Abstract
The present study investigated the effect of a nine-month physical activity (PA) intervention on children’s cardiorespiratory fitness levels and neuroelectric indices of conflict monitoring (i.e., error-related negativity [ERN]). Four hundred twenty-eight preadolescent children (8–9 years old) were randomized into a PA intervention or wait-list control group, and completed a fitness and cognitive control assessment (i.e., modified flanker task) at pre- and post-test. Following exclusion criterion, three hundred eight children were included in the analyses (PA intervention: n = 139; wait-list control: n = 169). Children in the intervention displayed greater improvements in fitness and response accuracy, which were accompanied by stability of ERN amplitude from pre- to post-test. In contrast, the control group revealed increased ERN amplitude at post-test compared to pre-test, despite no change in fitness or task performance. These findings demonstrate the efficacy of daily PA for promoting children’s fitness and underlying neural processes associated with effective conflict monitoring. Such findings have significant implications for promoting organized PA programs intended to foster overall physical and brain health in school age children.
Keywords: Exercise, Fitness, ERPs, ERN, Children
Research over the past decade is consonant in identifying physical activity (PA) as a marker for enhanced brain health and cognitive control performance (i.e., top-down goal directed behavior) in developing youth (Erickson, Hillman & Kramer, 2015). The implications surrounding these findings have considerable momentum for altering educational practice; yet current trends demonstrate that PA opportunities have been obviated from the school day (e.g., > 44% of school districts in the US report reductions in physical education classes; Centers for Education Policy, 2007). Such trends may be maladaptive for underlying brain processes that support academic success in the classroom. However, only a small number of randomized controlled trials (RCT) have utilized neuroimaging techniques to better understand functional brain mechanisms underlying these PA-induced changes in cognition (Chaddock-Heyman et al., 2013; Davis et al., 2011; Hillman et al., 2014; Kamijo, Pontifex, O’Leary, Scudder, Wu, Castelli, & Hillman, 2011; Krafft et al., 2014). Therefore, additional evidence is needed to establish strong empirical support for the importance of PA to cognitive and brain health, and provide the necessary rationale for driving public health change in developing youth.
Here, event related potentials (ERPs), recorded during a modified flanker task, were utilized to investigate the effect of a PA intervention on the neural underpinnings of conflict monitoring; one aspect of cognitive control (Larson, Clayson, & Clawson, 2014). Cognitive control encompasses a collection of higher-order cognitive operations responsible for guiding evaluative (i.e., conflict monitoring) and regulative (i.e., inhibition, attention) operations necessary for successful goal-directed behavior (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004). Regulative control includes the ability to allocate attention and adapt to a shifting environment by overriding prepotent responses to robust but irrelevant information (Bunge, Dudukovic, Thomason, Vaidya, & Gabrielli, 2002; Davidson, Amso, Anderson, & Diamond, 2006). Such processes have been found amenable to PA intervention in children (Hillman et al., 2014). For example, in a previous ERP investigation, preadolescent children assigned to a nine-month PA intervention demonstrated improved performance on a flanker task, with concomitant increases in neuroelectric measures of attentional resource allocation (i.e., P3-ERP component amplitude; Hillman et al., 2014). Such findings indicate that daily PA improves functional indices of cognitive control; specifically, attentional resource allocation, with associated behavioral improvements. However, this prior report focused primarily on regulative cognitive control operations and associated underlying functional processes (i.e., the P3 component). Accordingly, the present study sought to assess the effect of PA-induced changes in evaluative control processes via neuroelectric indices of conflict monitoring. Such findings will provide new insight into the link between PA engagement with frontally-mediated cognitive development and brain health in preadolescent children.
Effective cognitive control depends on the ability to detect conflict and make appropriate adjustments to improve subsequent behavior (i.e., conflict monitoring; Botvinick, et al., 2001, 2004; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999). The anterior cingulate cortex (ACC) represents a main cortical contributor of these processes with neuroimaging investigations identifying the error-related negativity (ERN) as a neural marker of ACC conflict monitoring (Brazdil, Roman, Daniel, & Rektor, 2005). The ERN is a response-locked negative deflection (most prominent over the ACC or front of the scalp) often engendered by an error of commission. While prior research has suggested that the ERN component represents reinforcement learning of error detection (Holroyd & Coles, 2002), other work has indicated that the ERN represents the concurrent activation of multiple competing response options (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Coles, Meyer, & Donchin, 1990). For instance, when conflict awareness is heightened during task instructions that emphasize response accuracy over speed (Gehring, Goss, Coles, Meyer, & Donchin, 1993; Yeung, Botvinick, & Cohen, 2004), ERN amplitude is upregulated suggesting greater salience of an error and the allocation of cognitive control to effectively manage sources of conflict (Larson, et al., 2014). Other modifications in task parameters that increase awareness to conflicting response options (i.e., spacing of flanking arrows or increasing size of stimuli; Danielmeier, Wessel, Steinhauser, & Ullsperger, 2009; Maier, di Pellegrino, & Steinhauser, 2012) also necessitate upregulation of cognitive control as reflected by larger ERN amplitude (Larson, et al., 2014). Conversely, under conditions of greater conflict, individual differences revealing a decrease in ERN amplitude may suggest a lower threshold for conflict detection and subsequent error signaling (Botvinick, et al., 2001; Carter, Macdonald, Botvinick, Ross, Stenger, Noll, & Cohen, 2000).
Pertinent to this investigation, Themanson and colleagues (2006, 2008) evaluated the relation of cardiorespiratory fitness to ERN amplitude and flanker performance in young adults under conditions that modulated error salience. In a cross-sectional study, they observed smaller ERN amplitude for higher-fit compared to their lower-fit peers when task parameters emphasized speed over accuracy. In contrast, when accuracy was emphasized, higher fitness levels were associated with increased ERN amplitude and improvements in task performance. Additionally, findings revealed greater change in ERN amplitude associated with higher fitness when comparing between task conditions (accuracy – speed). Collectively, these data suggest that higher fitness levels are associated with more efficient adjustments in conflict monitoring. That is, when error salience was heightened through task instruction, greater fitness was associated with efficient adjustments in top-down control necessary for appropriate corrective behavior.
However, recent investigations in children appear contrary to the ERN outcomes observed in young adults (Themanson, et al., 2006, 2008). Specifically, findings in youth samples indicate that higher-fit children exhibit smaller ERN amplitude relative to lower-fit children when task instructions emphasize response accuracy (Pontifex et al., 2011). However, cognitive development research suggests that adults are more effective at modulating response selection (i.e., reaction time) across difficult trial types to maintain response accuracy. Conversely, children demonstrate a more impulsive response selection; thus, response accuracy measures more precisely reflect up-regulation in inhibitory control processes in younger populations (Christakou, Halari, Smith, Ifkovits, Brammer, & Rubia, 2009; Davidson et al., 2006). Therefore, not surprising when accuracy is emphasized during task instruction in children, the effects of fitness on ERN demonstrate similar modulation patterns to that of previous adult investigations (emphasizing speed; Themanson et al., 2006), revealing smaller ERN amplitude for higher-fit compared to lower-fit children (Pontifex et al., 2011). These findings in children, together with the adult findings, affirm the relation of fitness to efficient adjustments in top-down control and further suggests that aerobic fitness may serve to influence underlying neural processes responsible for effective conflict monitoring and cognitive control operations across the lifespan. However, the directionality of the fitness-ERN relationship appears to be susceptible to multiple complex factors including stimulus characteristics, task instructions, and age.
