Abstract
Acute myelogenous leukemia (AML) has an overall poor survival rate and shows considerable molecular heterogeneity in its etiology. In the WHO classification there are >50 cytogenetic subgroups of AML, many showing highly specific chromosome translocations that lead to constitutive activation of individual kinases. In a rare stem cell leukemia/lymphoma syndrome, translocations involving 8p11 lead to constitutive activation of the fibroblast growth factor receptor 1 (FGFR1) kinase. This disorder shows myeloproliferative disease with almost invariably progresses to AML, and conventional therapeutic strategies are largely unsuccessful. Because of the rare nature of this syndrome, models that faithfully recapitulate the human disease are needed to evaluate therapeutic strategies. The t(8;13)(p11;q12) chromosome translocation is most common rearrangement seen in this syndrome and creates a ZMYM2-FGFR1 chimeric kinase. To understand more about the molecular etiology of AML induced by this particular rearrangement, we have created a model human CD34+ cells transplanted into immunocompromized mice which develop myeloproliferative disease that progresses to AML with a long (> 12 months) latency period. As in humans, these mice show hepatospenomegaly, hypercellular bone marrow and a CD45+CD34+CD13+ immunophenotype. Molecular studies demonstrate upregulation of genes such as KLF4 and FLT3 that promote stemness, and overexpression of MYC, which is associated with suppression of myeloid cell differentiation. This murine model, therefore, provides an opportunity to develop therapeutic strategies against the most common subtype within these FGFR1 driven neoplasms and opportunities for a more in depth study the molecular etiology of this disease.
Keywords: FGFR1, AML, myeloproliferative disease, mouse model, 8p11 translocation
Introduction
Myeloid and lymphoid neoplasms associated with rearrangements of the fibroblast growth factor receptor 1 (FGFR1) is the only example of a malignancy showing consistent abnormalities associated with this receptor kinase. Rearrangements involving FGFR1 kinase leads to the development of an atypical myeloproliferative disorder (MPD) that frequently (>80%) progresses to AML. These patients may also develop T- or B-cell lymphomas in addition to the AML, which carry the same FGFR1 rearrangements. Overall, survival of this disease is still very poor (overall survival <16 months),1 even following bone marrow transplantation. Immunophenotyping demonstrates bi-lineage disease, suggesting a stem cell origin. The FGFR1 abnormalities are derived from chromosome translocations that lead to the creation of chimeric kinases which involve dimerization domains from a wide variety of chromosome partners fused to the FGFR1 kinase domain which results in its constitutive, ligand-independent activation.2, 3 The t(8;13)(p12;q11) chromosome translocation is the most common rearrangement, generating a ZMYM2-FGFR1 fusion protein;1, 4,5 the next most frequent translocations generate BCR-FGFR1 and CNTRL-FGFR1 fusion proteins. Because this is a relatively rare disorder, it has been difficult to investigate the molecular etiology in the primary disease. To overcome this limitation, mouse models have been developed that are representative of the human disease. In early studies, transduction and transplantation of bone marrow derived cells in syngeneic mice demonstrated the development of MPD and rapid development and progression of T- and B-cell lymphomas, which were fatal.6–9 In contrast with the human disease, only the CNTRL-FGFR1 chimeric kinase has been reported to develop AML in the syngeneic mouse model8 and only after a long latency period. Recently, human leukemias have been modeled the NOD/SCID/IL2Rgnull (NSG) mouse strain through introduction of chimeric kinases into human cord blood-derived CD34+ cells which mirror the human disease.8 Using this approach, we now report the development and characterization of a humanized mouse model of AML resulting from the most common, ZMYM2-FGFR1 chimeric kinase.
