Abstract
Background
Infants aged <1 year are at highest risk for pertussis-related morbidity and mortality. In 2012, Tdap (tetanus toxoid, reduced diphtheria toxoid and acellular pertussis) vaccine was recommended for women during each pregnancy to protect infants in the first months of life; data on effectiveness of this strategy are currently limited.
Methods
We conducted a case-control evaluation among pertussis cases <2 months old with cough onset between January 1, 2011 and December 31, 2014 from six U.S. Emerging Infection Program Network states. Controls were hospital-matched and selected by birth certificate. Mothers were interviewed to collect information on demographics, household characteristics, and healthcare providers. Provider-verified immunization history was obtained on mothers and infants. Mothers were considered vaccinated during pregnancy if Tdap was received ≥14 days before delivery; trimester was calculated using Tdap date, infant’s date of birth, and gestational age. Odds ratios were calculated using multivariable conditional logistic regression; vaccine effectiveness (VE) was estimated as (1 – OR) × 100%.
Results
A total of 240 cases and 535 controls were included; 17 (7.1%) case-mothers and 90 (16.8%) control-mothers received Tdap during the 3rd trimester of pregnancy. The multivariable VE estimate for Tdap administered during the third trimester of pregnancy was 77.7% (48.3% – 90.4%); VE increased to 90.5% (65.2 – 97.4%) against hospitalized cases.
Conclusions
Vaccination during pregnancy is an effective way to protect infants during the early months of life. With a continuing resurgence in pertussis, efforts should focus on maximizing Tdap uptake among pregnant women.
Keywords: Infant, pertussis, Tdap, maternal immunization
BACKGROUND
Despite the dramatic impact of vaccines on the burden of B. pertussis in the United States, pertussis remains endemic, and reported cases have increased steadily since the late 1980s. In 2012, over 46,000 pertussis cases were reported in the U.S., the largest number since the mid-1950s. While causes for the increase are likely multifactorial, waning immunity from acellular pertussis vaccination has been documented to be a strong contributor [1].
Infants are at greatest risk for pertussis-related complications and mortality, especially during the first months of life [1]. Immunization against pertussis with the 5-dose childhood DTaP (diphtheria and tetanus toxoids, and acellular pertussis vaccine) series begins at 2 months of age, leaving young infants highly susceptible to pertussis. Among case-patients <2 months old, approximately 75% are hospitalized and 1 in 100 die [2]. Studies suggest that parents and siblings play an important role in transmitting pertussis to vulnerable infants [3, 4].
In 2005, two tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccines were licensed for use as a single booster dose among U.S. adolescents and adults [5, 6]. Although the primary goal of Tdap immunization was to provide direct protection to the vaccine recipient, recommendations also focused on indirectly protecting infants through vaccination of close contacts, a strategy known as “cocooning” [5, 6]. In 2011, the Advisory Committee on Immunization Practices (ACIP) recommended vaccinating women with Tdap during a single pregnancy as a strategy to protect young infants through the transplacental transfer of maternal antibodies [7]. Because maternal antibodies are short-lived and may not be sufficient to protect infants of subsequent pregnancies, the recommendation was expanded in 2012 to include a dose of Tdap during every pregnancy [8, 9]. Because the recommendation for vaccination during pregnancy was made with limited data, post-implementation evaluations are essential for monitoring effectiveness and longer term success of the strategy.
We conducted a multi-state, case-control evaluation to determine the effectiveness of maternal Tdap vaccination during pregnancy at preventing pertussis in U.S. infants <2 months old.
METHODS
A case of pertussis was defined as the onset of cough illness and at least one of the following: laboratory-confirmation (culture or PCR) of pertussis, epidemiological linkage to a laboratory-confirmed case, or clinically-compatible illness (cough ≥2 weeks with paroxysms, inspiratory whoop or post-tussive vomiting) in an infant <2 months old between January 1, 2011 and December 31, 2014. Cases were identified through surveillance in six Emerging Infection Program Network sites1[10]. Infants were eligible for enrollment if they were at least two days old and resided in the catchment area on their cough onset date, were born in a hospital in their state of residence, were ≥37 weeks gestational age at birth, were not adopted or in foster care, and did not live in a residential care facility. For each enrolled case-infant, we attempted to recruit three control-infants from birth certificates of infants born at the same hospital as the case-infant that were <2 months old on the case-infant’s cough onset date; once all potential controls meeting these criteria were exhausted, control enrollment ended for that case-infant. Eligibility criteria were the same for control-infants as for case-infants, and control-infants were additionally considered ineligible if they had a pertussis diagnosis prior to the cough onset date of the corresponding case-infant.