Developmental factors also warrant consideration in the present investigation given the protracted development of the ERN and underlying cortical contributors (i.e., ACC) in young populations. Prior research demonstrates marked ACC development and greater increases in ERN amplitude occurring during late adolescent periods and into adulthood (Crone, Zanolie, Van Leijenhorst, Westenberg, & Rombouts, 2008; Davies, Segalowitz, & Gavin, 2004; Adleman et al., 2002; Van Bogaert, Wikler, Damhaut, Szliwowski, & Goldman,1998; Segalowitz, Santesso, Murphy, Homan, Chantziantoniou, & Khan, 2010). However, the relation of larger ERN amplitude with age is not linear among pre-pubertal children and typically reveals maintenance of amplitude during the few years prior to pubertal onset (Meyer, Weinberg, Klein, & Hajcak, 2012; van Meel, Heslenfeld, Rommelse, Oosterlaan, & Sergeant, 2012). For example, Meyer and colleagues (2012) evaluated ERN in children 8–13 and found a non-significant trend for larger ERN amplitude with age only among older cohorts of children. Similar findings revealed no difference in ERN amplitude between younger (6–9 years old) and older (10–12 years old) pre-pubertal children (van Meel et al., 2012). Thus, based on these data, developmental factors in the present investigation are expected to be negligible given the young, pre-pubescent age (8–9 years old) and relatively short period between pre- and post-test assessments (nine-months). However, a robust literature base of individual differences research reveals greater increases in ERN amplitude in children with obsessive-compulsive disorder (Hajcak, Franklin, Foa, & Simons, 2008) and clinical anxiety disorder compared to healthy cohorts (Meyer et al., 2012; Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006), suggesting a maladaptive hyperactive error monitoring system during development (McDermott, Perez-Edgar, Henderson, Chronis-Tuscano, Pine, & Fox, 2009; Olvet & Hajcak, 2008). Taken together, younger children appear to maintain ERN amplitude with further evidence suggesting that greater increases in ERN may serve as a marker of atypical cognitive or psychological development. However, the investigation of other individual differences of health factors (i.e., physical activity) remain mostly unexplored.
The present investigation employed a nine-month afterschool PA intervention (Fitness Improves Thinking in Kids [FITKids]) to assess its impact on changes in children’s cardiorespiratory fitness, neuroelectric indices of conflict monitoring (i.e., ERN amplitude), and associated task performance during a flanker task that modulated inhibitory control. We predicted that improvements in fitness engendered by the PA intervention would foster similar differences in task performance and ERN amplitude observed in earlier cross-sectional investigations in children. That is, given task instructions emphasizing accuracy and speed (i.e., increased error salience), we predicted improvements in task performance while the ERN would coincide with developmental and fitness research demonstrating maintenance or a reduction in amplitude associated with greater increases in fitness following the PA intervention compared to a wait-list control group. Such findings may further promote public health programming regarding the effects of physical activity on cognitive and brain health in young children.
Method
The present investigation constitutes the evaluation of a subset of children who participated in either the FITKids (ClinicalTrials.gov: NCT01619826) or the FITKids2 (ClinicalTrials.gov: NCT01334359) clinical trials, and for simplicity sake will be identified as the FITKids intervention throughout. Accordingly, this investigation represents a secondary analysis of the previously published FITKids dataset (Hillman et al., 2014). In the previous paper, correct trial RT and response accuracy were reported along with the P3-ERP measure outcomes. In this secondary analysis, our aim was to assess conflict monitoring using the ERN and error-trial task performance. Task performance data reported herein from the subset of participants that overlap with Hillman et al. (2014) are presented only to better inform the novel ERN findings.
Study Design
The purpose of the FITKids intervention was to provide occasion for children to engage in moderate-to-vigorous PA for at least 70-minutes per weekday for one school year (nine months) with the intention of improving cardiorespiratory fitness. This was accomplished by randomizing all consenting participants into either the PA intervention or a wait-list control group following pre-test assessment. A trained staff member accomplished the randomization procedure following baseline pre-testing. Pairs of participants were matched on baseline demographics (age, gender, race, SES, IQ, and VO2) and then assigned to respective groups based on a coin flip. Additionally, group assignment was blinded for analyses by indicating ‘1’ or ‘0’ associated with each participant ID number. Those randomized to the wait-list group were guaranteed placement in the afterschool intervention the following school year (but not tested). Group assignment was blinded to all staff members involved with data collection. Pre-test and post-test measures were collected over a 2-day period that circumscribed academic years 2009 to 2016.
Afterschool Physical Activity Intervention
The FITKids intervention used a modified version of the American Academy of Pediatrics Community Access To Child Health (CATCH; www2.aap.org/catch/) program developed by the National Institutes of Health. This program is structured such that daily lessons provide intermittent bouts of PA integrated with educational lessons intended to facilitate and encourage healthy lifestyle behaviors outside the program. Additionally, the efficacy of the CATCH program has been demonstrated in prior research to reduce unhealthy weight status (Coleman, Tiller, Sanchez, Heath, Sy, Milliken, & Dzewaltowski, 2005) and increase PA behaviors among young children (Luepker et al., 1996).
Utilizing the CATCH curriculum, the FITKids intervention occurred every weekday (in accordance with the annual academic calendar for local public school districts) following completion of regular school hours. Children were either bussed or guardian-driven to a designated gymnasium rented from the University of Illinois Campus Recreation. During the intervention, children were provided at least 70-minutes of moderate-to-vigorous PA during a 2-hour period per day with intensity monitored utilizing Polar heart rate (HR) monitors (Polar E600, Polar Electro, Finland) worn for the duration of the program. Wait-list control children were instructed to maintain daily/normal activities between the nine-month pre- and post-test assessments; thus, providing un-intervened representation of physical and cognitive development in our sample.
Participants
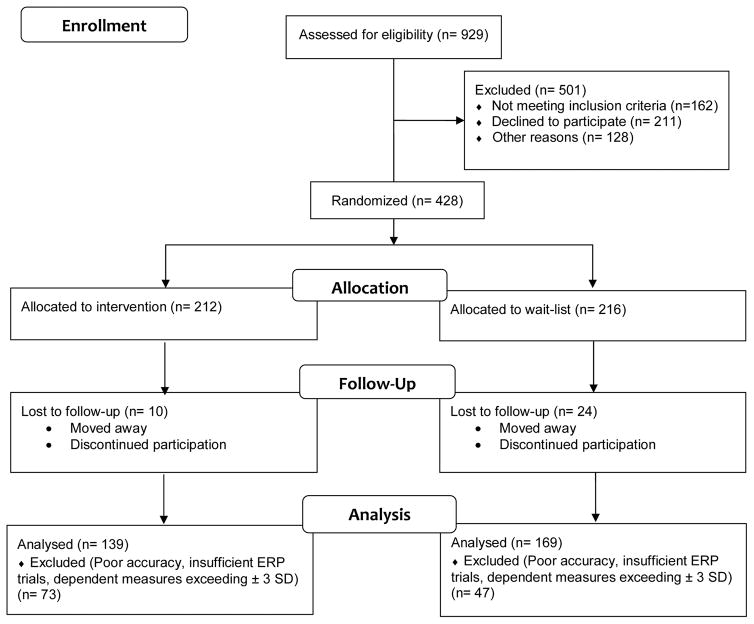
Nine hundred twenty-nine children were screened and 428 children were randomly assigned to either the intervention or wait-list (see Figure 1). All participants provided written informed assent and their legal guardians provided written informed consent in accordance with the Institutional Review Board (IRB) of the University of Illinois at Urbana-Champaign. Prior to testing, legal guardians completed a battery of health history and demographic questionnaires on behalf of the participant. Based on these questionnaires, all participants included in the analyses had not received special educational services from their school in connection with learning or attentional disorders, were free of neurological or physical disabilities, and had (corrected-to-) normal vision based on the minimal 20/20 standard.
Figure 1.
Flow diagram of the FITKids intervention.
Procedure
Pre- and post-test protocols were identical with each assessment divided across two separate days. All participants, on their first visit to the laboratory, completed informed assent/consent, preliminary screening, demographic information, intelligent quotient (IQ) assessment, and the cardiorespiratory fitness assessment. On the second laboratory visit, participants were fitted with an EEG cap and seated in a quiet testing chamber to complete the flanker task. All participants were instructed on appropriate completion of the flanker task and afforded practice prior to testing. Upon completion of post-testing, participants were briefed on the purpose of the study and received remuneration at a rate of $10/hour for their participation.
