Abstract
Regression analyses compared 41 type 2 diabetes (T2D) and 131 non-T2D cognitively normal elderly males on the associations of arterial wall function measures [large artery elasticity index (LAEI), small artery elasticity index (SAEI), systemic vascular resistance (SVR), and total vascular impedance (TVI)] with cognitive performance (memory, language, and executive functions), controlling for socio-demographic and cardiovascular factors. Higher LAEI and lower TVI were significantly associated with better executive functions performance in T2D but not in non-T2D subjects. Lower TVI was more associated with better language performance in T2D. Results suggest that arterial wall function is associated with cognition in T2D.
Keywords: Arterial wall function, cognitive function, large artery elasticity index, total vascular impedance, type 2 diabetes
INTRODUCTION
Type 2 diabetes (T2D) is associated with increased risk for cognitive decline [1] and dementia [2, 3]. Cerebral vasculature involvement in this relationship is plausible given the increased risk for micro- and macrovascular pathology in T2D [4]. Vascular abnormalities have been shown by some [5], but not all [6], to be associated with T2D-related cognitive compromise. A possible explanation for inconsistencies is that vascular abnormalities evolve, with functional typically preceding structural changes [7]. Nevertheless, research usually focuses on structural (primarily MRI), rather than functional assessments of vasculature. Impaired arterial wall function (AWF), which does not necessarily have a structural correlate identifiable by structural brain imaging, has been found in multiple brain regions in neurologically asymptomatic T2D subjects [8–10].
The present study compared T2D and non-T2D elderly subjects on the associations of measures of peripheral AWF with cognitive performance.
METHODS
Subjects
Subjects were recruited from the outpatient lists of the Computerized Patient Records System (CPRS) of the James J. Peters Veterans Affairs Medical Center in Bronx, NY. Subjects with diagnosis of dementia, cerebrovascular disease, or other neuropsychiatric disorders that may impair cognition, or prescribed dementia-related medications, were excluded. Inclusion criteria were: age ≥75, male (there are very few elderly women veterans), ambulatory patient status, and intact cognition. The study consisted of 172 consecutive participants who completed a neuropsychological assessment and pulse contour analysis to measure AWF. Informed consent was signed by all subjects.
Diagnosis of T2D was based on CPRS records (fully updated with the American Diabetes Association criteria [11]) and confirmation by the subjects.
Verification of intact cognition was based on the Clinical Dementia Rating (CDR) scale [12] and the Mini Mental State Examination (MMSE) [13]. The CDR assesses the severity of cognitive and functional impairment in six domains, through an interview with the subject and an informant. Subjects were required to have a CDR score of 0, reflecting absence of dementia or questionable dementia. The subjects’ score on the MMSE was required to be better than the 10th percentile of age and education adjusted norms [14]. Normal cognitive status was then confirmed by a clinical consensus conference.
Arterial pressures
Arterial pressures were measured using the HDI/Pulse-Wave CardioVascular Profiling Instrument (Hypertension Diagnostics, Eagan, MN). Following ≥ 5 min of rest, left brachial blood pressure was measured by oscillometer, with the patient in the supine position with head inclined up 30º. Tracing of the arterial waveforms from the right radial artery was performed using a calibrated stainless steel applanation tonometer with a connected ceramic piezoelectric element. A computer-based diastolic pulse wave contour analysis of radial artery was performed by evaluating the average diastolic pressure curve by a nonlinear parameter-estimating algorithm using a third-order, four-element Windkessel model of the circulation. The diastolic decay of a waveform was quantified for the large artery elasticity index (LAEI), representing capacitive or large arterial compliance (C1), and for the small artery elasticity index (SAEI), representing oscillatory or small arterial compliance (C2). The values of the C1 and C2 indices are weighted averages of the values obtained from waveforms recorded over 30 s.
