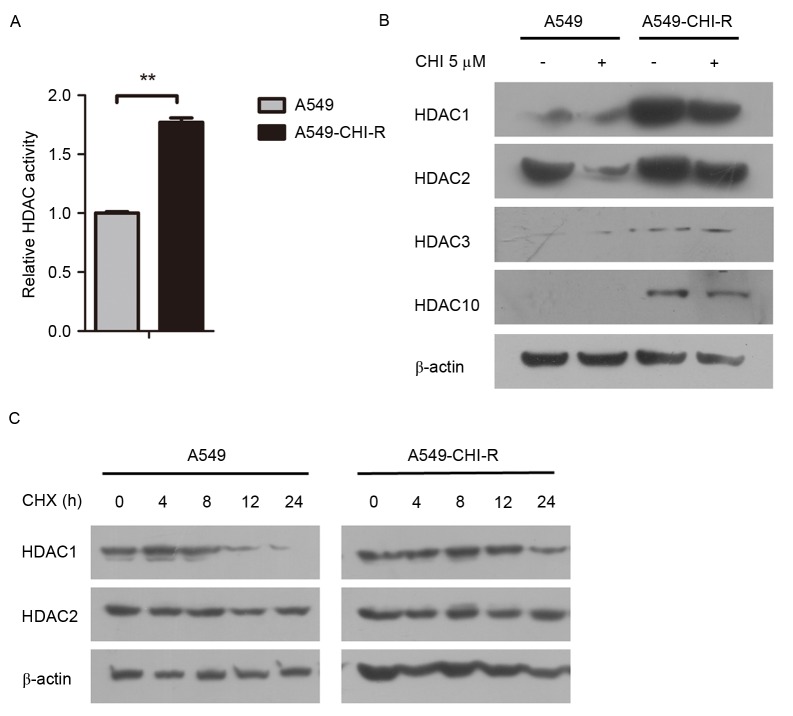
Intracellular HDAC activity was examined in the parental and resistant cell lines. The HDAC activity in the A549-CHI-R cells was 1.77-fold higher than in the parental A549 cells (Fig. 2A). Western blot analysis revealed that HDAC protein expression was markedly increased in the A549-CHI-R cells compared with the parental A549 cells (Fig. 2B). To analyze the mechanisms of HDAC activity increase in A549-CHI-R cells, protein synthesis was first inhibited by CHX. HDAC1 protein expression became negligible after 24 h in parental A549 cells (Fig. 2C, left panel), but slightly decreased after 24 h in A549-CHI-R cells (Fig. 2C, right panel). However, HDAC2 protein was slightly increased in parental A549 and A549-CHI-R cells after 24 h. These results indicated that the increased HDAC1 activity in A549-CHI-R cells was not induced by protein synthesis, but by the inhibition of protein degradation.
Figure 2.
HDAC protein accumulation in CHI-resistant lung cancer cells. (A) HDAC activity of A549 and A549-CHI-R cells. (B) Western blot analysis of HDACs with or without 5 µM CHI treatment for 72 h. (C) Protein level of HDAC1 and 2 in A549 and A549-CHI-R cells incubated with 10 µg/ml CHX for the indicated times. **P<0.005 vs. A549 cells. HDAC, histone deacetylase; CHI, chidamide; A549-CHI-R, chidamide-resistant A549 cells; CHX, cycloheximide.
HDAC1 contributes to chidamide resistance in lung cancer cells
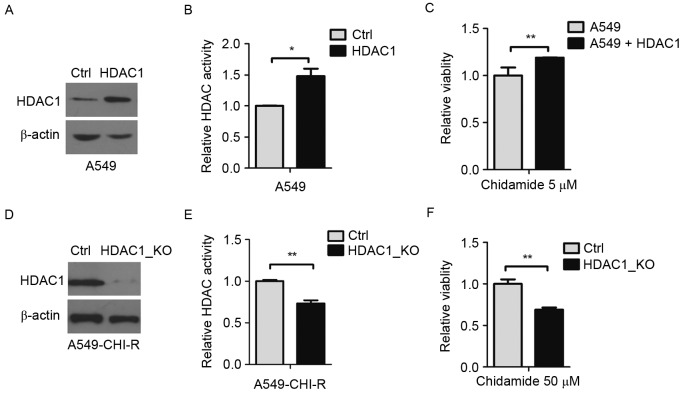
To investigate the pivotal role of HDAC1 in chidamide resistance, HDAC1 was overexpressed in A549 cells. The protein overexpression was confirmed by western blot analysis (Fig. 3A). Compared with parental cells, HDAC1-overexpressing cells exhibited ~1.5-fold increased intracellular HDAC activity (P<0.05; Fig. 3B). Following 5 µM of chidamide treatment, the survival rate of HDAC1-overexpressing A549 cells was 1.2-fold higher than the parental A549 cells (P<0.01; Fig. 3C), indicating that the overexpression of HDAC1 contributed to chidamide resistance.