Material and Methods
Retroviral transduction and transplantation
The production of retroviral particles, retroviral transduction of human CD34+ progenitor cells, and transplantation was performed as described previously.8 Anonymized human cord blood cells were obtained from the Georgia Regents University Cord Blood Bank under an approved institutional review board protocol (#1002143); approval was also obtained from the Georgia Regents University institutional review board for these studies. Informed consent was obtained according to the Declaration of Helsinki. CD34+ cells were isolated using the EasySep Cord Blood CD34 Positive Selection Kit (StemCell Technologies) following the manufacturer’s protocol, and expanded in StemSpan SFEM medium (StemCell) supplemented with recombinant human cytokines: low-density lipoprotein 10 mg/mL, Flt-3 100 ng/mL, stem cell factor 100 ng/mL, thrombopoietin 50 ng/mL, IL-3 20 ng/mL, and IL-6 20 ng/mL (R&D Systems). After 24 hours of prestimulation, CD34+ cells were transduced as described previously10 and transplanted into NSG mice.
Analysis of diseased mice
Mice that received transplants were evaluated daily for symptoms of disease as described previously8 to determine progression of the disease. During the course of this monitoring, peripheral blood (PB) samples were obtained from the tail veins to analyze the green fluorescent protein positive (GFP+)/CD45+ cells periodically after transplantation except as otherwise noted. All animal experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee of the Georgia Regents University.
Western blot analyses
Proteins were isolated using standard procedures. Whole-cell lysates containing 50 μg of proteins, were separated by SDS-PAGE and immunoblotted with the following specific antibodies: MYC, STAT5 and phospho-STAT5 (Cell Signaling Technologies, Danvers, MA, USA), ACTB (beta-actin) from (Sigma, St. Louis, MO, USA).
Molecular analyses
Total RNA was isolated using TRIzol (Invitrogen) reagent, and digested with RNA-free DNase to eliminate genomic DNA contamination. Reverse-transcription was achieved using a SuperScript first-strand synthesis system (Invitrogen), and amplified by PCR or quantitated by real-time RT-PCR using conditions described previously.8 The primer sequences for specific genes and PCR condition is available upon request.
Flow cytometric analysis
Spleen tissues were passed through a cell strainer (BD, Bedford, MA, USA) to generate single cell suspensions. Bone marrow cells were isolated by flushing mouse femurs with phosphate-buffered saline and stained using human-specific conjugated monoclonal antibodies as described.8 Following staining, cells were washed once in staining medium and then analyzed using an LSR II cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR, USA).
Results and Discussion
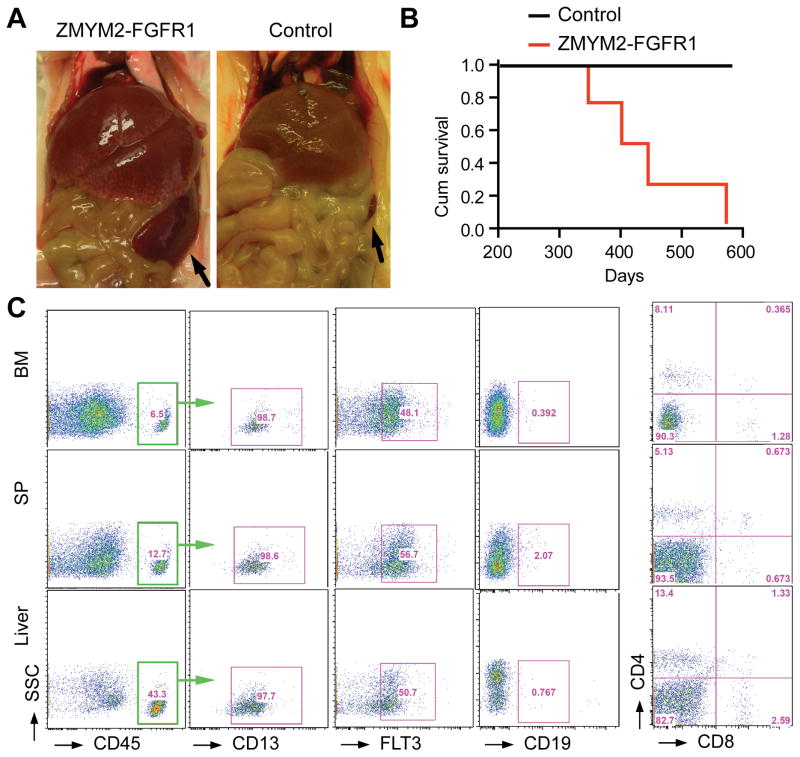
Although there have been more than 15 different rearrangements associated with FGFR1-driven myeloid and lymphoid malignancies,11, 12 the t(8;13) translocation that generates the ZMYM2-FGFR1 fusion kinase is by far the most common.1 It appears that there are subtle difference in disease presentation and progression depending on the specific fusion kinase involved.1 Developing a representative human disease for the ZMYM2-FGFR1 rearrangement in a mouse model would provide opportunities to better understand the associated molecular etiology, and possibly identify targets that may be used to treat the disease in preclinical studies. The ZMYM2-FGFR1 fusion gene was inserted into the MIEG3 vector