Arterial wall function was measured with a noninvasive device that uses blood pressure waveform analysis. Brachial and radial arterial elasticity measurements were obtained using a standard blood pressure cuff and an automated tonometer using an oscillometric technique. Systemic vascular resistance (SVR) was calculated as mean arterial pressure (MAP) divided by estimated cardiac output (ECO). ECO is obtained applying a multivariate algorithm considering patient age, heart rate, body surface area and cardiac ejection time, derived from the pulse wave analysis. The MAP is derived from waveform analysis by integrating the area under each beat and then calculating the average of all beats included in the analysis of recordings during 30 s. Total vascular impedance was determined from the modified Windkessel model evaluated at the frequency of the measured heart rate [15].
Neuropsychological testing
Neuropsychological testing was administered by certified psychometricians. Factor analysis using Varimax rotation derived three cognitive factors from principal components with eigenvalues >1, loading on the following tests: memory (Word List Memory Immediate Recall [16], Delayed Recall, Word List Recognition [16]), language (Verbal Fluency Test [17], Short Version of the Boston Naming Test [18], Shipley [19]), and executive functions (Trail Making Test (Parts A and B) [20], Diamond cancellation, TMX Cancellation [21]). All neuropsychological tests’ scores were transformed into Z scores and each cognitive domain was calculated as the sum of the z-scores of the cognitive tests pertaining to the particular domain based on the factor analyses results.
Statistical analysis
Statistical analysis was regression analyses of each cognitive factor (language, memory, and executive functions), evaluating the interaction of each AWF measure (LAEI, SAEI, SVR, and TVI) with the T2D dichotomy, controlling for sociodemographic and cardiovascular characteristics (age, years of education, body mass index (BMI), and average diastolic and systolic blood pressure). The interaction variables were calculated as the product of the T2D dichotomy and the AWF, both of which were additional control variables in the regressions. The effect size of the interaction was its partial correlation, with a positive sign if the association of the AWF with cognition was more positive in T2D than non-T2D. The Holm enhancement of the Bonferroni procedure was used to adjust significance for 12 interactions. For descriptive purposes, partial correlations of each cognitive factor with each AWF, with the same control variables, were calculated separately by T2D status. (Since the T2D and non-T2D partial correlations used the control variables differently, their interactions with T2D were also included as control variables in the regressions.) Student’s t-test was used to compare means of demographic, cardiovascular, and general cognitive measures.
RESULTS
The study included 172 subjects: 131 non-T2D and 41 T2D. Sample characteristics are presented in Table 1: mean age was 82.0 years and the MMSE averaged 28.2, reflecting normal cognitive function.
Table 1.
Socio-demographic, cardiovascular, and general cognitive characteristics of the sample
| Variable | Diabetes status | Mean (SD) | significance |
|---|---|---|---|