Mothers of case and control-infants were interviewed by telephone to collect information on demographics, mother and infant healthcare providers, and infant household contacts. The reference period for a case-infant and his or her matched controls was defined as the 30-day period prior to the case-infant cough onset date. Data used in the comparison of enrolled and unenrolled infants with pertussis were obtained from surveillance case report forms, maternal interviews, and birth certificate records; surveillance case report form data, which included hospitalization status, were collected via patient and physician interview.
Pertussis vaccination status, including brand, manufacturer and lot number, was collected through medical providers or state immunization registries for all enrolled case and control-infants and their mothers. When complete vaccine history was unavailable in registries, all medical providers identified during the interview were contacted. Additionally, birth hospitals were contacted to obtain case and control maternal Tdap histories. Tdap doses were considered valid if received at least two weeks before the case-infant’s cough onset date; for control-infants, the date of cough onset for the matched case-infant was used. Maternal vaccine history was considered complete when follow-up was exhausted with all providers; if there was incomplete follow-up with providers but at least one valid Tdap dose was identified, these individuals were included in the analysis. Mothers were considered unvaccinated if all medical providers were contacted and did not provide documentation of Tdap vaccination and no Tdap records were identified in the immunization registry. When more than one Tdap dose was verified, the most recent valid dose was included in the analysis. Mothers were classified as vaccinated before pregnancy if Tdap was received at any point prior to pregnancy with the case or control-infant, vaccinated during pregnancy if they received Tdap ≥14 days before delivery, and vaccinated post-partum if Tdap was received <2 months following the case or control-infant’s birth or in the 14 days before delivery; the trimester during which Tdap was administered was calculated from the vaccination date, infant’s date of birth, and infant’s gestational age at birth. When available, lot numbers were verified with vaccine manufacturers to confirm vaccine type and brand. An infant was considered enrolled when the maternal interview, and infant and maternal vaccine history were all completed.
Data were analyzed using SAS 9.3. Odds ratios were calculated using multivariable conditional logistic regression; vaccine effectiveness (VE) was estimated as (1 – OR) × 100%. Variables associated (p<0.05) with maternal Tdap vaccination in bivariate analyses were included in the multivariable models; those that retained significance in multivariable analysis after backwards elimination were included in the final model. Although not significant, we retained infant age (weeks) in the final models, because cases and controls were not matched on age. Case and control-mothers classified as unvaccinated were used as the reference group. Differences between proportions were tested using Pearson’s chi-squared test or Fisher’s exact test; differences in medians were assessed using the Wilcoxon Rank-Sum test.
RESULTS
A total of 788 infants <2 months old with pertussis were identified; of these, 29 (3.7%) were born prematurely, 6 (0.76%) were adopted or resided in foster care, 5 (0.63%) were born at a hospital outside their state of residence, and 3 (0.38%) were not born in a hospital. Of 745 infants eligible for enrollment, 251 (33.7%) were enrolled. The remaining 494 (66.3%) were not enrolled for the following reasons: 354 (71.7%) had mothers that could not be reached, 100 (20.2%) did not consent, 31 (6.3%) had incomplete maternal vaccination history follow-up, 7 (1.4%) did not speak English or Spanish, and 2 (0.4%) had another reason for non-enrollment. Compared with unenrolled infants with pertussis, mothers of enrolled infants were significantly more likely to have post-high school education; no significant differences were observed for sex, race, ethnicity, hospitalization, outcome, or insurance type (Table 1).
Table 1.
Characteristics of Enrolled and Non-enrolled Infants with Pertussis
| Enrolled (%) n=251 | Non-enrolled (%) n=537 | p-value | |
|---|---|---|---|
| Sexa, male | 123 (49.0) | 258 (49.3) | .99 |
| Raceb | .07 | ||
| White | 199 (80.9) | 356 (77.5) | |
| Black | 22 (8.9) | 30 (6.5) | |
| Other | 25 (10.1) | 73 (15.9) | |
| Hispanic Ethnicityc | 156 (62.2) | 297 (60.0) | .57 |
| Hospitalizedd | 164 (66.1) | 305 (67.6) | .69 |
| Died | 0 | 7 (1.3) | — |
| State | .0001 | ||
| California | 172 (68.5) | 418 (77.8) | |
| Connecticut | 14 (5.6) | 9 (1.7) | |
| Minnesota | 19 (7.6) | 30 (5.6) | |
| New Mexico | 22 (8.7) | 17 (3.2) | |