Demographic assessment
A dichotomous socioeconomic status (SES) index was used based on highest level of education obtained by the female guardian (Ogunwole, Drewery, & Rios-Vargas, 2012). Low SES was determined if the female guardian had no high school degree, a high school degree, or some college experience. High SES was determined if the female guardian obtained a bachelor or advanced degree. Hand dominance was determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Pubertal timing was determined using the Tanner Staging System Questionnaire (Tanner, 1962; Taylor, Whincup, Hindmarsh, Lampe, Odoki, & Cook, 2001), which was completed by a legal guardian in collaboration with the participant. Body mass index (BMI) was calculated as weight divided by the square of height (i.e., kg/m2). Per the Centers for Disease Control and Prevention BMI-for-age growth charts (Kuczmarski et al., 2002), children who were ≥85th percentile were classified as overweight, children who were ≥95th percentile were classified as obese. Lastly, an estimate of IQ was collected based on an age-normed standardized IQ examination, which was administered by a trained experimenter. IQ was assessed using the composite subtests of the Kaufman Brief Intelligence Test (K-BIT; Kaufman & Kaufman, 1990) and the Woodcock-Johnson III Test of Cognitive Abilities (WJ-III; Woodcock, McGrew, & Mather, 2001) with a standard score of 100 representing average IQ with a standard deviation of 15.
Cardiorespiratory fitness assessment
Each participant was fitted with a Polar heart rate (HR) monitor (Polar WearLink®+ 31, Polar Electro, Finland) and measurements of height and weight were recorded (stadiometer and a Tanita WB-300 Plus digital scale) prior to the fitness assessment test. A modified Balke Protocol (American College of Sports Medicine [ACSM], 2014) was used while participants ran on a motor driven treadmill (Life Fitness 93T classic; Brunswick Corporation, Schiller Park, IL, USA) at a constant speed. Sequentially, at two-minute intervals, treadmill incline increased 2.5% and the OMNI scale was used to collect ratings of perceived exertion (RPE; Utter, Robertson, Nieman, & Kang, 2002). Running was maintained until volitional exhaustion was achieved. Maximal aerobic capacity (VO2peak) was measured during treadmill running by a computerized indirect calorimetry system (ParvoMedics True Max 2400) with averages of respiratory exchange ratio (RER) and oxygen uptake (VO2) assessed every 20 seconds. Relative peak oxygen consumption was expressed in milliliters of oxygen consumed per kilogram of body weight per minute. VO2peak was based upon maximal effort as evidenced by a minimum of two of the following four criteria: 1) a plateau in oxygen uptake corresponding to an increase of less than 2 ml/kg·min−1 despite an increase in exercise workload; 2) a peak heart rate ≥ 185 beats per minute (ACSM, 2014) and a heart rate plateau (Freedson & Goodman, 1993); 3) RER ≥ 1.0 (Bar-Or, 1983); and/or 4) ratings on the children’s OMNI scale of perceived exertion ≥ 8 (Utter et al., 2002).
Flanker Task
Participants completed a modified version of the Eriksen flanker task (Eriksen & Eriksen, 1974). All stimuli were presented focally on a computer screen at a distance of approximately 1 m using Neuroscan Stim software (Compumedics NeuroScan, Charlotte, NC). Participants were instructed to respond with a thumb press on a response pad (Neuroscan STIM system response pad) to the direction of the centrally presented target (3 cm tall goldfish on a blue background) amid either congruent (e.g.,
 or
or
 ) or incongruent (e.g.,
) or incongruent (e.g.,
 or
or
 ) flanking non-targets (identical goldfish). Task instructions and encouragement to participants prior to and following each task block emphasized response accuracy (i.e., “It is important that you respond as accurately as possible…”) with secondary instructions encouraging response speed to maintain responding within the allotted response window (i.e., “…but we also want you to respond quickly so please make sure you respond before the next set of fish appear on the screen.”). Prior to testing, participants were given 40 practice trials to control for potential practice effects. Two blocks of 75 trials were presented randomly with equiprobable congruency (i.e., 50% congruent trials) and directionality (50% of targets facing right) for 200 ms with a fixed inter-trial interval (ITI) of 1700 ms. These parameters were slightly modified during FITKids2 intervention such that trials were presented for 250 ms with random variable ITI of 1600, 1800, and 2000 ms.
) flanking non-targets (identical goldfish). Task instructions and encouragement to participants prior to and following each task block emphasized response accuracy (i.e., “It is important that you respond as accurately as possible…”) with secondary instructions encouraging response speed to maintain responding within the allotted response window (i.e., “…but we also want you to respond quickly so please make sure you respond before the next set of fish appear on the screen.”). Prior to testing, participants were given 40 practice trials to control for potential practice effects. Two blocks of 75 trials were presented randomly with equiprobable congruency (i.e., 50% congruent trials) and directionality (50% of targets facing right) for 200 ms with a fixed inter-trial interval (ITI) of 1700 ms. These parameters were slightly modified during FITKids2 intervention such that trials were presented for 250 ms with random variable ITI of 1600, 1800, and 2000 ms.
ERP Recording
Electroencephalographic (EEG) activity was recorded from 64 Ag/AgCl sintered electrode sites (FPz, Fz, FCz, Cz, CPz, Pz, POz, Oz, FP1/2, F7/5/3/1/2/4/6/8, FT7/8, FC3/1/2/4, T7/8, C5/3/1/2/4/6, M1/2, TP7/8, CB1/2, P7/5/3/1/2/4/6/8, PO7/5/3/4/6/8, O1/2) arranged in an extended montage according to the international 10-10 system (Chatrian, Lettich, & Nelson, 1985) using a Neuroscan Quickcap (Compumedics NeuroScan, Charlotte, NC). Prior to EEG recordings, electrode impedance was maintained at < 10 kΩ. Online, continuous data were referenced to a midline electrode placed at midpoint between Cz and CPz with AFz serving as the ground electrode. Additional electrodes were placed above and below the left orbit and outer canthus of each eye to monitor electrooculographic (EOG) activity with separate bipolar recordings to monitor vertical (VEOG) and horizontal (HEOG) activity. Continuous online data were digitized at a sampling rate of 500 Hz, amplified 500 times with a DC to 70 Hz filter, and a 60-Hz notch filter was applied using Neuroscan SynAmps2 amplifier.
Offline, continuous EEG data were processed utilizing Matlab (R2012b) and various toolbox plugins including EEGLAB (Delorme & Makeig, 2004) and ERPLAB (Lopez-Calderon & Luck, 2010). EEG data were re-referenced to averaged mastoids (M1, M2). Independent component analysis (ICA) were conducted to identify stereotypical eye-blink artifact (Comon, 1994) followed by an auto-correlation procedure developed for rejecting ICA components related to VEOG activity. This was accomplished by correlating point-by-point raw VEOG data with separate ICA activation waveforms (i.e., EEG.icaact matrix generated by the ICA procedure). No more than two ICA components with a correlation coefficient greater than 0.30 were removed.
Response-locked epochs were extracted for correct and commission-error trials (−600 to 1000 ms relative to response onset), baseline corrected (−400 to −200 ms), and bandpass filtered (zero phase shift 1 to 12 Hz with 24 dB/octave roll-off). Epochs were rejected if a moving window peak-to-peak amplitude exceeded 100 μV (100 ms window width and 50 ms window step) and if the overall variance of the epoch exceeded ± 3 SDs of the mean of local (by electrode site) and global (all electrode sites) accepted epochs. ERP average waveforms (averaged across congruent and incongruent trial types) were computed as the difference between commission-error (ERN) and correct (CRN) response-locked averages (ERNerror – correct) to account for artifact that may be due to activity beyond the confines of error-specific activity (Torpey, Hajcak, Kim, Kujawa, & Klein, 2012). The CRN, ERN, and ERNerror – correct components were characterized using a peak interval technique that measured the mean amplitude (30-ms window) encompassing the largest negative-going peak identified within a −50 to 100 ms latency window relative to response onset.