| Age (years) | Total (n = 172) | 82.01 (4.41) | |
| Non-T2D (n = 131) | 82.23 (4.59) | 0.249 | |
| T2D (n = 41) | 81.32 (3.76) | ||
| BMI (kg/m2) | Total (n = 171) | 26.84 (4.08) | |
| Non-T2D (n = 130) | 26.39 (3.71) | 0.026 | |
| T2D (n = 41) | 28.27 (4.85) | ||
| Systolic blood pressure (mmHg) | Total (n = 172) | 136.12 (20.02) | |
| Non-T2D (n = 131) | 135.92 (20.54) | 0.81 | |
| T2D (n = 41) | 136.78 (18.49) | ||
| Diastolic blood pressure (mmHg) | Total (n = 172) | 71.42 (10.39) | |
| Non-T2D (n = 131) | 72.41 (9.96) | 0.037 | |
| T2D (n = 41) | 68.24 (11.19) | ||
| Diagnosis of hypertension %* | Total (n = 143) | 65.6 | |
| Non-T2D (n = 108) | 60.7 | 0.042 | |
| T2D (n = 35) | 84.4 | ||
| Cholesterol levels* | Total (n = 143) | 170.85 (37.88) | |
| Non-T2D (n = 108) | 173.75 (36.69) | 0.108 | |
| T2D (n = 35) | 161.91 (40.60) | ||
| HDL cholesterol* | Total (n = 143) | 52.27 (14.93) | |
| Non-T2D (n = 108) | 53.58 (15.93) | 0.025 | |
| T2D (n = 35) | 48.23 (10.49) | ||
| LDL cholesterol* | Total (n = 143) | 97.86 (57.30) | |
| Non-T2D (n = 108) | 101.94 (62.98) | 0.131 | |
| T2D (n = 34) | 84.89 (30.67) | ||
| Triglycerides* | Total (n = 143) | 136.94 (73.15) | |
| Non-T2D (n = 108) | 131.08 (69.33) | 0.092 | |
| T2D (n = 35) | 155.03 (82.30) | ||
| Glucose* | Total (n = 154) | 113.91 (47.87) | |
| Non-T2D (n = 117) | 97.13 (15.83) | <0.0001 | |
| T2D (n = 37) | 166.97 (71.56) | ||
| HbA1c* | Total (n = 154) | 5.9 (105) | |
| Non-T2D (n = 120) | 5.62 (0.45) | <0.0001 | |
| T2D (n = 34) | 7.03 (1.69) | ||
| Creatinine | Total (n = 154) | 1.22 (0.46) | |
| Non-T2D (n = 117) | 1.15 (0.32) | P=0.01 | |
| T2D (n = 37) | 1.47 (0.69) | ||
| LAEI | Total (n = 173) | 12.53 (5.51) | |
| Non-T2D (n = 132) | 11.98 (4.72) | 0.016 | |
| T2D (n = 41) | 14.33 (7.31) | ||
| SAEI | Total (n = 172) | 3.41 (2.10) | |
| Non-T2D (n = 131) | 3.24 (1.92) | 0.05 | |
| T2D (n = 41) | 3.98 (2.54) | ||
| SVR | Total (n = 172) | 1848.30 (427.044) | |
| Non-T2D (n = 131) | 1907.63 (437.89) | <0.0001 | |
| T2D (n = 41) | 1658.73 (328.74) | ||
| TVI | Total (n = 172) | 190.49 (76.56) | |
| Non-T2D (n = 131) | 197.23 (79.58) | 0.02 | |
| T2D (n = 41) | 168.95 (62.06) | ||
| Education (years) | Total (n = 168) | 13.68 (3.51) | |
| Non-T2D (n = 129) | 13.69 (3.49) | 0.971 | |
| T2D (n = 39) | 13.67 (3.62) | ||
| MMSE score | Total (n = 169) | 28.20 (1.60) | |
| Non-T2D (n = 129) | 28.16 (1.53) | 0.897 | |
| T2D (n = 40) | 28.20 (1.74) | ||
| Language z score | Total (n = 172) | −0.093 (1.10) | |
| Non-T2D (n = 131) | −0.06 (1.0) | 0.42 | |
| T2D (n = 41) | −0.22 (1.29) | ||
| Episodic memory z score | Total (n = 172) | 0.04 (0.93) | |
| Non-T2D (n = 131) | 0.02 (0.94) | 0.54 | |
| T2D (n = 41) | 0.12 (0.91) | ||
| Executive function z score | Total (n = 172) | 0.01 (1.07) | |
| Non-T2D (n = 131) | −0.08 (0.93) | 0.08 | |
| T2D (n = 41) | 0.32 (1.39) |
data on diagnosis of hypertension, Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, blood glucose levels and HbA1c (hemoglobin A1c) was available for n = 108–120 non-T2D subjects and for n = 34–35 T2D subjects. T2D, type 2 diabetes; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LAEI, large artery elasticity index; SAEI, small artery elasticity index; SVR, systemic vascular resistance; TVI, total vascular impedance; MMSE, Mini-Mental State Examination.