| New York | 12 (4.8) | 42 (7.8) | |
| Oregon | 12 (4.8) | 21 (3.9) | |
| Case Classificatione | 0.31 | ||
| Laboratory-confirmed | 235 (93.6) | 492 (91.6) | |
| Epidemiologically-linked | 1 (0.4) | 9 (1.7) | |
| Clinically-compatible | 15 (6.0) | 35 (6.5) | |
| Insurance Typef | .05 | ||
| Medicaid or no insurance | 131 (59.8) | 299 (67.5) | |
| Private, self-pay, or other | 88 (40.2) | 144 (32.5) | |
| Maternal Educationg | .001 | ||
| High School or less | 120 (53.6) | 295 (66.6) | |
| More than High School | 104 (46.4) | 148 (33.4) |
Calculated from those with known sex (n=251 for enrolled; n=523 for non-enrolled)
Calculated from those with known race (n=246 for enrolled; n=459 for non-enrolled)
Calculated from those with known ethnicity (n=495 non-enrolled)
Calculated from those with known hospitalization status (n=248 enrolled; n=451 non-enrolled)
Calculated from those with known case classification (n=251 enrolled; n=536 non-enrolled)
Calculated from those with known insurance type (n=219 enrolled; n=443 non-enrolled)
Calculated from those with known maternal education (n=224 enrolled; n=443 non-enrolled)
We identified 5,507 infants eligible to be enrolled as controls, 682 (12.4%) of whom were enrolled in the evaluation. The remaining 4,825 were not enrolled for the following reasons: 4,124 (85.5%) had mothers that could not be reached, 623 (12.9%) did not consent, 53 (1.1%) had incomplete maternal vaccination history follow-up, 6 (0.1%) resided outside of the catchment area on corresponding case-infant cough onset date, 4 (0.1%) did not speak English or Spanish, and 15 (0.3%) had another reason for non-enrollment. Demographics of enrolled case and control-infants are shown in Table 2. Because there was a disproportionate number of enrolled control-infants compared to enrolled case-infants among those <2 weeks old, we restricted our analysis to case-infants who were ≥2 weeks old on their cough onset date and control-infants who were ≥2 weeks old on their corresponding case-infant’s cough onset date (Table 2); 11 (4.4%) case-infants and 147 (21.6%) control-infants were excluded. The population for analysis included 240 case-infants and 535 control-infants.
Table 2.
Demographics of Enrolled Infants, by Case Status
| Cases (%) n=251 | Controls (%) n=682 | p-value | |
|---|---|---|---|
| Sex, male | 123 (49.0) | 330 (48.4) | .87 |
| Racea | |||
| White | 199 (80.9) | 543 (81.9) | .62 |
| Black | 22 (8.9) | 47 (7.1) | |
| Other | 25 (10.2) | 73 (11.0) | |
| Hispanic Ethnicity | 156 (62.2) | 344 (50.4) | .002 |
| Age in weeksb | |||
| 0–1 weeks | 11 (4.4) | 147 (21.6) | <.0001 |
| 2–3 weeks | 66 (26.3) | 147 (21.6) | |
| 4–5 weeks | 70 (27.9) | 153 (22.4) | |
| 6–7 weeks | 79 (31.5) | 178 (26.1) | |
| 8 weeks | 25 (9.9) | 57 (8.3) | |
| Infants with a DTaP dose | 2 (0.8) | 3 (0.4) | .62 |
Calculated from those with known race (n=246 for case-infants; n=663 for control-infants)
Age at date of cough onset (for case-infants) or date of cough onset of matched case-infant (for control-infants)
Overall, 136 (56.7%) case-mothers and 358 (66.9%) control-mothers had at least one valid Tdap dose identified. Eighteen (13.2%) of 136 vaccinated case-mothers and 50 (14.0%) of 358 vaccinated control-mothers had more than one valid dose of Tdap reported (p=0.83); 61 mothers had two documented doses of Tdap, and 7 had three. Among the 61 women with two Tdap doses, the median time between doses was 1,022 days (range: 7 – 2,744 days) and did not differ significantly between case and control-mothers (p=0.32).
Figures 1a–1c show the distribution of Tdap doses included in the final model and their timing of administration in relation to pregnancy. Of Tdap doses received during pregnancy (associated with 22 cases and 117 controls), approximately 77% were received during the third trimester, most during the ACIP-recommended 27–36 weeks of gestation (Figure 1a). For the 24 case-associated and 67 control-associated doses received before pregnancy, 6 (25.0%) case-mothers and 46 (68.7%) control-mothers received Tdap ≤2 years before pregnancy (Figure 1b). For doses classified as post-partum, 75.9% of control-associated doses and 74.4% of case-associated doses were given within the first two days following birth; 5 (5.6%) doses among case-mothers and 7 (4.0%) among control-mothers were received during the last two weeks of pregnancy and therefore classified as post-partum (Figure 1c).
Figure 1a. Timing of Tdap Doses Classified as During Pregnancy.

The white bars represent Tdap doses received by control-associated mothers during pregnancy, and the gray bars represent Tdap doses received by case-associated mothers during pregnancy.
*2 cases were missing gestational age so the exact week of Tdap administration could not be calculated; based on date of birth and Tdap date, both cases were included in the second trimester of pregnancy in the analysis models.