Figure 3.
HDAC1 contributed to chidamide resistance in the A549 lung cancer cell line. (A) HDAC1 expression in A549 cells transfected with empty vector controls or a HDAC1 plasmid. (B) HDAC activity in A549 cells with or without HDAC1 overexpression. (C) Relative viability of HDAC1-overexpressing and parental A549 cells following treatment with 5 µM chidamide for 72 h. The (D) protein level of HDAC1, (E) relative HDAC activity and (F) relative viability following treatment with 50 µM chidamide for 72 h in A549-CHI-R and HDAC1-KO A549-CHI-R cells. *P<0.01 vs. control; **P<0.005 vs. control or A549 cells. All experiments were performed three times. HDAC, histone deacetylase; KO, knockout; Ctrl, control; A549-CHI-R, chidamide-resistant A549 cells.
HDAC1 was then knocked out in the chidamide-resistant A549-CHI-R cell line. HDAC1 protein decreased in HDAC1-knockout A549-CHI-R cells (Fig. 3D). Compared with A549-CHI-R cells, intracellular HDAC activity rate was decreased to 0.27-fold following HDAC1-knockout (Fig. 3E). When treated with 50 µM chidamide, the survival rate of HDAC1-knockout A549-CHI-R cells was 0.32-fold lower than the A549-CHI-R cells (P<0.01; Fig. 3F).
Cross-resistance of the chidamide-resistant lung cancer cell line
It was investigated whether the chidamide-resistant A549-CHI-R cell line was cross-resistant to other chemotherapeutic drugs. IC50 values revealed that A549-CHI-R cells remained sensitive to CDDP and 5-FU. However, compared with the parental A549 cell line, the A549-CHI-R cells were 10.55-, 13.23- and >100-fold (P<0.01) more resistant to GEM, VNR and TAX, respectively (Table II).
Table II.
Drug sensitivity in parental A549 and A549-CHI-R cells.
| A549 | A549-CHI-R | ||||
|---|---|---|---|---|---|
| Drug | IC50 | 95% CI | IC50 | 95% CI | Resistance index |
| Chidamide | 9.07 | 6.00–11.32 | 78.34 | 51.30–111.87 | 8.64 |
| 5-fluorouracil | 4.03 | 2.09–7.75 | 5.17 | 2.77–7.58 | 1.28 |
| Cisplatin | 9.13 | 8.90–9.37 | 7.87 | 5.75–10.76 | 0.86 |
| Gemcitabine | 0.21 | 0.01–3.65 | 2.25 | 1.67–3.03 | 10.55 |
| Vinorelbine | 31.06 | 11.32–85.17 | 411.00 | 282.10–598.80 | 13.23 |
| Paclitaxel | 0.89 | 0.11–1.98 | 123.40 | 6.00–260.00 | 123.40 |
Sensitivity to the drugs was determined by MTT assay after 72 h. Resistance index is a comparison between the IC50 for the drug in A549-CHI-R cells and the IC50 for the parental A549 cells. A549-CHI-R, chidamide-resistant A549 cells; CI, confidence interval.
The cell cycle of A549-CHI-R and A549 cells was analyzed by flow cytometry. Cells were incubated with 1 nM TAX or 20 nM VNR for 72 h. Compared with untreated cells, the percentage of A549 cells in the G2/M phase markedly increased, from 28.2% to 77.7 (TAX) and 79.4% (VNR). However, following the same treatment, compared with control cells, the percentage of A549-CHI-R cells in the G2/M phase increased from 30.5 to 52.1 and 42.3%. Therefore, A549-CHI-R cells were more resistant to G2/M arrest caused by TAX and VNR (Table III).