Compared to non-T2D, T2D subjects had higher BMI (p = 0.026), higher rates of hypertension (p = 0.042), higher values of glucose (p < 0.0001) and Hemoglobin A1c (p < 0.0001), lower diastolic blood pressure (p = 0.037), lower HDL-cholesterol levels (p = 0.025), higher LAEI (p = 0.016), higher SAEI (p = 0.050), lower SVR (p < 0.0001), and lower TVI (p = 0.020) The groups did not differ in other socio-demographic, cardiovascular factors, or cognitive scores (Table 1).
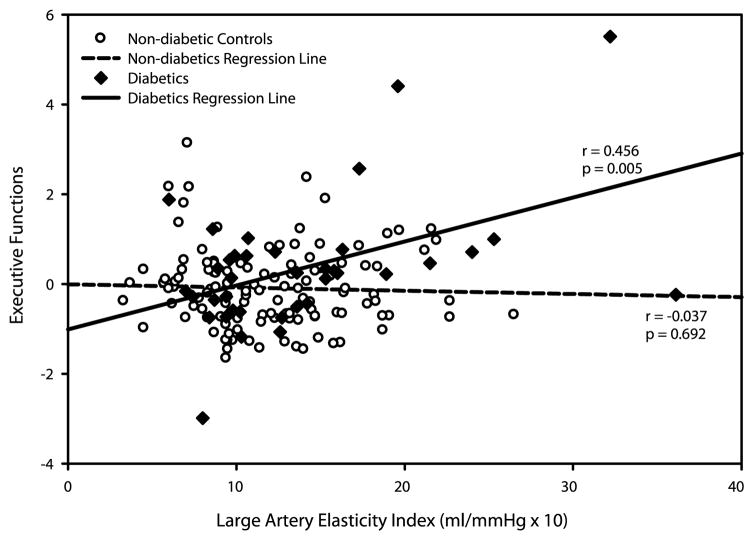
Regression analysis showed a significant interaction for LAEI in executive functions (p = 0.004)—higher LAEI was associated with better performance in T2D (partial r = 0.47; p = 0.007) but not in non-T2D subjects (partial r = 0.05, p = 0.58) (Fig. 1). TVI results were similar, with the direction of association reversed—higher TVI was significantly associated with worse performance in T2D (p for interaction = 0.003; partial r = −0.39, p = 0.03 for T2D and r = 0.02, p = 0.82 for non-T2D). Both results had p < 0.05 after adjusting for multiple comparisons. Language interaction for TVI was in the same direction as for executive functions (p for interaction = 0.039; r = −0.27, p = 0.13 for T2D and r = 0.04, p = 0.67 for non-T2D). The interactions of T2D with SAEI and SVR were not significant in any cognitive domains (Table 2).
Fig. 1.
Partial correlations of LAEI with executive functions in T2D and non-T2D subjects (unadjusted).
Table 2.
Interactions of AWF with T2D, and partial correlations* of AWF with cognitive domains by T2D status
| AWF | Cognitive domain | Interaction of AWF with T2D effect size (p) | Non-T2D partial r (p) | T2D partial r (p) |
|---|---|---|---|---|
| LAEI | Language | 0.096 (0.250) | 0.016 (0.865) | 0.201 (0.269) |
| Memory | 0.026 (0.752) | 0.094 (0.315) | 0.202 (0.267) | |
| Executive functions | 0.238 (0.004) | 0.052 (0.581) | 0.467 (0.007) | |
| SAEI | Language | 0.014 (0.867) | 0.036 (0.703) | 0.059 (0.749) |
| Memory | −0.069 (0.410) | 0.230 (0.013) | 0.137 (0.455) | |
| Executive functions | 0.118 (0.159) | 0.076 (0.417) | 0.270 (0.135) | |
| SVR | Language | −0.135 (0.102) | 0.033 (0.723) | −0.211 (0.239) |
| Memory | −0.034 (0.686) | −0.146 (0.117) | −0.180 (0.315) | |
| Executive functions | −0.075 (0.363) | −0.072 (0.442) | −0.147 (0.414) | |
| TVI | Language | −0.170 (0.039) | 0.040 (0.669) | −0.271 (0.127) |
| Memory | −0.047 (0.572) | −0.154 (0.098) | −0.248 (0.164) | |
| Executive functions | −0.246 (0.003) | 0.022 (0.818) | −0.386 (0.027) |
Controlling for age, years of education, body mass index, and average diastolic and systolic blood pressure. AWF, arterial wall function; T2D, type 2 diabetes; LAEI, large artery elasticity index; SAEI, small artery elasticity index; SVR, systemic vascular resistance; TVI, total vascular impedance.