Figure 1c. Timing of Tdap Doses Classified as Post-partum.

The white bars represent Tdap doses received by control-associated mothers during the post-partum period, and the gray bars represent Tdap doses received by case-associated mothers during the post-partum period.
Figure 1b. Timing of Tdap Doses Classified as Before Pregnancy.
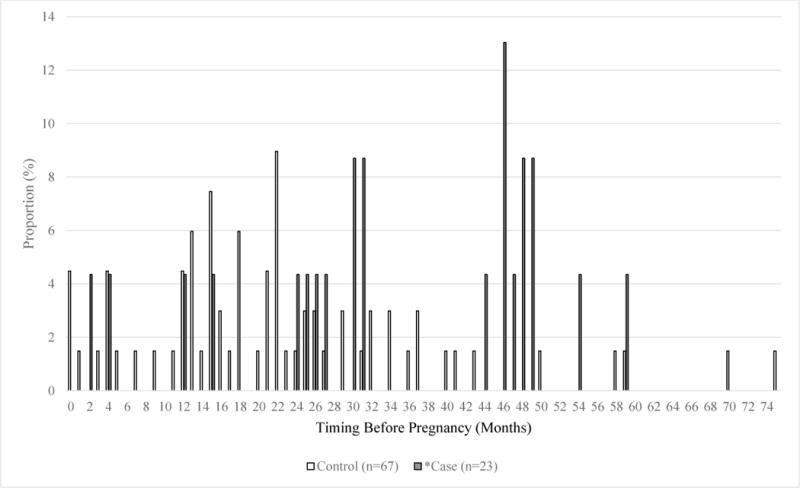
The white bars represent Tdap doses received by control-associated mothers before pregnancy, and the gray bars represent Tdap doses received by case-associated mothers before pregnancy.
*1 case missing gestational age so the exact month of Tdap administration before pregnancy could not be calculated; based on date of birth and Tdap date, this case was included in the ≤2 year group in the analysis models.
The overall effectiveness of vaccination at any time during the third trimester of pregnancy was 77.7% (48.3% – 90.4%; Table 3); the effectiveness of Tdap given during the first or second trimester was 64.3% (−13.8% – 88.8%), but confidence bounds overlapped with those for the third trimester. When restricting third-trimester doses to the recommended window of 27 – 36 weeks, VE was 78.4% (49.8% – 90.7%). There was no effectiveness when a dose of Tdap was given to the mother during the postpartum period (4.9%; −49.3% – 39.5%; Table 3).
Table 3.
Effectiveness of Maternal Tdap Vaccination at Preventing Infant Pertussis, by Timing of Vaccination
| Cases | Controls | Multivariable VEa, % (95% CI) | |||
|---|---|---|---|---|---|
| Total | 240 | % | 535 | % | |
| Unvaccinated | 104 | 43.3 | 177 | 33.1 | REFERENCE |
| Before pregnancy | 24 | 10.0 | 67 | 12.5 | 50.8 (2.1 – 75.2) |
| 1st or 2nd trimester | 5 | 2.1 | 27 | 5.1 | 64.3 (−13.8 – 88.8) |
| 3rd trimester | 17 | 7.1 | 90 | 16.8 | 77.7 (48.3 – 90.4) |
| After pregnancy | 90 | 37.5 | 174 | 32.5 | 4.9 (−49.3 – 39.5) |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness
The following variables were included in the final model: household size >2 persons, maternal education, household member with pertussis diagnosis, and infant age (weeks).
Tdap given at any point before pregnancy was 50.8% (2.1% – 75.2%) effective at preventing infant pertussis (Table 3). We further stratified this analysis by examining Tdap doses given ≤2 years before pregnancy and Tdap doses given >2 years before pregnancy; the effectiveness of Tdap given >2 years before pregnancy was −25.6% (−207.4 – 48.7%) and the effectiveness of Tdap given ≤2 years before pregnancy was 83.0% (49.6% – 94.3%). The point estimate for Tdap given ≤2 years before pregnancy was not significantly different than the point estimate for Tdap given during the 3rd trimester (p=0.6034).
Approximately 65.4% (157/240) of case-infants were hospitalized during the course of their pertussis infection. When we examined the effectiveness of Tdap at preventing infant pertussis hospitalizations, the VE point estimate for vaccination during the 3rd trimester increased to 90.5% (65.2 – 97.4%). An increase in VE point estimates was also observed for doses given at any point before pregnancy, during the 1st or 2nd trimester, and post-partum; however, the post-partum estimate remained non-significant (Table 4).
Table 4.