Table III.
Effects of TAX or VNR on the cell cycle distribution in parental A549 and A549-CHI-R cells.
| A549 cells, % | A549-CHI-R cells, % | |||||
|---|---|---|---|---|---|---|
| Cell cycle phase | Ctrl | TAX | VNR | Ctrl | TAX | VNR |
| Sub-G1 | 1.7 | 0.3 | 0.5 | 0.2 | 0.1 | 0.1 |
| G1 | 60.2 | 15.1 | 10.8 | 53.4 | 34.8 | 41.7 |
| S | 9.9 | 6.9 | 9.3 | 15.9 | 13.0 | 15.9 |
| G2/M | 28.2 | 77.7 | 79.4 | 30.5 | 52.1 | 42.3 |
Cell cycle distribution was examined by propidium iodide staining and analyzed with fluorescence-activated cell sorting. Data represent the results of three independent experiments. TAX, paclitaxel; VNR, vinorelbine; Ctrl, control; A549-CHI-R, chidamide-resistant A549 cells.
Discussion
In the present study, a chidamide-resistant NSCLC cell line, A549-CHI-R, was established. With growth inhibition and colony formation assays, it was revealed that A549 and A549-CHI-R cell lines exhibited a ~9-fold difference in sensitivity to chidamide (IC50 of A549 cell line, 9.07 µM; IC50 of the A549-CHI-R cell line, 78.34 µM). Compared with parental A549 cells, A549-CHI-R cells exhibited a slower growth rate and a reduced colony formation rate. A previous study demonstrated that HL-60/LR cells, human acute myeloid leukemia cells resistant to LAQ824 (a hydroxamic acid analog pan-HDACi), exhibited a markedly higher growth compared with parental HL-60 cells (21). A vorinostat-induced subline, HCT116/vorinostat, exhibited a slightly slower growth rate compared with the parental HCT116 cell line (20). The different results indicate that the growth rate of HDACi-resistant cell lines may be drug-specific, cell type-specific or even case-specific.
A number of HDACi-resistant cancer cell lines have already been reported (20,21,25). The mechanisms of HDACi resistance include the upregulation of P-glycoprotein, other ATP-binding cassette transporters, cell cycle proteins and signaling proteins, alterations to HDAC protein level, increases in thioredoxin level, nuclear factor-κB activation and anti-apoptotic/prosurvival mechanisms (26). The molecular mechanism for acquired resistance varies in different HDACi-resistant cells. The acquired resistance of HCT116/VOR cells was associated with a reduction in histone acetylation, G2/M checkpoint activation and apoptosis susceptibility (20). Pan-HDACi-resistant HL-60/LR cells expressed higher levels of HDAC1, 2 and 4, but lacked expression of HDAC6, with concomitant hyper-acetylation of heat shock protein 90 (21).
Chidamide was revealed to be a low nanomolar inhibitor of HDAC1, 2, 3 and 10 (15). HDAC1 belongs to class I HDACs, which are the most closely associated with malignant phenotypes (27). HDAC1 overexpression has been reported to be positively associated with cell division, differentiation and tumorigenesis (28–30). Loss of HDAC1 resulted in a 60% reduction in total HDAC activity and a loss of stem cell viability (28). Consistently, total HDAC activity was also elevated in A549-CHI-R and A549 HDAC1-overexpressed cells. Similarly, HDAC1 was also accumulated in chidamide-resistant A549-CHI-R cells, consistent with other HDACi-resistant cells (23).
Post-translational modifications of HDAC have been demonstrated to perform pivotal roles in the regulation of gene expression. HDACs modified by ubiquitination are targeted for degradation (31). Certain chemicals can target HDAC1 to induce proteasome-mediated degradation (32). Specifically, treatment with diesel exhaust particulate induced degradation of HDAC1 in a human bronchial epithelial cell line, BEAS-2B (33). The present study reported a decreased degradation of HDAC1 in A549-CHI-R cells. Overexpression of HDAC1 in cervical cancer cells restrained cell proliferation and induced premature senescence (34). Taken together, HDAC1 accumulation may be a predictive marker for the resistance to chidamide.