There were 3 subjects with T2D for whom LAEI was > 30. Excluding them from the analysis did not change substantially the results for executive function and LAEI (partial r = 0.42, p = 0.02) and for executive function with TVI (partial r = −0.37, p = 0.04), reflecting the robustness of the results. In addition, for 70% of the subjects, we had data on creatinine, triglycerides, total cholesterol, and smoking. Results of analysis including these variables were essentially unchanged (data not shown).
DISCUSSION
In cognitively normal very old male subjects, significant interactions were demonstrated between measures of AWF and T2D in executive functions and language. Higher large arterial compliance and lower total vascular impedance were significantly associated with better executive functions performance in T2D but not non-T2D subjects. Lower total vascular impedance was also more associated with better language performance in T2D than non-T2D subjects. These analyses accounted for socio-demographic and cardiovascular factors that have been associated with cognition and with vascular disease [22]. The cognitive domain most strongly involved in this relationship, executive functions, is consistent with a profile of vascular insults to the brain [23].
The cognitive impairment observed in T2D has previously been attributed to neurodegenerative and to cerebrovascular mechanisms [24]. However, the structural component of these mechanisms, as detected by MRI scans, cannot fully explain the lower cognitive performance observed in T2D, suggesting the involvement of additional factors [24]. Measurement of AWF can demonstrate the functional precedents of structural vascular pathology. Moreover, AWF may also contribute to neurodegeneration [6],[25] and brain atrophy [25]. Thus its assessment may enhance understanding of the direct and indirect roles of functional vascular pathology in T2D-related cognitive compromise.
Previous cross-sectional studies showed that higher pulse wave velocity (PWV)—a marker of arterial wall stiffness—was associated with lower cognitive performance, neurodegenerative and vascular [26] pathologies in stroke- and dementia-free subjects. PWV was significantly higher in subjects with dementia or mild cognitive impairment compared with normal cognition [27]. Higher PWV was associated with lower scores in episodic memory in cognitively normal subjects aged 45–65[28] and with lower performance in executive functions, and—in contrast to our results—worse verbal episodic memory (primarily affected by AD) in similarly aged T2D subjects [29]. In non-T2D, untreated hypertensive subjects, lower MMSE scores were associated with increased large artery stiffness [30]. In longitudinal studies, higher PWV was associated with a faster rate of cognitive decline in community dwelling older adults [31], very old institutionalized subjects [32] and AD patients [33]. Our study innovates by directly comparing large and small artery compliance measures in elderly with and without T2D and suggests that results of studies that included T2D patients or other subjects prone for vascular pathology (e.g., hypertensive subjects) may be primarily led by them. Lower cognitive performance, even within the normal range, is associated with increased risk for dementia [34]. Thus improvement of AWF, which is modifiable [35], could potentially contribute to prevention or postponement of dementia in elderly T2D subjects.