Effectiveness of Maternal Tdap Vaccination at Preventing Infant Pertussis Hospitalizations, by Timing of Vaccination
| Cases | Controls | Multivariable VEa, % (95% CI) | |||
|---|---|---|---|---|---|
| Total | 157 | % | 336 | % | |
| Unvaccinated | 76 | 48.4 | 109 | 32.4 | REFERENCE |
| Before pregnancy | 16 | 10.2 | 46 | 13.7 | 76.2 (37.2 – 91.0) |
| 1st or 2nd trimester | 2 | 1.3 | 20 | 6.0 | 91.4 (24.8 – 99.0) |
| 3rd trimester | 6 | 3.8 | 47 | 14.0 | 90.5 (65.2 – 97.4) |
| After pregnancy | 57 | 36.3 | 114 | 33.9 | 32.5 (−23.5 – 63.1) |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness
The following variables were included in the final model: household size >2 persons, maternal education, household member with pertussis diagnosis, and infant age (weeks).
A total of 43/136 (31.6%) vaccinated case-mothers and 102/358 (28.5%) vaccinated control-mothers received Boostrix™, and 76/136 (55.9%) vaccinated case-mothers and 207/358 (57.8%) vaccinated control-mothers received Adacel®; brand information was not available for the remaining 17/136 (12.5%) case-mothers and 49/358 (13.7%) control-mothers who had record of Tdap receipt. When we calculated the effectiveness of either vaccine product at preventing infant disease when administered during the 3rd trimester, point estimates were not statistically different from one another (p=0.85).
DISCUSSION
Our findings, using data from multiple U.S. states, add to the growing body of evidence that vaccination during pregnancy is effective, underscoring its importance as a key strategy for preventing pertussis in young infants. The United Kingdom was first to evaluate Tdap vaccination during pregnancy, demonstrating high effectiveness at preventing pertussis during the first two months of life [11, 12]. Vaccination during pregnancy was recommended in the U.K in late 2012 owing to a sudden increase in pertussis morbidity and mortality among infants, and high Tdap coverage (60%) was rapidly achieved among pregnant women [11]. Three years post-introduction, high effectiveness has been sustained [13]. Contrary to the U.K. experience, U.S. uptake of the recommendation has progressed at a much slower pace. A recent survey reports that 48.8% of U.S. pregnant women received Tdap during the 2015–16 influenza season, an increase of 21.8% from the 2013–14 season [14]. Although coverage continues to increase, there is likely considerable variation in vaccine uptake across the U.S. and among medical providers caring for pregnant women. Emphasis should be placed on maximizing Tdap uptake during pregnancy in order to optimize the benefits of the strategy.
Although our results are consistent with those from a U.S. cohort analysis, we found slightly lower effectiveness of vaccination during pregnancy than in the U.K., where estimates were obtained using similar case-control methodology; a second, more recent U.S. cohort study also found higher point estimates of VE, but the CIs were wide and included our estimated effectiveness [11, 15, 16]. Ninety percent of infants <1 year old with pertussis reported in the U.K. between 2002 and 2009 were hospitalized [17]. In contrast, U.S. data indicate that 34%–69% of infants with pertussis are hospitalized [3, 6, 18, 19], with the proportion of hospitalizations decreasing in recent years [2]. While there could be real differences in the epidemiology of severe disease or the threshold for hospitalization of suspect infant cases, this disparity more likely reflects differences in case ascertainment practices, and the ability of U.S. surveillance to capture more outpatient illness [10]. Interestingly, when our models were restricted to hospitalized cases, higher vaccine effectiveness was observed, closely mirroring the U.K. estimates. In the U.K. study, all cases were laboratory-confirmed which could also lead to a higher VE estimate; however, the majority of our cases were also laboratory-confirmed (94%), suggesting that this does not account for our lower estimate.
Sixty-eight percent of our cases were from California (Table 1) which has experienced large pertussis epidemics in recent years, with a proportion of cases in Hispanic infants that is greater than the proportion of Hispanics in the California birth cohort [20]. As a result, 62% of our enrolled cases were Hispanic (Table 1), which is higher than U.S. national estimates (49% of pertussis cases aged <2 months with known ethnicity, unpublished data, US National Notifiable Disease Surveillance System, 2011–2014). While an increased risk of pertussis in Hispanic infants has been reported, additional study is needed to fully understand why Hispanic infants are at increased risk [21]. It is reassuring that vaccination during pregnancy is highly effective in a population with a high proportion of Hispanic cases, further underscoring the importance of this strategy.