Cross-resistance is a common response to chemotherapy. HDACi-resistant cell lines, HL-60/LR and HCT116/vorinostat, exhibited cross-resistance to other HDACis (20,21). Pemetrexed-resistant NSCLC cell lines exhibited cross-resistance to CDDP (35). A549-CHI-R cells, which exhibit enhanced HDAC activity, demonstrated cross-resistance to GEM, TAX and VNR in the present study. Consistent with this, paclitaxel-resistant NSCLC cells exhibited enhanced HDAC activity and tumorigenicity (36). Increased HDAC1 expression in NSCLC tissue predicted a poor prognosis for patients treated with paclitaxel (36). TAX and VNR are microtubule-targeting drugs; their most potent cytotoxic action is the suppression of microtubule dynamics, leading to mitotic arrest and subsequent cell death (37). Gong et al (38) demonstrated that chidamide inhibits cell proliferation by inducing cell cycle arrest. The present study revealed that G2/M arrest decreased in A549-CHI-R cells compared with parental A549 cells following treatment with TAX or VNR. However, the chidamide-resistant cells retained sensitivity and susceptibility to the drugs CDDP and 5-FU. These drugs induce cell death by inhibiting DNA synthesis (39,40). A combination of CDDP or 5-FU with chidamide may synergistically induce apoptosis (17,18). The results of the present study are consistent with these previous studies.
In conclusion, a chidamide-resistant cell line was established, and it was proposed that HDAC1 accumulation may contribute to chidamide resistance. In addition, the chidamide-resistant cell line remained sensitive to 5-FU and CDDP, but cross-resistant to TAX and VNR, indicating a potential strategy for cancer therapy.
Acknowledgements
The present study was supported by the National Natural Science Foundation (grant nos. 81321091, 81452761 and 31171322) and the National Basic Research Program of China (grant no. 2011CB910700704). The authors thank Shenzhen ChipScreen Biosciences Ltd., (Shenzhen, China) for providing chidamide used in the present study.
Glossary
Abbreviations
- HDAC
histone deacetylase
- A549-CHI-R
chidamide-resistant A549 cells
- CDDP
cisplatin
- GEM
gemcitabine
- 5-FU
5-fluorouracil
- TAX
paclitaxel
- VNR
vinorelbine
- NSCLC
non-small cell lung cancer
References
- 1.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 2.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 3.Song J, Noh JH, Lee JH, Eun JW, Ahn YM, Kim SY, Lee SH, Park WS, Yoo NJ, Lee JY, Nam SW. Increased expression of histone deacetylase 2 is found in human gastric cancer. APMIS. 2005;113:264–268. doi: 10.1111/j.1600-0463.2005.apm_04.x. [DOI] [PubMed] [Google Scholar]
- 4.Toh Y, Yamamoto M, Endo K, Ikeda Y, Baba H, Kohnoe S, Yonemasu H, Hachitanda Y, Okamura T, Sugimachi K. Histone H4 acetylation and histone deacetylase 1 expression in esophageal squamous cell carcinoma. Oncol Rep. 2003;10:333–338. [PubMed] [Google Scholar]
- 5.Giannini R, Cavallini A. Expression analysis of a subset of coregulators and three nuclear receptors in human colorectal carcinoma. Anticancer Res. 2005;25:4287–4292. [PubMed] [Google Scholar]
- 6.Waltregny D, North B, Van Mellaert F, de Leval J, Verdin E, Castronovo V. Screening of histone deacetylases (HDAC) expression in human prostate cancer reveals distinct class I HDAC profiles between epithelial and stromal cells. Eur J Histochem. 2004;48:273–290. [PubMed] [Google Scholar]
- 7.Bartling B, Hofmann HS, Boettger T, Hansen G, Burdach S, Silber RE, Simm A. Comparative application of antibody and gene array for expression profiling in human squamous cell lung carcinoma. Lung Cancer. 2005;49:145–154. doi: 10.1016/j.lungcan.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber K. Purchase of Aton spotlights HDAC inhibitors. Nat Biotechnol. 2004;22:364–365. doi: 10.1038/nbt0404-364. [DOI] [PubMed] [Google Scholar]