that co-expresses GFP from the murine stem cell virus promoter. This construct was transfected into enriched populations of human CD34+ cells derived from umbilical cord blood. The transfected cells were then introduced into un-irradiated NSG mice. Development of the disease was followed though regular sampling of the peripheral blood, beginning 4 weeks after engraftment. Human-specific CD45 antibodies were used to distinguish between the human and mouse cells and populations of these cells developed progressively over six months. The first mouse in the cohort died after ~350 days; subsequent animals died between 350–575 days with features of AML such as enlarged liver and spleen (Figure 1). In the syngeneic mouse transplantation models, the ZMYM2-FGFR1 expressing tumors were exclusively CD4+CD8+ T-cell lymphomas, which developed rapidly, killing the mice within 4–8 weeks.7 This rapid development of an aggressive T-cell disease may have precluded AML development which appears much later in other syngeneic models.9 Slow progression was also seen for the ZMYM2-FGFR1 transduced CD34+ cells in NSG mice (over 12–15 months). Progression to AML in this case was likely facilitated by the absence of functional T- and B-cells in this mouse strain which might otherwise have allowed the development of lymphomas that could overwhelm the mice before AML developed. Peripheral blood, spleen and bone marrow cells from the ZMYM2-FGFR1 transduced mice were analysed using flow cytometry at the time of death, all of which showed the same immunophenotype (Figure 1b), where ~10–40% of the cells were positive for the human-specific CD45 antigen. The majority of these CD45+ cells expressed human CD13 and FLT3, which is characteristic of immature myeloid cells (Figure 1b). Consistent with the primary human disease, mouse leukemias transformed with FGFR1 chimeric kinases, often showed mixed lineage markers indicative of a stem cell origin.7 The cells from the ZMYM2-FGFR1 AML were CD19 negative, precluding a B cell lineage, and CD4 and CD8 negative (Figure 1b) precluding a T-cell lineage, which is not surprising due to the absence of functional B- and T- cells in these mice. In a previous report,13 a similar approach was used by Agerstam and colleagues in attempts to develop a mouse model of ZMYM2-FGFR1 driven AML and, although expression in stem cells resulted in increased proliferation in vitro, a transformed transplantable leukemia was not observed, even though there was an increase in the myeloid cell population. Surprisingly, and in contrast to our studies, many of the mice described in this report died within 6–7 weeks, and the remainder were only followed for 10 weeks13 during which time only an apparent chronic phase of the disease had developed, which was not transplantable.13 In our study, the ZMYM2-FGFR1 AML has been successively transplanted through three generations, demonstrating that fully transformed stem cells are present in the transplant.
Figure 1. ZMYM2-FGFR1 driven AML development in NSG mice engrafted with transduced human CD34+ progenitor cells.
a) Enlarged liver and spleen (arrows) is seen in a representative mouse with ZMYM2-FGFR1 AML. b) Survival accumulation was determined using Kaplan-Meier analysis and log-rank (Mantel-Cox) tests (p < 0.04) for mice with ZMYM-FGFR1 AML (n=4) compared with mice engrafted with empty vector transfected CD34+ cells (n=4). c) Flow cytometry analysis with human-specific antibodies shows a myeloid progenitor immunophenotype (CD45+CD13+FLT3+) in bone marrow (BM), spleen (SP) and liver cells.