The recommendation for cocooning has been in place since the U.S. introduction of Tdap; however, programmatic implementation of the strategy has encountered substantial logistical challenges resulting in poor Tdap uptake and incomplete coverage among infant close contacts [22, 23]. Consistent with published evaluations of the cocooning strategy, point estimates from our analysis, although not reaching significance, show no benefit of a dose of Tdap administered to the mother post-partum [16, 24, 25]. Recent data from non-human primates has suggested that acellular vaccination, unlike vaccination with whole-cell pertussis vaccine, may not preclude B. pertussis colonization and further transmission of the organism [26]. In an era of exclusively acellular vaccine use in the U.S., vaccinated individuals could remain a significant source of pertussis transmission to young infants, highlighting additional shortcomings of cocooning and further underscoring the critical role of effective strategies such as vaccination during pregnancy.
Recent studies have brought into question the ideal timing of Tdap administration during pregnancy, providing evidence that vaccination during the 2nd or early 3rd trimester may maximize transplacental transfer of maternal pertussis antibodies to the infant [27–29]. While active transport of maternal antibodies is thought to be minimal before 30 weeks’ gestation, vaccination too late in pregnancy could diminish protection provided to the infant [30]. Because most Tdap-vaccinated mothers in our evaluation received vaccine during the recommended 3rd trimester, our sample size was not adequate to detect a significant difference in VE between 3rd trimester doses and doses administered earlier in pregnancy, and small numbers left us unable to evaluate narrower time periods. Our analysis did find benefit of a dose of Tdap given in the 2 years prior to pregnancy, with a VE point estimate not significantly different than that of a dose administered during the 3rd trimester. While we were not powered to detect a significant difference between point estimates, this finding is consistent with other VE studies that have shown benefit of Tdap prior to pregnancy [16, 31]. Future studies should not only continue to evaluate the infant immune response to Tdap doses administered prior to the third trimester of pregnancy, but also assess how transferred antibody correlates with protection against clinical disease.
Although Tdap vaccination during pregnancy is effective at preventing infant pertussis, important questions remain. Reduced Tdap effectiveness has been documented among adolescents primed with acellular pertussis vaccines compared to cohorts vaccinated with whole-cell vaccine [32–34]. Whether the effectiveness of vaccination during or in the two years prior to pregnancy will diminish as the number of aP-primed pregnant women increases is yet to be determined; the exact timing of Tdap administration could become more critical among aP-vaccinated mothers if antibodies diminish more rapidly in these women. Maternal immunization during pregnancy also has the potential to blunt an infant’s immune response to the DTaP childhood series. While circulating maternal antibodies decline rapidly and potential interference is expected to be short-lived, studies have generated conflicting evidence on blunting, and its clinical relevance is still largely unknown [35–40]. Continued monitoring of the epidemiology of pertussis will be important to detect potential shifts in the age distribution of childhood disease.
Maximizing the protection of young infants remains a high priority, especially in the setting of increased pertussis activity. Efforts should focus on the promotion of maternal immunization through provider and patient education in order to increase Tdap uptake among pregnant women (www.cdc.gov/pertussis/pregnant). Pertussis vaccines with improved duration of immunity are needed, but new vaccines remain on the distant horizon. While maternal immunization during pregnancy will help bridge the gap until next-generation pertussis vaccines are licensed and available for use, this highly effective strategy will likely remain an integral component of pertussis prevention and control, even in the setting of new vaccines.
Summary of Main Points.
Tdap vaccine administered to a mother during the third trimester of pregnancy is 77.7% effective at preventing pertussis in infants <2 months of age.
Acknowledgments
We’d like to thank the following individuals for their work on the evaluation: Matt Griffith, Nancy Messonnier, Thomas Clark, Adria Lee (CDC); Pam Daily, Mohammed Khan, Sarah New, Tara Scheuer, Kathy Harriman, Kathleen Winter (California); Roxanne Ryan (Connecticut); Emily Banerjee, Rachel Ostadkar, Cynthia Kenyon, Pam Gahr (Minnesota); Kevin Aicher, Julianna Ferreira, Bernadette Gutierrez, Dana Moore-Smith, Emily Hancock (New Mexico); Prescela Perez, Kathrine Woodworth (New York); Juventila Liko, Beletshachew Shiferaw, Allison Ryan, Laura Reynolds, Rita McConathy (Oregon).
Funding: This work was supported by a Centers for Disease Control and Prevention cooperative agreement with the Emerging Infections Program Network (EIP).
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The Emerging Infections Program Network is a collaborative network between CDC and state and local health departments, academic institutions, and laboratories that serves as a national resource for surveillance, prevention, and control of emerging infectious diseases. This case-control evaluation was conducted statewide in California, Connecticut, Minnesota, and New Mexico, and in select counties of New York (Albany, Allegany, Cattaraugus, Chautauqua, Chemung, Clinton, Columbia, Delaware, Erie, Essex, Franklin, Fulton, Genesee, Greene, Hamilton, Livingstone, Montgomery, Monroe, Niagara, Ontario, Orleans, Otsego, Rensselaer, Saratoga, Schenectady, Schoharie, Schuyler, Seneca, Steuben, Warren, Washington, Wayne, Wyoming, and Yates) and Oregon (Clackamas, Multnomah, and Washington).