- 10.Duvic M, Vu J. Vorinostat: A new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin Investig Drugs. 2007;16:1111–1120. doi: 10.1517/13543784.16.7.1111. [DOI] [PubMed] [Google Scholar]
- 11.Grant C, Rahman F, Piekarz R, Peer C, Frye R, Robey RW, Gardner ER, Figg WD, Bates SE. Romidepsin: A new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther. 2010;10:997–1008. doi: 10.1586/era.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poole RM. Belinostat: First global approval. Drugs. 2014;74:1543–1554. doi: 10.1007/s40265-014-0275-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Choy ML, Marks PA. Mechanisms of resistance to histone deacetylase inhibitors. Adv Cancer Res. 2012;116:39–86. doi: 10.1016/B978-0-12-394387-3.00002-1. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766–1771. doi: 10.1093/annonc/mdv237. [DOI] [PubMed] [Google Scholar]
- 15.Ning ZQ, Li ZB, Newman MJ, Shan S, Wang XH, Pan DS, Zhang J, Dong M, Du X, Lu XP. Chidamide (CS055/HBI-8000): A new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol. 2012;69:901–909. doi: 10.1007/s00280-011-1766-x. [DOI] [PubMed] [Google Scholar]
- 16.Dong M, Ning ZQ, Xing PY, Xu JL, Cao HX, Dou GF, Meng ZY, Shi YK, Lu XP, Feng FY. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother Pharmacol. 2012;69:1413–1422. doi: 10.1007/s00280-012-1847-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Pan DS, Shan S, Zhu JZ, Zhang K, Yue XP, Nie LP, Wan J, Lu XP, Zhang W, Ning ZQ. Non-toxic dose chidamide synergistically enhances platinum-induced DNA damage responses and apoptosis in non-small-cell lung cancer cells. Biomed Pharmacother. 2014;68:483–491. doi: 10.1016/j.biopha.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Qiu S, Liu Y, Liu Z, Zheng Y, Su X, Chen B, Chen H. Chidamide and 5-flurouracil show a synergistic antitumor effect on human colon cancer xenografts in nude mice. Neoplasma. 2016;63:193–200. doi: 10.4149/203_150422N214. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Guo Y, Fu M, Liang X, Zhang X, Wang R, Lin C, Qian H. Antitumor activity of Chidamide in hepatocellular carcinoma cell lines. Mol Med Rep. 2012;5:1503–1508. doi: 10.3892/mmr.2012.858. [DOI] [PubMed] [Google Scholar]
- 20.Dedes KJ, Dedes I, Imesch P, von Bueren AO, Fink D, Fedier A. Acquired vorinostat resistance shows partial cross-resistance to ‘second-generation’ HDAC inhibitors and correlates with loss of histone acetylation and apoptosis but not with altered HDAC and HAT activities. Anticancer Drugs. 2009;20:321–333. doi: 10.1097/CAD.0b013e3283262a32. [DOI] [PubMed] [Google Scholar]
- 21.Fiskus W, Rao R, Fernandez P, Herger B, Yang Y, Chen J, Kolhe R, Mandawat A, Wang Y, Joshi R, et al. Molecular and biologic characterization and drug sensitivity of pan-histone deacetylase inhibitor-resistant acute myeloid leukemia cells. Blood. 2008;112:2896–2905. doi: 10.1182/blood-2007-10-116319. [DOI] [PubMed] [Google Scholar]
- 22.Xiao JJ, Huang Y, Dai Z, Sadée W, Chen J, Liu S, Marcucci G, Byrd J, Covey JM, Wright J, et al. Chemoresistance to depsipeptide FK228 [(E)-(1S,4S,10S,21R)-7-[(Z)-ethylidene]-4,21-diisopropyl-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8,7,6]-tricos-16-ene-3,6,9,22-pentanone] is mediated by reversible MDR1 induction in human cancer cell lines. J Pharmacol Exp Ther. 2005;314:467–475. doi: 10.1124/jpet.105.083956. [DOI] [PubMed] [Google Scholar]
- 23.Yamada H, Arakawa Y, Saito S, Agawa M, Kano Y, Horiguchi-Yamada J. Depsipeptide-resistant KU812 cells show reversible P-glycoprotein expression, hyper-acetylated histones, and modulated gene expression profile. Leukemia Res. 2006;30:723–734. doi: 10.1016/j.leukres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Juengel E, Makarević J, Tsaur I, Bartsch G, Nelson K, Haferkamp A, Blaheta RA. Resistance after chronic application of the HDAC-inhibitor valproic acid is associated with elevated Akt activation in renal cell carcinoma in vivo. PLoS One. 2013;8:e53100. doi: 10.1371/journal.pone.0053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Das K, Wu J, Lee MH, Tan P. RNH1 regulation of reactive oxygen species contributes to histone deacetylase inhibitor resistance in gastric cancer cells. Oncogene. 2014;33:1527–1537. doi: 10.1038/onc.2013.104. [DOI] [PubMed] [Google Scholar]