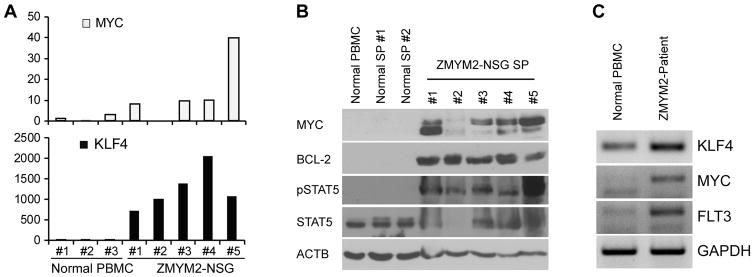
The CNTRL-FGFR1-induced AML described previously8 identified consistent genetic events in the leukemias that developed, which included consistent upregulation of FLT3, MYC and BCL2. Upregulation of FLT3, MYC and KLF4 was also seen in the humanized ZMYM2-FGFR1 AML cells (Figure 2a and b) as well as in AML cells from a primary, patient-derived, ZMYM2-FGFR1 containing AML,14 compared with the normal control (Figure 2c). KLF4 was also transcriptionally upregulated in ZMYM2-FGFR1 induced AML. In addition, activation of the STAT signaling pathway was seen in the ZMYM2-FGFR1 driven AML in our NSG mouse model (Figure 2b), which is consistent with previous reports from in vitro and in vivo experimental models, as well as primary disease with various FGFR1 rearrangements.2, 15, 16 Thus, there appear to be consistent genetic changes accompanying the development of AML driven by different chimeric FGFR1 kinases, although differences were also observed, which possibly accounts for the slightly different clinical presentation between the subtypes1.
Figure 2. Upregulation of multiple signaling pathways in ZMYM2-FGFR1 induced AML.
a) Quantitative RT-PCR analysis shows the comparison of gene expression levels in ZMYM2-FGFR1 mice (ZMYM2-NSG) compared with normal, healthy human PB mononuclear cells (Normal PBMC). b) Western blot analysis shows protein levels of MYC, BCL2, and an FGFR1 downstream signaling intermediate, phospho-STAT5, in leukemic mouse spleens compared with normal human PBMC and normal NSG mouse spleens. c) RT-PCR analysis shows the transcriptional levels of genes in AML cells from a ZMYM2-FGFR1 patient-derived AML compared with PBMC from a healthy donor.
In this report we described the first successful development of a model of human ZMYM2-FGFR1 driven AML in immunocompromised mice, which shows an etiology consistent with the development of the primary human disease. This model provides an opportunity to investigate both the underlying molecular mechanisms behind the most common ZMYM2-FGFR1 driven disease and to investigate potential therapeutic strategies to treat this disease.
Novelty and impact.
This is the first report of a transplantable, humanized model of ZMYM2-FGFR1 driven AML in immuncompromized mice. The disease is faithfully recapitulated in this mouse model and molecular analysis shows genetic changes consistent with stem cell leukemias. The close similarity with the primary human disease makes this mouse model the ideal opportunity to develop novel therapeutic approaches to treat this almost invariably lethal disease.