Financial Disclosure: None of the authors have financial relationships relevant to this article to disclose.
Conflict of Interest: None of the authors have conflicts of interest to disclose.
References
- 1.Clark T. Changing pertussis epidemiology: everything old is new again. The Journal of infectious diseases. 2014;209(7):978–81. doi: 10.1093/infdis/jiu001. [DOI] [PubMed] [Google Scholar]
- 2.Bozio C, Skoff TH, Pondo T, Liang J. Epidemiology and Trends of Pertussis among Infants – United States, 2000–2015; 2017 Annual Epidemic Intelligence Service Conference; Atlanta, GA. April 24–27, 2017. [Google Scholar]
- 3.Skoff TH, Kenyon C, Cocoros N, et al. Sources of Infant Pertussis Infection in the United States. Pediatrics. 2015;136(4):635–41. doi: 10.1542/peds.2015-1120. [DOI] [PubMed] [Google Scholar]
- 4.Wendelboe AM, Njamkepo E, Bourillon A, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007:26. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 5.Kretsinger K, Broder KR, Cortese MM, et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep. 2006;55(RR-17):1–37. [PubMed] [Google Scholar]
- 6.CDC. Preventing Tetanus, Diphtheria, and Pertussis Among Adolescents: Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccines Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2006;55(RR-3):1–43. [PubMed] [Google Scholar]
- 7.Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months — Advisory Committee on Immunization Practices (ACIP), 2011. MMWR. 2011;60(41):1424–6. [PubMed] [Google Scholar]
- 8.Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women–Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Healy CM, Rench M, Baker C. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clinical infectious diseases. 2013;56(4):539–44. doi: 10.1093/cid/cis923. [DOI] [PubMed] [Google Scholar]
- 10.Skoff T, Baumbach J, Cieslak P. Tracking Pertussis and Evaluating Control Measures through Enhanced Pertussis Surveillance, Emerging Infections Program, United States. Emerging infectious diseases. 2015;21(9):1568–73. doi: 10.3201/eid2109.150023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dabrera G, Amirthalingam G, Andrews N, et al. A Case-Control Study to Estimate the Effectiveness of Maternal Pertussis Vaccination in Protecting Newborn Infants in England and Wales, 2012–2013. Clinical Infectious Diseases. 2015;60(3):333–7. doi: 10.1093/cid/ciu821. [DOI] [PubMed] [Google Scholar]
- 12.Amirthalingam G, Andrews N, Campbell H, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. The Lancet. 2014;384(9953):1521–8. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 13.Amirthalingam G, Campbell H, Ribeiro S, et al. Sustained Effectiveness of the Maternal Pertussis Immunization Program in England 3 Years Following Introduction. Clinical Infectious Diseases. 2016;63(suppl 4):S236–S43. doi: 10.1093/cid/ciw559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn KE, Black CL, Ding H, et al. Pregnant Women and Tdap Vaccination, Internet Panel Survey, United States. 2016 Apr; Available at: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/tdap-report-2016.html.
- 15.Winter K, Nickell S, Powell M, Harriman K. Effectiveness of Prenatal Versus Postpartum Tetanus, Diphtheria, and Acellular Pertussis Vaccination in Preventing Infant Pertussis. Clinical infectious diseases. 2017;64(1):3–8. doi: 10.1093/cid/ciw634. [DOI] [PubMed] [Google Scholar]
- 16.Baxter R, Bartlett J, Fireman B, Lewis E, Klein NP. Effectiveness of Vaccination During Pregnancy to Prevent Infant Pertussis. Pediatrics. 2017 doi: 10.1542/peds.2016-4091. [DOI] [PubMed] [Google Scholar]
- 17.Campbell H, Amirthalingam G, Andrews N, et al. Accelerating control of pertussis in England and Wales. Emerging infectious diseases. 2012;18(1):38–47. doi: 10.3201/eid1801.110784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. Pertussis–United States, 2001–2003. MMWR Morb Mortal Wkly Rep. 2005;54(50):1283–6. [PubMed] [Google Scholar]
- 19.CDC. Pertussis–United States, 1997–2000. MMWR Morb Mortal Wkly Rep. 2002;51(4):73–6. [PubMed] [Google Scholar]