Statistical Analysis
Prior to statistical analyses, participants were excluded for insufficient response-locked trials (n = 75) necessary to characterize ERP components (< 6 commission error trials; Pontifex, Scudder, Brown, O’Leary, Wu, Themanson, & Hillman, 2010), poor performance on the flanker task (< 50% accuracy; n = 20), and dependent measures exceeding ± 3 SD (n = 25). Thus, statistical and multiple imputation procedures, utilizing SPSS (SPSS v. 22, Chicago, IL), were conducted on the remaining participants (n = 308; n = 151 stem from previously published work [Hillman et al., 2014]) following these exclusion criterions.
To account for missing data at post-test (~12%), all missing values were imputed with 20 iterations for each data point. Pre-test demographic measures, cardiorespiratory fitness, and BMI change scores [i.e. (post – pre)] were evaluated between wait-list and intervention groups with independent t-tests. Multivariate repeated measures MANOVAs were performed to evaluate the efficacy of the intervention with group as the between-subject factor. Findings are reported with a family-wise alpha threshold for all tests set at p = .05. Subsidiary univariate ANOVAs were used for post hoc procedures with contrasting p-values adjusted using the Sidak correction method for multiple comparisons (i.e., a reported p-value of .05 is significant). Further reporting included partial η2 and estimated effect size for main effects and interactions. Task performance (accuracy, median reaction time) were separately analyzed utilizing a 2 (group: intervention, wait-list) × 2 (time: pre-test, post-test) × 2 (type: congruent, incongruent) statistical model. Remaining behavioral analyses (i.e., commission and omission errors and error runs, post-error accuracy, post-error latency) were separately analyzed utilizing a 2 (group: intervention, wait-list) × 2 (time: pre-test, post-test) statistical model with decomposition of non-parametric interactions accomplished utilizing Mann-Whitney (between-subjects) and Wilcoxon signed-rank tests (within-subjects). ERP analyses were performed at single electrode sites based on scalp distribution and previous research indicating component maximum at fronto-central (FCz) site (Clayson, & Larson, 2012; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003). ERN, CRN, and ERNerror – correct were analyzed separately utilizing a 2 (group: treatment, wait-list) × 2 (time: pre-test, post-test) statistical model. Lastly, planned Pearson partial correlations were performed to evaluate the relation of change in fitness from pre- to post-test to task performance and ERN changes observed separately for each treatment group. This was accomplished by performing within-group partial correlations on change scores [(i.e., (post – pre)] while controlling for statistically correlated demographic and pre-test confounding factors including age, IQ, sex, pre-test BMI, and pre-test fitness. Lastly, previous analyses of the FITKids intervention (Hillman et al., 2014) demonstrated significant differences in flanker performance at baseline between groups, such that children in the intervention demonstrated poorer performance compared to children in the wait-list group. Thus, the present investigation performed confirmatory analyses on a subsample of participants matched on flanker accuracy at baseline to more accurately represent PA-induced changes unbiased by pre-test differences. This was accomplished by utilizing a case-controlled matching technique in SPSS pairing treatment and wait-list groups per overall flanker accuracy with a match tolerance set at five and treatment as the group indicator. Subsample analyses (treatment: n = 139; wait-list: n = 139) are reported post hoc.
Results
Participants
Means and standard error of the mean (± 1 SEM) are reported for demographic measures provided in Table 1. Analyses of baseline demographics revealed no significant differences between groups on measures of age, cardiorespiratory fitness, BMI, IQ, and pubertal timing [t’s (306) ≤ 1.43, p’s ≥ .15]. Children in the FITKids intervention attended 122 (± 1.7) sessions, which equated to an attendance rate of 85.4 % (± 0.8%). Analyses were conducted on the number of ERP error-trials to ensure that group differences were not influenced by trial count included in response-locked averages. Results revealed no main effect or interaction involving group [F’s (1, 306) ≤ 2.1, p’s ≥ 0.16, ηp2 ≤ 0.01] indicating an equivalent trial count across groups with an average of 21 (± 0.6) error trials per participant. Lastly, analysis of change scores revealed a 3-fold increase in fitness from pre- to post-test for the intervention group (pre: 39.1 ± 0.6 mL/kg/min, post: 40.9 ± 0.6 mL/kg/min; Δ1.8 ± 0.4 mL/kg/min; 5.4% change) compared to the wait-list group (pre: 39.9 ± 0.6 mL/kg/min; post: 40.5 ± 0.6 mL/kg/min; Δ0.6 ± 0.3 mL/kg/min; 2.1% change; see Table 1) [t (306) = 2.3, p = 0.02], demonstrating the efficacy of the PA intervention.
Table 1.
Mean (±1 SEM) values for participant demographic and fitness measures.
| Measure | Intervention | Wait-list |
|---|---|---|
| n | 139 | 169 |
| Females, n (%) | 71 (51) | 86 (51) |
| Race, n (%) | ||
| Asian | 17 (12) | 15 (9) |
| African American | 30 (22) | 37 (22) |
| White | 63 (46) | 88 (52) |
| Other or mixed | 28 (20) | 26 (15) |
| Hispanic | 13 (9) | 12 (7) |
| Low SES (%) | 55 (40) | 70 (41) |
| Overweight/obese (%) | 62 (45) | 77 (46) |
| Age (years) | 8.72 ± 0.05 a | 8.75 ± 0.04 a |
| IQ | 108.04 ± 1.18 a | 109.24 ±1.01 a |
| Puberty timing | 1.42 ± 0.04 a | 1.45 ± 0.04 a |
| ΔBMI (kg/m2) | 0.15 ± 0.11 a | 0.70 ± 0.19 b |
| ΔVO2max (mL/kg/min) | 1.8 ± 0.40 a | 0.61 ± 0.35 b |
| Days attended | 122 ± 1.68 | - |
| Attendance rate (%) | 85.4 ± 0.84 | - |
Note: Low SES was determined if the female guardian had no high school degree, a high school degree, or some college experience; IQ = intelligent quotient; BMI = body mass index; Data are presented as mean (±1 SEM) unless noted otherwise. Values sharing a common superscript are not statistically different at p ≤ .05.
Flanker Performance
Reaction Time
The omnibus analysis for RT revealed a main effect of time [F (1, 306) = 51.0, p ≤ 0.01, ηp2 = 0.14], demonstrating shorter RT at post-test (467.2 ± 5.2 ms) compared to pre-test (505.2 ± 6.5 ms) for all participants. In addition, a main effect of type was observed [F (1, 306) = 385.2, p ≤ 0.01, ηp2 = 0.56], indicating shorter RT for congruent (465.4 ± 5.0 ms) compared to incongruent (506.9 ± 5.7 ms) trials. No other main effects or interactions were observed for RT [F’s (1, 306) ≤ 2.1, p’s ≥ 0.15, ηp2’s ≤ 0.01].
Accuracy
The omnibus analysis for response accuracy revealed a main effect of type [F (1, 306) = 502.1, p ≤ 0.01, ηp2 = 0.62] indicating higher accuracy for congruent (80.5 ± 0.7%) compared to incongruent (72.8 ± 0.7%) trials. In addition, a main effect of time was observed [F (1, 306) = 118.1, p ≤ 0.01, ηp2 = 0.28], that was superseded by a group × time interaction [F (1, 306) = 5.3, p = 0.02, ηp2 = 0.02]. Decomposition of this interaction revealed increased response accuracy for both the wait-list and intervention groups at post-test (wait-list: 80.4 ± 0.9%, intervention: 80.5 ± 1.0%) compared to pre-test (wait-list: 74.4 ± 1.1%, intervention: 71.3 ± 1.2%; see Table 2) [F’s (1, 306) ≥ 40.7, p’s ≤ 0.01]. Between-group comparisons at pre- and post-test revealed higher accuracy for the wait-list group relative to the intervention group only at pre-test [F (1, 306) = 3.9, p = 0.05]. Although post-test measures were not significant between groups [F (1, 306) = 0.0, p = 0.93], change score analysis demonstrated greater change from pre- to post-test for the intervention group (9.3 ± 1.0%) compared to the wait-list group (6.0 ± 1.0%) [F (1, 306) = 5.1, p = 0.02]. No other main effects or interactions were observed for accuracy [F’s (1, 306) ≤ 1.6, p’s ≥ 0.21, ηp2 ≤ 0.01]. Lastly, subsampling results that matched participants between groups based on pre-test response accuracy scores were consonant with the above observed effects (i.e., greater change from pre- to post-test for the intervention group [F (1, 276) = 4.3, p = 0.04]). Confirming the success of the matching procedure, no significant difference in performance were observed at pre-test between groups [F (1, 276) = 2.9, p = 0.09] (see Table 2), suggesting that greater changes in flanker performance for the intervention group remain regardless of pre-test behavior differences.
