Abstract
OBJECTIVES:
Snakebites are a significant and severe global health problem. Till date, anti-snake venom serum is the only beneficial remedy existing on treating the snakebite victims. As antivenom was reported to induce early or late adverse reactions to human beings, snake venom neutralizing potential for Cyclea peltata root extract was tested for the present research by ex vivo and in vivo approaches on Naja naja toxin.
MATERIALS AND METHODS:
Ex vivo evaluation of venom toxicity and neutralization assays was carried out. The root extracts from C. peltata were used to evaluate the Ex vivo neutralization tests such as acetylcholinesterase, protease, direct hemolysis assay, phospholipase activity, and procoagulant activity. Gas chromatography-mass spectrometry (GC-MS) analysis from root extracts of C. peltata was done to investigate the bioactive compounds.
RESULTS:
The in vivo calculation of venom toxicity (LD50) of N. naja venom remained to be 0.301 μg. C. peltata root extracts were efficiently deactivated the venom lethality, and effective dose (ED50) remained to be 7.24 mg/3LD50 of N. naja venom. C. peltata root extract was found effective in counteracting all the lethal effects of venom. GC-MS analysis of the plant extract revealed the presence of antivenom compounds such as tetradecanoic and octadecadienoic acid which have neutralizing properties on N. naja venom.
CONCLUSION:
The result from the ex vivo and in vivo analysis indicates that C. peltata plant root extract possesses significant compounds such as tetradecanoic acid hexadecanoic acid, heptadecanoic acid, and octadecadienoic acid which can counteract the toxins present in N. naja.
Key words: Acetylcholinesterase, Cyclea peltata, Naja naja, octadecenoic acid, phospholipase A2
Introduction
Snake venom consists of various growth factors, neurolysin, coagulants, de-coagulants, and constituents with cytotoxic effects. Enzymes existing in snake venom hydrolyze protein which leads to tissue gangrene and blood coagulation.[1] Phosphodiesterase A2 causes hemolysis by lysing cell membrane of red blood cells (RBCs). Oxidases and proteases are used for digestion. Snake venoms have been categorised into 3 groups (Cytotoxin, Neurotoxin, Hemotoxin) according to its mode of actions and effects. Neurotoxins target central nervous system causing heart failure and breathing problems. Hemotoxins affects cardiovascular system and blood functions. Cytotoxic venoms target specific cellular sites or muscles. Phospholipase A2, venom enzymatic constituent, was reported to be responsible for different toxic effects such as heart failure, deformations, renal failure, and amputations.[2] The snake venom of Naja naja selected in this research was reported as a potential neurotoxin affects normally the central nervous system in human beings which leads to sudden death, paralysis, and other central nervous disorders.[3]
The statistical analysis showed that in India alone 25,000 people dies every year, especially in rural areas. This analysis influences the requirement of antivenom in all parts of the world; snake venom is essential for the production of antivenom to treat potentially fatal snakebites. India has a large polyvalent antivenom production unit at Central Research Institute, Kasauli and Simla. Even though it was reported that antivenom plays a major role in snakebites, researchers have highlighted its limitations with justifications. Antivenoms are usually costly and may be available in limited supply and mostly available in freeze-dried ampoules and may not be stable in other storage conditions. Intramuscular route of administration may not be uniformly effective. Liquid antivenom may get turned opaque due to the formation of precipitation of protein, indicating the lack of activity of antivenom. This may increase the risk of reactions against humans.[4] Antivenom does not provide whole security against bleeding, death, kidney failure induced during envenomation.[5] Therefore, finding ways to counteract the multiple poisonousness-caused postenvenomation is a major task to the therapists.