- 20.Winter K, Harriman K, Zipprich J, et al. California pertussis epidemic, 2010. J Pediatr. 2012:1. doi: 10.1016/j.jpeds.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 21.Levri KM, Reynolds L, Liko J, Dott M, Robinson BF, Cieslak PR. Risk Factors for Pertussis Among Hispanic Infants: Metropolitan Portland, Oregon, 2010–2012. The Pediatric Infectious Disease Journal. 2016;35(5):488–93. doi: 10.1097/INF.0000000000001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urwyler P, Heininger U. Protecting newborns from pertussis - the challenge of complete cocooning. BMC Infectious Diseases. 2014;14(1):397. doi: 10.1186/1471-2334-14-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blain A, Lewis M, Banerjee E, et al. An Assessment of the Cocooning Strategy for Preventing Infant Pertussis-United States, 2011. Clinical infectious diseases. 2016;63(suppl 4):S221–S6. doi: 10.1093/cid/ciw528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carcione D, Regan AK, Tracey L, et al. The impact of parental postpartum pertussis vaccination on infection in infants: A population-based study of cocooning in Western Australia. Vaccine. 2015;33(42):5654–61. doi: 10.1016/j.vaccine.2015.08.066. [DOI] [PubMed] [Google Scholar]
- 25.Castagnini LA, Healy CM, Rench MA, Wootton SH, Munoz FM, Baker CJ. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin Infect Dis. 2012:54. doi: 10.1093/cid/cir765. [DOI] [PubMed] [Google Scholar]
- 26.Warfel J, Zimmerman L, Merkel T. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):787–92. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eberhardt CS, Blanchard-Rohner G, Lemaître B, et al. Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis. Clinical Infectious Diseases. 2016;62(7):829–36. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu Raya B, Srugo I, Bamberger E. Optimal Timing of Immunization Against Pertussis During Pregnancy. Clinical Infectious Diseases. 2016;63(1):143–4. doi: 10.1093/cid/ciw233. [DOI] [PubMed] [Google Scholar]
- 29.Naidu M, Muljadi R, Davies Tuck M, Wallace E, Giles M. The optimal gestation for pertussis vaccination during pregnancy: a prospective cohort study. American journal of obstetrics and gynecology. 2016;215(2):237.e1–e6. doi: 10.1016/j.ajog.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Englund JA. The influence of maternal immunization on infant immune responses. Journal of comparative pathology. 2007;137(Suppl 1):S16–S9. doi: 10.1016/j.jcpa.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Quinn HE, Snelling TL, Habig A, Chiu C, Spokes PJ, McIntyre PB. Parental Tdap Boosters and Infant Pertussis: A Case-Control Study. Pediatrics. 2014;134(4):713–20. doi: 10.1542/peds.2014-1105. [DOI] [PubMed] [Google Scholar]
- 32.Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308(5):454–6. doi: 10.1001/jama.2012.6364. [DOI] [PubMed] [Google Scholar]
- 33.Liko J, Robison SG, Cieslak PR. Priming with Whole-Cell versus Acellular Pertussis Vaccine. New England Journal of Medicine. 2013;368(6):581–2. doi: 10.1056/NEJMc1212006. [DOI] [PubMed] [Google Scholar]
- 34.Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. Comparative Effectiveness of Acellular Versus Whole-Cell Pertussis Vaccines in Teenagers. Pediatrics. 2013;131(6):e1716–e22. doi: 10.1542/peds.2012-3836. [DOI] [PubMed] [Google Scholar]
- 35.Hardy Fairbanks A, Pan S, Decker M, et al. Immune responses in infants whose mothers received Tdap vaccine during pregnancy. The Pediatric infectious disease journal. 2013;32(11):1257–60. doi: 10.1097/INF.0b013e3182a09b6a. [DOI] [PubMed] [Google Scholar]
- 36.Munoz F, Bond N, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA: the Journal of the American Medical Association. 2014;311(17):1760–9. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoang HTT, Leuridan E, Maertens K, et al. Pertussis vaccination during pregnancy in Vietnam: Results of a randomized controlled trial Pertussis vaccination during pregnancy. Vaccine. 2016;34(1):151–9. doi: 10.1016/j.vaccine.2015.10.098. [DOI] [PubMed] [Google Scholar]
- 38.Ladhani S, Andrews N, Southern J, et al. Antibody responses after primary immunization in infants born to women receiving a pertussis-containing vaccine during pregnancy: single arm observational study with a historical comparator. Clinical infectious diseases. 2015;61(11):1637–44. doi: 10.1093/cid/civ695. [DOI] [PubMed] [Google Scholar]
- 39.Maertens K, Hoang TTH, Nguyen TD, et al. The Effect of Maternal Pertussis Immunization on Infant Vaccine Responses to a Booster Pertussis-Containing Vaccine in Vietnam. Clinical Infectious Diseases. 2016;63(suppl 4):S197–S204. doi: 10.1093/cid/ciw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kent A, Ladhani S, Andrews N, et al. Pertussis Antibody Concentrations in Infants Born Prematurely to Mothers Vaccinated in Pregnancy. Pediatrics. 2016;138(1) doi: 10.1542/peds.2015-3854. [DOI] [PubMed] [Google Scholar]