Table 2.
Mean (±1 SEM) values for participant flanker task performance measures.
| Measure | Intervention
|
Wait-list
|
||
|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | |
| Reaction time (ms) | 498.8 ± 9.9a | 468.4 ± 7.5b | 507.0 ± 8.2a | 460.5 ± 7.3b |
| Accuracy (%) | 71.4 ± 1.3a | 80.5 ± 1.0b | 74.4 ± 1.0c | 80.4 ± 1.0b |
| Subsampling accuracy (%) | 71.4 ± 1.3a | 80.5 ± 1.0b | 74.1 ± 1.1a | 80.3 ± 1.0b |
| Commission errors (#) | 24.2 ± 1.0a | 17.3 ± 0.8b | 23.0 ± 1.0a | 17.2 ± 0.9b |
| Commission error runs (#) | 4.2 ± 0.3a | 2.6 ± 0.3b | 3.8 ± 0.3a | 2.6 ± 0.2b |
| Omission errors (#) | 19.6 ± 1.3a | 10.7 ± 0.9b | 16.1 ± 1.2a | 11.7 ± 0.8b |
| Omission error runs (#) | 3.7 ± 0.3a | 1.6 ± 0.2b | 2.6 ± 0.3a | 1.9 ± 0.2b |
| Post-error reaction time (ms) | 554.8 ± 11.9a | 496.5 ± 9.0b | 560.0 ± 10.6a | 492.4 ± 8.9b |
| Post-error accuracy (%) | 69.8 ±1.5a | 78.3 ± 1.5b | 71.8 ± 1.4a | 78.1 ± 1.6b |
Note: Values for each measure are collapsed across congruent and incongruent trials; Subsampling accuracy represents participants matched on flanker accuracy at baseline (treatment: n = 139; wait-list: n = 139); Values sharing a common superscript are not statistically different at p ≤ .05.
Error performance
The omnibus analysis for commission errors, commission error runs, omission errors, and omission error runs revealed a main effect of time [F’s (1, 306) ≥ 38.0, p’s ≤ 0.01, ηp2’s ≥ 0.11] with fewer errors and error runs at post-test (16.4 ± 0.6 commission errors, 2.5 ± 0.2 commission error runs, 10.7 ± 0.6 omission errors, 1.6 ± 0.1 omission error runs) compared to pre-test (22.5 ± 0.7 commission errors, 3.8 ± 0.2 commission error runs, 17.0 ± 0.8 omission errors, 3.0 ± 0.2 omission error runs). Additionally, only omission errors and omission error runs revealed an interaction of group × time [F’s (1, 306) ≥ 6.1, p’s ≤ 0.02, ηp2’s ≥ 0.02]. Decomposition of these interactions utilizing Mann-Whitney (between-subjects) and Wilcoxon signed-rank tests (within-subjects) for non-parametric comparisons revealed that the wait-list and intervention groups both exhibited greater omission errors and error runs at pre-test (intervention: 19.6 ± 1.3 omission errors, 3.7 ± 0.3 omission error runs; wait-list: 16.1 ± 1.2 omission errors, 2.6 ± 0.3 omission error runs) compared to post-test (intervention: 10.7 ± 0.9 omission errors, 1.6 ± 0.2 omission error runs; wait-list: 11.7 ± 0.8 omission errors, 1.8 ± 0.2 omission error runs; see Table 2) [W’s ≥ 4,527, z’s ≥ 3.7, p’s ≤ 0.01], with no differences observed between groups at pre- and post-test [U’s ≤ 12,754, z’s ≤ 1.5, p’s ≥ 0.13]. However, analyses of change scores revealed a greater reduction in omission errors and error runs from pre- to post-test for the intervention group (−8.5 ± 1.3 omission errors, −2.0 ± 0.3 omission error runs) compared to the wait-list group (−4.1 ± 1.2 omission errors, −0.7 ± 0.3 omission error runs) [F’s (1, 306) ≥ 6.1, p’s ≤ 0.02, ηp2’s ≥ 0.02].
The omnibus analysis for post-error accuracy and RT revealed a main effect of time [F’s (1, 306) ≥ 42.3, p ≤ 0.01, ηp2 ≥ 0.12] demonstrating increase accuracy and shorter RT at post-test (accuracy: 78.3 ± 1.0%, RT: 495.2 ± 6.2 ms) compared to pre-test (accuracy: 70.8 ± 1.0%, RT: 557.4 ± 8.0 ms; see Table 2). No main effect of group or interaction of group × time were revealed for post-error accuracy or RT [F’s (1, 306) ≤ 1.2, p’s ≥ 0.30, ηp2’s ≤ 0.01].
ERPs
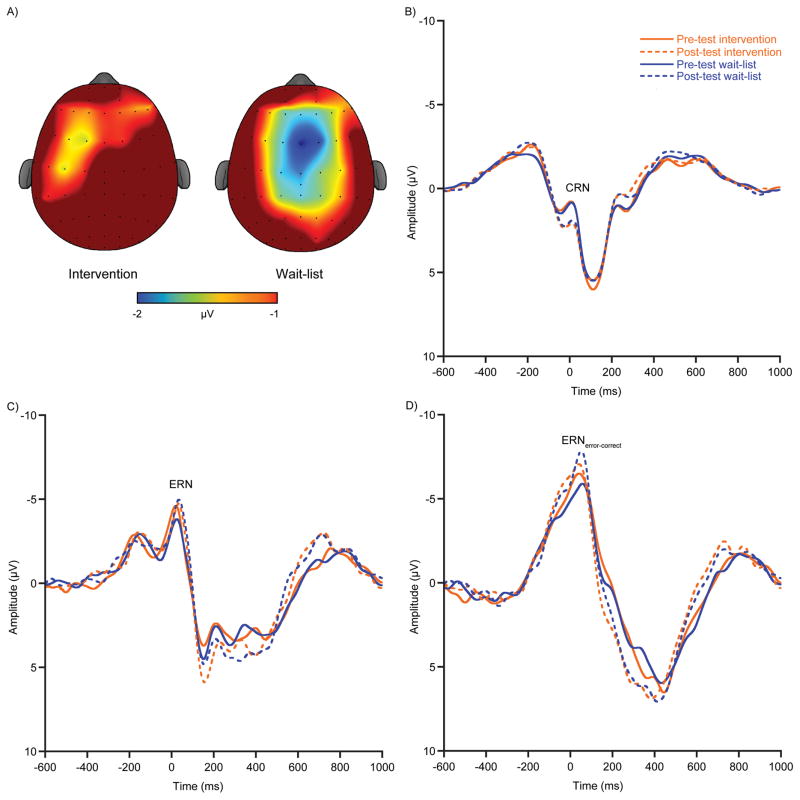
The omnibus analysis for CRN amplitude revealed a main effect of time [F (1, 306) = 9.9, p ≤ 0.01, ηp2 = 0.03] demonstrating larger negative-going amplitude at pre-test (0.4 ± 0.2 μV) compared to post-test (1.0 ± 0.2 μV). No main effect of group or interaction of group × time was observed [F’s (1, 306) ≤ 0.2, p’s ≥ 0.70, ηp2’s ≤ 0.01]. The omnibus analyses for ERN and ERNerror – correct amplitude revealed a main effect of time [F’s (1, 306) ≥ 5.6, p’s ≤ 0.04, ηp2’s ≥ 0.02] that was superseded by a group × time interaction [F’s (1, 306) ≥ 4.4, p’s ≤ 0.05, ηp2’s ≥ 0.01]. Decomposition of the interaction revealed larger negative-going ERN and ERNerror – correct amplitude at post-test (ERN: −6.3 ± 0.4 μV; ERNerror – correct: −9.9 ± 0.5 μV) compared to pre-test (ERN: −5.0 ± 0.3 μV; ERNerror – correct: −7.7 ± 0.3 μV), only for the wait-list group [F’s (1, 306) ≥ 9.6, p’s ≤ 0.01]. Conversely, the intervention group demonstrated stability of ERN amplitude with no differences observed from pre-test (ERN: −5.7 ± 0.3 μV; ERNerror – correct: −8.5 ± 0.4 μV) to post-test (ERN: −5.8 ± 0.4 μV; ERNerror – correct: −9.0 ± 0.4 μV; see Figure 2) [F’s (1, 306) ≤ 0.6, p’s ≥ 0.46]. Additionally, analyses of change scores revealed increased negative-going ERN amplitude from pre- to post-test only for the wait-list group (ΔERN: −1.1 ± 0.5 μV; ΔERNerror – correct: −2.1± 0.5 μV) with no such effect observed for the intervention group (ΔERN: −0.1 ± 0.4 μV; ΔERNerror – correct: −0.5 ± 0.4 μV; see Figure 2a) [F’s (1, 306) ≥ 10.2, p’s ≤ 0.01]. Lastly, subsampling results revealed no effect for ΔERN [F (1, 276) = 3.39, p = 0.13]); however, ΔERNerror – correct results were consonant with the initially observed effects [F (1, 276) = 8.2, p = 0.02], indicating greater changes in ERNerror – correct from pre- to post-test for the wait-list group regardless of task performance differences at pre-test.