- 26.Robey RW, Chakraborty AR, Basseville A, Luchenko V, Bahr J, Zhan Z, Bates SE. Histone deacetylase inhibitors: Emerging mechanisms of resistance. Mol Pharm. 2011;8:2021–2031. doi: 10.1021/mp200329f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa M, Oda Y, Eguchi T, Aishima S, Yao T, Hosoi F, Basaki Y, Ono M, Kuwano M, Tanaka M, Tsuneyoshi M. Expression profile of class I histone deacetylases in human cancer tissues. Oncol Rep. 2007;18:769–774. [PubMed] [Google Scholar]
- 28.Jamaladdin S, Kelly RD, O'Regan L, Dovey OM, Hodson GE, Millard CJ, Portolano N, Fry AM, Schwabe JW, Cowley SM. Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc Natl Acad Sci USA. 2014;111:9840–9845. doi: 10.1073/pnas.1321330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JH, Kwon HJ, Yoon BI, Kim JH, Han SU, Joo HJ, Kim DY. Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res. 2001;92:1300–1304. doi: 10.1111/j.1349-7006.2001.tb02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korfei M, Skwarna S, Henneke I, MacKenzie B, Klymenko O, Saito S, Ruppert C, von der Beck D, Mahavadi P, Klepetko W, et al. Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax. 2015;70:1022–1032. doi: 10.1136/thoraxjnl-2014-206411. [DOI] [PubMed] [Google Scholar]
- 31.Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, Khochbin S. Identification of components of the murine histone deacetylase 6 complex: Link between acetylation and ubiquitination signaling pathways. Mol Cell Biol. 2001;21:8035–8044. doi: 10.1128/MCB.21.23.8035-8044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D, Zhou P, Zhang L, Zheng Y, He F. HPV16 activates the promoter of Oct4 gene by sequestering HDAC1 from repressor complex to target it to proteasomal degradation. Med Hypotheses. 2012;79:531–534. doi: 10.1016/j.mehy.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Cao D, Bromberg PA, Samet JM. COX-2 expression induced by diesel particles involves chromatin modification and degradation of HDAC1. Am J Respir Cell Mol Biol. 2007;37:232–239. doi: 10.1165/rcmb.2006-0449OC. [DOI] [PubMed] [Google Scholar]
- 34.Chuang JY, Hung JJ. Overexpression of HDAC1 induces cellular senescence by Sp1/PP2A/pRb pathway. Biochem Biophys Res Commun. 2011;407:587–592. doi: 10.1016/j.bbrc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Ochi N, Takigawa N, Tanimoto Y, Chen Y, Ichihara E, Hotta K, Tabata M, Tanimoto M, Kiura K. Establishment of pemetrexed-resistant non-small cell lung cancer cell lines. Cancer Lett. 2011;309:228–235. doi: 10.1016/j.canlet.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Li H, Ren Y, Zou S, Fang W, Jiang X, Jia L, Li M, Liu X, Yuan X, et al. Targeting HDAC with a novel inhibitor effectively reverses paclitaxel resistance in non-small cell lung cancer via multiple mechanisms. Cell Death Dis. 2016;7:e2063. doi: 10.1038/cddis.2015.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 38.Gong K, Xie J, Yi H, Li W. CS055 (Chidamide/HBI-8000), a novel histone deacetylase inhibitor, induces G1 arrest, ROS-dependent apoptosis and differentiation in human leukaemia cells. Biochem J. 2012;443:735–746. doi: 10.1042/BJ20111685. [DOI] [PubMed] [Google Scholar]
- 39.Sorenson CM, Eastman A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: Role of G2 arrest and DNA double-strand breaks. Cancer Res. 1988;48:4484–4488. [PubMed] [Google Scholar]
- 40.Santi DV, McHenry CS, Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974;13:471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]