Worse glycemic control has previously been demonstrated to be associated with increased arterial stiffness [36]. In the present study, T2D subjects had higher large and small artery elasticity, perhaps reflecting more aggressive treatment of other factors affecting arterial stiffness (e.g., hypertension) as advised by international clinical practice guidelines which recommend lower target blood pressure levels in T2D compared to non-T2D subjects [37]. Such an approach may underlie the observed lower values of diastolic blood pressure in T2D subjects participating in the current study despite higher prevalence of hypertension diagnosis. The differences between T2D and non-T2D subjects in the relationship of cognitive performance with worse AWF have not been reported previously. Hyperglycemic and insulin resistance have been more strongly associated with cognitive performance in T2D subjects compared to non-T2D subjects supporting our findings, and, perhaps pointing to a greater vulnerability of the brain in T2D [38]. The mechanisms underlying this vulnerability are beyond the scope of the present study.
Increased intracranial vascular resistance has previously been shown to be associated with poorer cognitive performance in dementia-free individuals [39]. Thus, use of peripheral rather than central nervous system measures of AWF is a limitation of the present study. Nevertheless, the heterogenic effect of vasculopathy on different organs, as demonstrated by the 10-year difference between the peak incidence of myocardial infarction and that of stroke [40, 41], suggests that cerebral vessels are affected at a later stage than coronary vessels and stresses the relevance of the relationship of peripheral extra-cranial vessels function with cognition. An additional limitation is lack of data on carotid artery stenosis, previously demonstrated to be associated with cognitive function, independently of intracranial vascular changes (e.g., silent MRI infarcts, white matter lesions) [39, 42–44], and lack of structural imaging of brain parenchyma and vasculature, precluding any conclusions about the relationship of peripheral AWF with these factors. The study included only male subjects, limiting the generalizability of the findings to women. Nevertheless, this cohort pertains to the very elderly—the most rapidly growing segment of the population [45] with the highest rates of cognitive decline and dementia [46], and thus of particular importance when developing tailored dementia preventive strategies.
Acknowledgments
This study was supported by the American Federation for Aging Research (AFAR), Young investigator award 2011 and NIRG-11-205083 Alzheimer’s Association, 2012 to Dr. Ravona-Springer, NIA grant R01 AG034087 to Dr. Beeri, NIA-AG02219 (VH), the Helen Bader Foundation and the Irma T. Hirschl Scholar Award as well as the Leroy Schecter Foundation Award to Dr. Beeri, National Institute of Aging grant P01-AG02219 (to Mary Sano), a Merit Award to Dr. Silverman from the United States Department of Veterans Affairs.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=2561) .
References
- 1.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 2.Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: A population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 3.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 4.Reijmer YD, van den Berg E, de Bresser J, Kessels RP, Kappelle LJ, Algra A, Biessels GJ. Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev. 2011;27:195–202. doi: 10.1002/dmrr.1163. [DOI] [PubMed] [Google Scholar]
- 5.Reijmer YD, Leemans A, Brundel M, Kappelle LJ, Biessels GJ. Disruption of the cerebral white matter network is related to slowing of information processing speed in patients with type 2 diabetes. Diabetes. 2013;62:2112–2115. doi: 10.2337/db12-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Munch G, Wood AG, Forbes J, Greenaway TM, Pearson S, Srikanth V. Brain atrophy in type 2 diabetes: Regional distribution and influence on cognition. Diabetes Care. 2013;36:4036–4042. doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi R, Nuzzo A, Olaru AI, Origliani G, Modena MG. Endothelial function affects early carotid atherosclerosis progression in hypertensive postmenopausal women. J Hypertens. 2011;29:1136–1144. doi: 10.1097/HJH.0b013e328345d950. [DOI] [PubMed] [Google Scholar]