Figure 2.
A) Topographic scalp distribution of change in ERNerror-correct amplitude from pre- to post-test (i.e., [post-pre]) by intervention (left) and wait-list (right) groups (time window plotted between −2 and −1 μV) for commission errors committed during the flanker task. B) Response-locked grand-average waveforms at site FCz for CRN, C) ERN, D) and ERNerror-correct separated by group and pre- and post-test.
Correlation
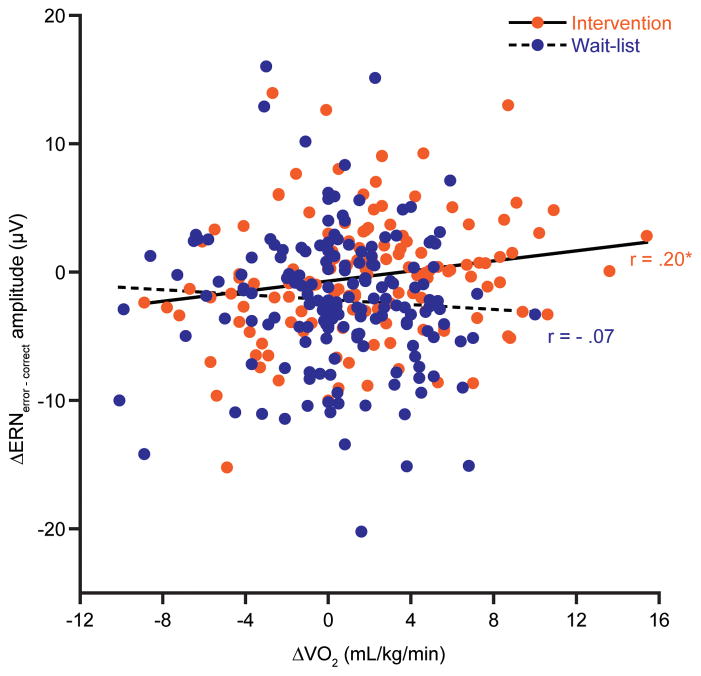
Planned Pearson partial correlations for the intervention group revealed a significant association with increases in fitness related to decreases in ERNerror – correct amplitude from pre- to post-test [r = .20, p = 0.02; see Figure 3]. No further correlations of task performance measures or ERN with Δfitness were observed for the intervention [r’s ≤ .09, p’s ≥ 0.26] or wait-list [r’s ≤ .12, p’s ≥ 0.10] groups. Lastly, a one-tailed Fisher’s z-test for difference of correlation strength was performed to determine if the relation of Δfitness to ΔERNerror – correct observed for the intervention group was meaningfully different from the null results observed for the wait-list group. Results were significant [z = 2.4, p ≤ 0.01] indicating that improving fitness by means of PA intervention has significant effect on reductions in ERN amplitude.
Figure 3.
Scatter plot for the bivariate relation between changes in cardiorespiratory fitness and changes in ERN amplitude separated by groups. This figure illustrates the partial correlation results (controlling for age, IQ, sex, pre-test fitness, and pre-test BMI) demonstrating a positive relation of increased fitness with reductions in ERN amplitude from pre- to post-test for the intervention (r = .20), with no such relation observed for the wait-list group (r = −.07).
Discussion
This investigation demonstrated that a nine-month PA intervention improved fitness and behavioral indices of cognitive control in preadolescent children relative to children randomized to a wait-list control group. Additionally, children randomized to the PA intervention exhibited maintenance of ERN amplitude following the intervention, while those assigned to the wait-list group exhibited larger ERN amplitude nine months later. Lastly, for those enrolled in the intervention group, greater increases in fitness were related to greater reductions in ERN amplitude, with no such effect observed for the wait-list group. Collectively, these results support the promotion of organized PA interventions in school age children, with positive implications for brain health and function that underlie effective school-based learning.
As expected, behavioral results are consonant with previous analyses of the FITKids intervention indicating greater improvements in task performance for the intervention compared to the wait-list group (Chaddock-Heyman et al., 2013; Hillman et al., 2014). However, unique to the present investigation, error analyses revealed greater reductions in omission errors and omission error runs for the intervention group. These findings align closely with prior cross-sectional results wherein higher-fit children demonstrated fewer omission errors and error runs on a flanker task compared to lower-fit children, suggesting poorer attentional vigilance among lower-fit children (Pontifex, Scudder, Drollette, & Hillman, 2012). Previous research suggests that these performance patterns occur in response to attentional resource depletion and subsequent temporal remedial measures for regeneration of neural resources and reengagement of attention (Fisk & Schneider, 1981). Accordingly, the present data further indicate that changes in fitness engendered by a daily PA intervention are associated with enhanced sustained attention necessary for superior cognitive control performance.
The ERN patterns observed in the current study provide further support for the a priori hypothesis regarding the effect of PA on adjustments in conflict monitoring. That is, larger increases in ERN amplitude were observed for the wait-list group, with no change observed for children randomized to the intervention. The maintenance of ERN amplitude among the intervention group, along with larger improvements in task performance, suggest greater neural efficiency as evidenced by stable levels of conflict monitoring despite improved performance at post-test. Further, correlational analyses demonstrated a dose-response relationship with change in fitness, such that greater improvements in fitness from pre- to post-test were related to greater reductions in ERN amplitude; a finding that was selective to children in the intervention. These results, together with prior cross-sectional fitness investigations in children (Pontifex et al., 2011) and young adults (Themanson et al., 2006, 2008), are in accordance with conflict monitoring theory (Botvinick et al., 2001), which suggests that reductions in ACC activation and ERN amplitude reflect a relative reduction in error-salient conflict facilitating greater increases top-down cognitive control (Carter et al., 2000). Similar reductions in neural response patterns are observed in separate neuroimaging interventions in children indicating decreased hemodynamic activation in the frontal cortex (Chaddock-Heyman et al., 2013) and decreased resting state synchrony (i.e., task-free resting state activation) associated with the default mode network (i.e., predisposition for goal directed cognitive operations; Krafft et al., 2014) for children randomized to PA interventions. These prior reports, in conjunction with our present findings, suggest that PA participation at a young age influences frontally mediated brain networks during preadolescent development in a manner that appears more efficient (i.e., down-regulation in activation) for effective cognitive control and performance monitoring behavior.