Acknowledgments
This study was supported by the National Institute of Health (NIH), National Cancer Institute grant CA076167.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
References
- 1.Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010;41:461–76. doi: 10.1016/j.humpath.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Baumann H, Kunapuli P, Tracy E, Cowell JK. The oncogenic fusion protein-tyrosine kinase ZNF198/fibroblast growth factor receptor-1 has signaling function comparable with interleukin-6 cytokine receptors. The Journal of biological chemistry. 2003;278:16198–208. doi: 10.1074/jbc.M300018200. [DOI] [PubMed] [Google Scholar]
- 3.Guasch G, Mack GJ, Popovici C, Dastugue N, Birnbaum D, Rattner JB, Pebusque MJ. FGFR1 is fused to the centrosome-associated protein CEP110 in the 8p12 stem cell myeloproliferative disorder with t(8;9)(p12;q33) Blood. 2000;95:1788–96. [PubMed] [Google Scholar]
- 4.Abruzzo LV, Jaffe ES, Cotelingam JD, Whang-Peng J, Del Duca V, Jr, Medeiros LJ. T-cell lymphoblastic lymphoma with eosinophilia associated with subsequent myeloid malignancy. The American journal of surgical pathology. 1992;16:236–45. doi: 10.1097/00000478-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Still IH, Cowell JK. The t(8;13) atypical myeloproliferative disorder: further analysis of the ZNF198 gene and lack of evidence for multiple genes disrupted on chromosome 13. Blood. 1998;92:1456–8. [PubMed] [Google Scholar]
- 6.Roumiantsev S, Krause DS, Neumann CA, Dimitri CA, Asiedu F, Cross NC, Van Etten RA. Distinct stem cell myeloproliferative/T lymphoma syndromes induced by ZNF198-FGFR1 and BCR-FGFR1 fusion genes from 8p11 translocations. Cancer Cell. 2004;5:287–98. doi: 10.1016/s1535-6108(04)00053-4. [DOI] [PubMed] [Google Scholar]
- 7.Ren M, Li X, Cowell JK. Genetic fingerprinting of the development and progression of T-cell lymphoma in a murine model of atypical myeloproliferative disorder initiated by the ZNF198-fibroblast growth factor receptor-1 chimeric tyrosine kinase. Blood. 2009;114:1576–84. doi: 10.1182/blood-2009-03-212704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren M, Qin H, Kitamura E, Cowell JK. Dysregulated signaling pathways in the development of CNTRL-FGFR1-induced myeloid and lymphoid malignancies associated with FGFR1 in human and mouse models. Blood. 2013;122:1007–16. doi: 10.1182/blood-2013-03-489823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren M, Tidwell JA, Sharma S, Cowell JK. Acute progression of BCR-FGFR1 induced murine B-lympho/myeloproliferative disorder suggests involvement of lineages at the pro-B cell stage. PLoS One. 2012;7:e38265. doi: 10.1371/journal.pone.0038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren M, Qin H, Ren R, Tidwell J, Cowell JK. Src activation plays an important key role in lymphomagenesis induced by FGFR1 fusion kinases. Cancer Res. 2011;71:7312–22. doi: 10.1158/0008-5472.CAN-11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura Y, Ito Y, Wakimoto N, Kakegawa E, Uchida Y, Bessho M. A novel fusion of SQSTM1 and FGFR1 in a patient with acute myelomonocytic leukemia with t(5;8)(q35;p11) translocation. Blood cancer journal. 2014;4:e265. doi: 10.1038/bcj.2014.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savage N, George TI, Gotlib J. Myeloid neoplasms associated with eosinophilia and rearrangement of PDGFRA, PDGFRB, and FGFR1: a review. International journal of laboratory hematology. 2013;35:491–500. doi: 10.1111/ijlh.12057. [DOI] [PubMed] [Google Scholar]
- 13.Agerstam H, Jaras M, Andersson A, Johnels P, Hansen N, Lassen C, Rissler M, Gisselsson D, Olofsson T, Richter J, Fan X, Ehinger M, et al. Modeling the human 8p11-myeloproliferative syndrome in immunodeficient mice. Blood. 2010;116:2103–11. doi: 10.1182/blood-2009-05-217182. [DOI] [PubMed] [Google Scholar]
- 14.Savage NM, Johnson RC, Gotlib J, George TI. Myeloid and lymphoid neoplasms with FGFR1 abnormalities: diagnostic and therapeutic challenges. American journal of hematology. 2013;88:427–30. doi: 10.1002/ajh.23296. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Deangelo DJ, Kutok JL, Williams IR, Lee BH, Wadleigh M, Duclos N, Cohen S, Adelsperger J, Okabe R, Coburn A, Galinsky I, et al. PKC412 inhibits the zinc finger 198-fibroblast growth factor receptor 1 fusion tyrosine kinase and is active in treatment of stem cell myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101:14479–84. doi: 10.1073/pnas.0404438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren M, Qin H, Ren R, Cowell JK. Ponatinib suppresses the development of myeloid and lymphoid malignancies associated with FGFR1 abnormalities. Leukemia. 2013;27:32–40. doi: 10.1038/leu.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]