- 8.Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulesdi B, Limburg M, Bereczki D, Kaplar M, Molnar C, Kappelmayer J, Neuwirth G, Csiba L. Cerebrovascular reactivity and reserve capacity in type II diabetes mellitus. J Diabetes Complications. 1999;13:191–199. doi: 10.1016/s1056-8727(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 10.Kadoi Y, Saito S, Goto F, Fujita N. The effect of diabetes on the interrelationship between jugular venous oxygen saturation responsiveness to phenylephrine infusion and cerebrovascular carbon dioxide reactivity. Anesth Analg. 2004;99:325–331. doi: 10.1213/01.ANE.0000132693.69567.70. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: The CERAD experience. Consortium to Establish a Registry for Alzheimer’s Disease. Aging (Milano) 1996;8:379–385. doi: 10.1007/BF03339599. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 15.Zimlichman R, Shargorodsky M, Boaz M, Duprez D, Rahn KH, Rizzoni D, Payeras AC, Hamm C, McVeigh G. Determination of arterial compliance using blood pressure waveform analysis with the CR-2000 system: Reliability, repeatability, and establishment of normal values for healthy European population–the seven European sites study (SESS) Am J Hypertens. 2005;18:65–71. doi: 10.1016/j.amjhyper.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Beeri MS, Schmeidler J, Sano M, Wang J, Lally R, Grossman H, Silverman JM. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology. 2006;67:1006–1010. doi: 10.1212/01.wnl.0000237548.15734.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cauthen NR. Verbal fluency: Normative data. J Clin Psychol. 1978;34:126–129. doi: 10.1002/1097-4679(197801)34:1<126::aid-jclp2270340129>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.LaBarge E, Edwards D, Knesevich JW. Performance of normal elderly on the Boston Naming Test. Brain Lang. 1986;27:380–384. doi: 10.1016/0093-934x(86)90026-x. [DOI] [PubMed] [Google Scholar]
- 19.Shipley WC. A self-administering scale for measuring intellectual impairment and deterioration. J Psychol. 1940;9:371–377. [Google Scholar]
- 20.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 21.Diller L. Studies in cognition and rehabilitation in hemiplegia (Rehabilitation monograph) New York: 1974. [Google Scholar]
- 22.Zeki Al Hazzouri A, Haan MN, Neuhaus JM, Pletcher M, Peralta CA, Lopez L, Perez Stable EJ. Cardiovascular risk score, cognitive decline, and dementia in older Mexican Americans: The role of sex and education. J Am Heart Assoc. 2013;2:e004978. doi: 10.1161/JAHA.113.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66:545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- 24.Qiu C, Sigurdsson S, Zhang Q, Jonsdottir MK, Kjartansson O, Eiriksdottir G, Garcia ME, Harris TB, Vanbuchem MA, Gudnason V, Launer LJ. Diabetes, markers of brain pathology, and cognitive cognition: The AGES-Reykjavik study. Ann Neurol. 2014;75:138–146. doi: 10.1002/ana.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cumming TB, Brodtmann A. Can stroke cause neurodegenerative dementia? Int J Stroke. 2011;6:416–424. doi: 10.1111/j.1747-4949.2011.00666.x. [DOI] [PubMed] [Google Scholar]
- 26.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- 28.Pase MP, Pipingas A, Kras M, Nolidin K, Gibbs AL, Wesnes KA, Scholey AB, Stough C. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J Hypertens. 2010;28:1724–1729. doi: 10.1097/HJH.0b013e32833b1ee7. [DOI] [PubMed] [Google Scholar]
- 29.Mehrabian S, Raycheva M, Gateva A, Todorova G, Angelova P, Traykova M, Stankova T, Kamenov Z, Traykov L. Cognitive dysfunction profile and arterial stiffness in type 2 diabetes. J Neurol Sci. 2012;322:152–156. doi: 10.1016/j.jns.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 30.Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, Trivilou P, Zerva L, Stamboulis E, Kremastinos DT. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am J Hypertens. 2009;22:525–530. doi: 10.1038/ajh.2009.35. [DOI] [PubMed] [Google Scholar]