Following this line of reasoning, the present results further suggest that physical inactivity may be a marker for atypical cognitive development among young children. Although literature investigating between-subject variation in ERN and aspects of cognitive control is limited, previous research investigating ERN as a biomarker of abnormal personality and psychological disorders may shed light on this speculation such that increased ERN was observed in youth and young adults with symptoms of obsessive-compulsive disorder (Hajcak & Simons, 2002; Hajcak & Simons, 2008; Santesso, Segalowitz, & Schmidt, 2006), heighted levels of negative affect (Hajcak, McDonald, & Simons 2004; Luu, Collins, & Tucker, 2000), and anxiety (Ladouceur et al., 2006; Meyer et al., 2011) compared to typically developing cohorts. However, assessments of psychological disorders were beyond the scope of the present investigation. Thus, additional longitudinal studies are needed to evaluate the influence of PA and fitness on the developmental trajectory of the ERN and its relations with atypical psychological development in children. Regardless, this area of research warrants further consideration relative to how inactivity might influence brain and behavior, especially in developing children.
Further, our ERN results provide compelling evidence that link changes in brain function to changes in fitness stemming from an afterschool PA program. However, no PA-induced changes in post-error task performance were observed. Prior investigations suggest that post-error slowing is a behavioral marker of remedial adjustments associated with ERN production (see Larson et al., 2014 for review). However, such relations are not consistent in the literature suggesting that conflict signaled by the ERN may not be directly related to remedial behavior measures (Hajcak & Simons, 2008). Therefore, it is possible that behavioral responses to conflict are necessitated by additional and separable neural systems (Larson et al., 2014), and that dynamic changes in ERN amplitude may not relate to sustained adjustments in cognitive control following errors (Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001). Thus, the absence of significant findings for post-error performance in the present investigation may highlight the selectivity of PA participation on conflict monitoring particularly for neural processes involved with conflict monitoring and ERN production.
One possible mechanism for the observed PA-induced changes may include the upregulation and co-expression of neurotrophic growth factors (i.e., brain derived neurotophic factors [BDNF]; insulin like growth factor-1 [IGF-1]) and dopamine. Specifically, research reveals co-dependency of these two factors in non-human animal models (Williams & Undieh, 2009; Hyman et al., 1991; Kuppers & Beyer, 2001), demonstrating that prolonged PA engagement upregulates production and binding of dopamine (Marques et al., 2008; Foley & Fleshner, 2008) and BDNF (see Knaepen, Goekint, Heyman, & Meeusen, 2010 for review). Interestingly, increased dopamine in the brain is associated with ERN modulation in humans (see Jocham & Ullsperger, 2009 for review). Given that these neurochemicals facilitate proliferation and cell survival in various brain structures associated with cognitive control and memory operations (van Praag, Christie, Sejnowski, & Gage, 1999), the current ERN findings suggest that PA may facilitate neurochemical activation necessary for appropriate neurocognitive function. However, this remains speculative at this time given that BDNF and dopamine measures were not assessed in the present investigation. Regardless, there remains untapped avenues for future investigations to extend these findings and potentially isolate mechanistic factors associated with PA and brain development in young children.
As such, certain limitations are worth noting. Most importantly, a control group that received a non-physical activity experience was not included in the study design. Thus, it is difficult to attribute the observed effects solely to PA because other factors may have differed between groups including social interaction, physical education instruction, and continual positive affirmation from adult figures. Additionally, the present investigation restricted the age range to 8 and 9-year-old children, therefore, the extent to which the present results are observed in other domains of cognition, as well as across developmental age groups, remains unknown. Future research encompassing a developmental perspective may provide additional insight into the interaction of PA behaviors and the developmental time course of brain and cognitive operations.
In summary, this investigation provides further evidence for the utility of PA interventions as a means of optimizing cognition in preadolescent children, with selective effects observed for frontally mediated performance monitoring systems associated with cognitive control. Such findings provide valuable information for future investigations aimed at isolating and identifying cognitive operations and cortical mechanisms modified by PA participation. Further, these patterns of improved cognitive performance, combined with fitness changes, advocate for the importance of interventions aimed at all preadolescent children, not only for overall health but also for improving cognitive faculties necessary for effective learning.
Acknowledgments
The Fitness Improves Thinking in Kids (FITKids) trial was supported by the National Institute of Child Health and Human Development (NICHD RO1 HD055352 to Charles Hillman). The Fitness Improves Thinking in Kids 2 (FITKids2) trial is supported by the National Institute of Child Health and Human Development (NICHD RO1 HD069381 to Charles Hillman and Arthur Kramer). Additional support for this project was provided by the National Institute of Food and Agriculture, US Department of Agriculture, (2011-67001-30101) and by the University of Illinois at Urbana-Champaign and Abbott Nutrition through the Center for Nutrition, Learning, and Memory (ANGC1204 to Charles Hillman and Naiman Khan).
Footnotes
Author Disclosure
No conflicting financial interests exist.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the stroop color-word task. NeuroImage. 2001;16:61–75. doi: 10.1006/nimg.2001.1046. https://doi.org/10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9. New York: Loppincott Williams & Wilkins; 2014. [DOI] [PubMed] [Google Scholar]
- Bar-Or O. Pediatric Sports Medicine for the Practitioner: From Physiologic Principles to Clinical Applications. New York: Springer-Verlag; 1983. [Google Scholar]
- Botvinick MM, Braver TS, Barch D, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. https://doi.org/10.1037//0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. https://doi.org/10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection for-action in anterior cingulate cortex. Letters to Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Roman R, Daniel P, Rektor I. Intracerebral error-related negativity in a simple go/no-go task. Journal of Psychophysiology. 2005;19:244–255. https://doi.org/10.1027/0269-8803.19.4.244. [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrielli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. https://doi.org/10.1016/S0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger A, Noll D, Cohen JD. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. PNAS. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. https://doi.org/10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Education Policy. Choices, Changes, and Challenges: Curriculum and Instruction in the NCLB Era. Washington DC: Center for Education Policy; 2007. [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Voss MW, Knecht AM, Pontifex MB, Castelli DM, … Kramer AF. The effects of physical activity on functional MRI activation associated with cognitive control in children: a randomized controlled intervention. Frontiers in Human Neuroscience. 2013;7:1–13. doi: 10.3389/fnhum.2013.00072. https://doi.org/10.3389/fnhum.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. American Journal of EEG Technology. 1985;25:83–92. [Google Scholar]
- Christakou A, Halari R, Smith AB, Ifkovits E, Brammer M, Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. NeuroImage. 2009;48:223–236. doi: 10.1016/j.neuroimage.2009.06.070. https://doi.org/10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Clayson PE, Larson MJ. Cognitive performance and electrophysiological indices of cognitive control: A validation study of conflict adaptation. Psychophysiology. 2012;49:627–637. doi: 10.1111/j.1469-8986.2011.01345.x. https://doi.org/10.1111/j.1469-8986.2011.01345.x. [DOI] [PubMed] [Google Scholar]
- Coleman KJ, Tiller CL, Sanchez J, Heath EM, Sy O, Milliken G, Dzewaltowski DA. Prevention of the epidemic increase in child risk of overweight in low-income schools: the El Paso coordinated approach to child health. Archives of Pediatric Adolescent Medicine. 2005;159:217–224. doi: 10.1001/archpedi.159.3.217. https://doi.org/10.1001/archpedi.159.3.217. [DOI] [PubMed] [Google Scholar]
- Comon P. Independent component analysis - a new concept? Signal Processing. 1994;36:287–314. https://doi.org/10.1016/0165-1684(94)90029-9. [Google Scholar]
- Crone E, Zanolie K, Van Leijenhorst L, Westenberg P, Rombouts S. Neural mechanisms supporting flexible performance adjustment during development. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:165–177. doi: 10.3758/cabn.8.2.165. https://doi.org/10.3758/CABN.8.2.165. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Wessel JR, Steinhauser M, Ullsperger M. Modulation of the error-related negativity by response conflict. Psychophysiology. 2009;46:1288–1298. doi: 10.1111/j.1469-8986.2009.00860.x. https://doi.org/10.1111/j.1469-8986.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. https://doi.org/10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. https://doi.org/10.1207/z15326942dn25036. [DOI] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, … Naglieri JA. Exercise improves executive function and achievement and alters brain activation in overweight children: A randomized, controlled trial. Health Psychology. 2011;30:91–98. doi: 10.1037/a0021766. https://doi.org/10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeif S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. https://doi.org/10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Hillman CH, Kramer AF. Physical activity, brain, and cognition. Current Opinion in Behavioral Sciences. 2015;4:27–32. https://doi.org/10.1016/j.cobeha.2015.01.005. [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. https://doi.org/10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fisk A, Schneider W. Control and automatic processing during tasks requiring sustained attention: A new approach to vigilance. Human Factors. 1981:737–750. [Google Scholar]
- Foley TE, Fleshner M. Neuroplasticity of dopanine circuits after exercise: Implications for central fatigue. NeuroMolecular Medicine. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3. https://doi.org/10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Goodman TL. Measurement of oxygen consumption. In: Rowland TW, editor. Pediatric laboratory exercise testing: Clinical guidelines. Champaign, IL: Human Kinetics; 1993. pp. 91–113. [Google Scholar]
- Gehring WJ, Coles MG, Meyer DE, Donchin E. The error-related negativity: An event-related potential accompanying errors. Psychophysiology. 1990;27:S34. [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-cumpulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. https://doi.org/10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. https://doi.org/10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Research. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. https://doi.org/10.1016/S0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Oops!.. I did it again: An ERP and behavioral study of double-errors. Brain and Cognition. 2008;68:15–21. doi: 10.1016/j.bandc.2008.02.118. https://doi.org/10.1016/j.bandc.2008.02.118. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB, Scudder MR, … Kamijo K. Physical activity intervention improves cognitive and brain health in children. Pediatrics. 2014;134:e1063–e1071. doi: 10.1542/peds.2013-3219. https://doi.org/10.1542/peds.2013-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. https://doi.org/10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde Y, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. https://doi.org/10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Jocham G, Ullsperger M. Neuropharmacology of performance monitoring. Neuroscience & Biobehavioral Reviews. 2009;33:48–60. doi: 10.1016/j.neubiorev.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Kamijo K, Pontifex MB, O’Leary KC, Scudder MR, Wu CT, Castelli DM, Hillman CH. The effects of an afterschool physical activity program on working memory in preadolescent children. Developmental Science. 2011;14:1046–1058. doi: 10.1111/j.1467-7687.2011.01054.x. https://doi.org/10.1111/j.1467-7687.2011.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor. Sports Medicine. 2010;40:765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Krafft CE, Pierce JE, Schwarz NF, Chi L, Weinberger AL, Schaeffer DJ, … McDowell JE. An eight month randomized controlled exercise intervention alters resting state synchrony in overweight children. Neuroscience. 2014;256:445–455. doi: 10.1016/j.neuroscience.2013.09.052. https://doi.org/10.1016/j.neuroscience.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, … Johnson CL. 2000 CDC growth charts for the United States: methods and development. Vital & Health Statistics. 2002;246:1–190. [PubMed] [Google Scholar]
- Kuppers E, Beyer C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport. 2001;12:1175–1179. doi: 10.1097/00001756-200105080-00025. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. https://doi.org/10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Clayson PE, Clawson A. Making sense of all the conflict: A theoretical review and critique of conflict-related ERPS. International Journal of Psychophysiology. 2014;93:283–297. doi: 10.1016/j.ijpsycho.2014.06.007. https://doi.org/10.1016/j.ijpsycho.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB (version 1.0.0.33a) (Computer Software) UC-Davis Center for Mind & Brain. 2010 Retrieved from http://erpinfo.org/erplab/erplab-download.
- Luepker RV, Perry CL, McKinlay SM, Nader PR, Parcel GS, Stone EJ, … Wu M. Outcomes of a field trial to improve children’s dietary patterns and physical activity. The Child and Adolescent Trial for Cardiovascular Health. CATCH collaborative group. JAMA. 1996;275:768–776. doi: 10.1001/jama.1996.03530340032026. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. https://doi.org/10.1037/0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Maier ME, di Pellegrino G, Steinhauser M. Enhanced error-related negativity on flanker errors: error expectancy or error significance? Psychophysiology. 2012;49:899–908. doi: 10.1111/j.1469-8986.2012.01373.x. https://doi.org/10.1111/j.1469-8986.2012.01373.x. [DOI] [PubMed] [Google Scholar]
- Marques E, Vasconcelos F, Rolo MR, Pereira FC, Silva AP, Macedo TR, Ribeiro CF. Influence of chronic exercise on the amphetamine-induced dopamine release and neurodegeneration in the striatum of the rat. Annals of the New York Academy of Sciences. 2008;1139:222–231. doi: 10.1196/annals.1432.041. https://doi.org/10.1196/annals.1432.041. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. https://doi.org/10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein D, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. https://doi.org/10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. https://doi.org/10.1111/1469-8986.3850752. [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Ogunwole SU, Drewery MP, Rios-Vargas M. The population with a bachelor’s degree or higher by rase and Hispanic origin: 2006–2010. United States Census Bureau; 2012. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. https://doi.org/10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathologh: toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. https://doi.org/10.1016/j.cpr/2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, Cohen NJ, … Hillman CH. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. Journal of Cognitive Neuroscience. 2011;23:1332–1345. doi: 10.1162/jocn.2010.21528. https://doi.org/10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Brown ML, O’Leary KC, Wu C, Themanson JR, Hillman CH. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. https://doi.org/10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Scudder MR, Drollette ES, Hillman CH. Fit and vigilant: The relationship between poorer aerobic fitness and failures in sustained attention during preadolescence. Neuropsychology. 2012;26:407–413. doi: 10.1037/a0028795. https://doi.org/10.1037/a0028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Developmental Neuropsychology. 2006;29:431–445. doi: 10.1207/s15326942dn2903_3. https://doi.org/10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Murphy TI, Homan D, Chantziantoniou DK, Khan S. Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology. 2010;47:260–270. doi: 10.1111/j.1469-8986.2009.00942.x. https://doi.org/10.1111/j.1469-8986.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence: With a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: A preliminary study. Paediatric and Perinatal Epidemiology. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Hillman CH. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience. 2006;141:757–767. doi: 10.1016/j.neuroscience.2006.04.004. https://doi.org/10.1016/j.neuroscience.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Themanson JR, Pontifex MB, Hillman CH. Fitness and action monitoring: Evidence for improved cognitive flexibility in young adults. Neuroscience. 2008;157:319–328. doi: 10.1016/j.neuroscience.2008.09.014. https://doi.org/10.1016/j.neuroscience.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa A, Klein DN. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology. 2012;54:139–150. doi: 10.1002/dev.20590. https://doi.org/10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter AC, Robertson RJ, Nieman DC, Kang J. Children’s OMNI scale of perceived exertion: walking/running evaluation. Medicine & Science in Sports & Excercise. 2002;34:139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- Van Bogaert P, Wikler D, Damhaut P, Szliwowski HB, Goldman S. Regional changes in glucose metabolism during brain development from the age of 6 years. NeuroImage. 1998;8:62–68. doi: 10.1006/nimg.1998.0346. https://doi.org/10.1006/nimg.1998.0346. [DOI] [PubMed] [Google Scholar]
- Van Meel CS, Heslenfeld DJ, Rommelse NN, Oosterlaan J, Sergeant JA. Developmental trajectories of neural mechanisms supporting conflict and error processing in middle childhood. Developmental Neuropsychology. 2012;37:358–378. doi: 10.1080/87565641.2011.653062. https://doi.org/10.1080/87565641.2011.653062. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Neurobiology. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. https://doi.org/10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SN, Undieh AS. Dopamine D1-like receptor activation induces brain-derived neurotrophic factor protein expression. Neuroreport. 2009;20:606–610. doi: 10.1097/WNR.0b013e32832a0a98. https://doi.org/10.1097/WNR.0b013e32832a0a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities. Rolling Meadows, IL: Riverside Publishing; 2001. [Google Scholar]
- Yeung N, Botvinick MW, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. https://doi.org/10.1037/0033-295X.111.4.931. [DOI] [PubMed] [Google Scholar]