- 31.Zeki Al Hazzouri A, Newman AB, Simonsick E, Sink KM, Sutton Tyrrell K, Watson N, Satterfield S, Harris T, Yaffe K. Pulse wave velocity and cognitive decline in elders: The Health, Aging, and Body Composition study. Stroke. 2013;44:388–393. doi: 10.1161/STROKEAHA.112.673533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benetos A, Watfa G, Hanon O, Salvi P, Fantin F, Toulza O, Manckoundia P, Agnoletti D, Labat C, Gautier S. Pulse wave velocity is associated with 1-year cognitive decline in the elderly older than 80 years: The PARTAGE study. J Am Med Dir Assoc. 2012;13:239–243. doi: 10.1016/j.jamda.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, Moffa F, Provinciali L, Vernieri F. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006;37:1010–1015. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein G, Beiser AS, Decarli C, Au R, Wolf PA, Seshadri S. Brain imaging and cognitive predictors of stroke and Alzheimer disease in the Framingham Heart Study. Stroke. 2013;44:2787–2794. doi: 10.1161/STROKEAHA.113.000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anfossi G, Russo I, Bonomo K, Trovati M. The cardiovascular effects of metformin: Further reasons to consider an old drug as a cornerstone in the therapy of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2010;8:327–337. doi: 10.2174/157016110791112359. [DOI] [PubMed] [Google Scholar]
- 36.Lukich E, Matas Z, Boaz M, Shargorodsky M. Increasing derangement of glucose homeostasis is associated with increased arterial stiffness in patients with diabetes, impaired fasting glucose and normal controls. Diabetes Metab Res Rev. 2010;26:365–370. doi: 10.1002/dmrr.1086. [DOI] [PubMed] [Google Scholar]
- 37.Rabi DM, Padwal R, Tobe SW, Gilbert RE, Leiter LA, Quinn RR, Khan N. Risks and benefits of intensive blood pressure lowering in patients with type 2 diabetes. CMAJ. 2013;185:963–967. doi: 10.1503/cmaj.120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Euser SM, Sattar N, Witteman JC, Bollen EL, Sijbrands EJ, Hofman A, Perry IJ, Breteler MM, Westendorp RG. A prospective analysis of elevated fasting glucose levels and cognitive function in older people: Results from PROSPER and the Rotterdam Study. Diabetes. 2010;59:1601–1607. doi: 10.2337/db09-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Oloriz J, Lopez-Cancio E, Arenillas JF, Hernandez M, Jimenez M, Dorado L, Barrios M, Soriano-Raya JJ, Miralbell J, Caceres C, Fores R, Pera G, Davalos A, Mataro M. Asymptomatic cervicocerebral atherosclerosis, intracranial vascular resistance and cognition: The AsIA-neuropsychology study. Atherosclerosis. 2013;230:330–335. doi: 10.1016/j.atherosclerosis.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Drouet L. Atherothrombosis as a systemic disease. Cerebrovasc Dis. 2002;13(Suppl 1):1–6. doi: 10.1159/000047782. [DOI] [PubMed] [Google Scholar]
- 41.Rosengarten B, Grebe M, Muller A, Voss RK, Kaps M. Severity of coronary artery disease but not degree of coronary stenosis is correlated to cerebrovascular reactivity. Cerebrovasc Dis. 2009;28:290–297. doi: 10.1159/000228712. [DOI] [PubMed] [Google Scholar]
- 42.Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bonaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis: The Tromso Study. Neurology. 2004;62:695–701. doi: 10.1212/01.wnl.0000113759.80877.1f. [DOI] [PubMed] [Google Scholar]
- 43.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, Au R, DeCarli C, Wolf PA. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: The Framingham study. Stroke. 2009;40:1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong W, Cruickshanks KJ, Huang GH, Klein BE, Klein R, Nieto FJ, Pankow JS, Schubert CR. Carotid atherosclerosis and cognitive function in midlife: The Beaver Dam Offspring Study. Atherosclerosis. 2011;219:330–333. doi: 10.1016/j.atherosclerosis.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Population Division USCB. NP2008 D1: Projected population by single year of age, sex, race, and Hispanic origin for the United States: 2000 to 2050. 2008. [Google Scholar]
- 46.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: The 90+study. Ann Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]