Maximum of the world's inhabitants still depend completely on plant-based treatments.[6] Medicinal florae are a rich source of many usual inhibitors and pharmacologically active compounds.[7,8] The importance of medicinal plants for curing snake bites and stings by poisonous insects has been discussed in the historical as well as present literature.[9]
It was reported that each plant and its specific parts such as root, leaves, and barks have herbal antidote properties against specific snake venoms. It was investigated through various instrumental analyses such as gas chromatography-mass spectrometry (GC-MS) and Liquid chromatography-MS, and high-performance thin-layer chromatography. Specific plant constituents or its phytochemicals play a major role in providing the antidote properties rather than the whole plant itself.[10] Herbal compounds active against snake envenomation were already reported. Herbal plants such as Hemidesmus indicus, Pimpinella anisum, Salix alba contain aristolochic acid, anisic acid, salicylic acid, respectively.[11] Alkaloids such as atropine and AIPLAI as herbal antidote are present in Dendroaspis polylepis and Azadirachta indica.[12] Edunol, a pterocarpanes, is present in Harpalyce brasiliana.[13] Terpenoids such as glycyrrhizin, neo-clerodane, and ursolic acid are present in Glycyrrhiza glabra, Baccharis trimera, and Eriobotrya japonica.[14]
In the present research, Cyclea peltata, a significant medicinal herb, was screened for its antivenom properties. C. peltata exists to Menispermaceae family, in Ayurveda, known as Rajpatha and its local name is Kariballi,[15] it is a mounting shrub. The medical significance of C. peltata was earlier reported. It is extensively used in the management of cold, illness, kidney disorder, urinary disorder, and snakebite. Powdered roots of the plants were used for the management of various diseases.[16] Phytoconstituent of therapeutic plants revealed the presence of alkaloids, carbohydrates, glycosides, phytosterols compounds and proteins and amino acids. Other significant plant constituents such as fangchinoline, perpamine, cycleahomine chloride, burmannaline, cycleapeltine, cycleamine, cycleadrine, chondocurine, cycleacurine, magnoflorine, cycleanorine, and isotetradrine were present in different parts of the plants. The gas chromatographic of the plants revealed other significant constituents such as tetradecanoic acid, hexadecanoic acid, eicosane, palmitic acid vinyl ester, and docosane.[17]
Although the plant has been a widely used as folklore medicine with reportedly high diuretic and anti-inflammatory properties, the antivenom potential remains still uncharacterized. Hence, in this research, the neutralization efficacy of aqueous C. peltata root extract against N. naja snake venom was dogged.
Materials and Methods
Snake venom
N. naja venom was procured as lyophilized powder from Chennai. The lyophilized venom powder was stored at 4°C before experiments. Stock solution was prepared by dissolving 1 mg of lyophilized venom in 1 ml of physiological saline (1 mg/ml).
Collection and authentication of plant material
C. peltata was collected from Anakkal region, Malampuzha, Palakkad district, Kerala after questionnaire with tribal people and from vaidyars in and around Palakkad district. It was authenticated by Botanical Survey of India Southern Regional Centre, Coimbatore (Herbarium voucher specimen number 2491).
Preparation of extract
20 g of C. peltata powder was extracted using 180 ml of distilled water. The mixture in the beaker was agitated for 6 min and kept for overnight. The extract was filtered in What man No. 1 filter paper. The filtrate was vapored at reduced pressure below 40°C.
Acute oral toxicity
Acute oral toxicity of all the selected plant extracts was performed as per OECD guidelines 423. A limit test at 2000 mg/kg body weight of the extracts was administered. Briefly, two thousand milligrams of the test substance per kilogram of body weight were administered to 3 healthy mice by oral gavages. The animals were observed for mortality, signs of gross toxicity, and behavioral changes at least once daily for 14 days. Body weights were recorded before administration and again on days 7 and 14 (day of termination). Necropsies were performed on all animals at terminal sacrifice (Ethics committee approval number: JSSCP/IAEC/PH. D/PH. COLOGY/02/2014-15).
Ex vivo analysis
Acetyl cholinesterase activity
Acetylcholinesterase inhibition assay was carried out according to the modified technique of Ellman et al.[18] At different dilutions of plant extract (200, 250, 300 μg), 200 μg of venom (1 mg/ml) was preincubated (1 h) and supernatant was added to the assay mixture which consists of 100 μl of 75 mM acetylcholine iodide in 1 ml of phosphate buffer. By taking the absorbance at 412 nm, the activity was measured. Control was considered as venom without plant extract. The calculation of Inhibition % was done by the formula,
Inhibition % = Control-test/control × 100
Proteolytic activity
According to the method of Satake et al.,[19] proteolytic activity was studied with the aid of % casein as substrate in 0.02M Tris-HCl buffer (pH 8.5). Venom 200μg (1mg/ml) and different dilutions of plant extract 200μg, 250μg, 300μg were pre-incubated with 1ml of substrate for 2h at 37°C. vThe undigested casein was precipitated by the addition of 1.5 ml of 0.44M trichloroacetic acid and centrifuged. Using Folin-Ciocalteu's reagent, the digested casein in the supernatant was determined. N. Naja venom deprived by C. peltata extract was used as control. The calculation of Inhibition % was done by the formula,
Inhibition % = Control-test/control × 100
Direct hemolysis assay
By using RBC, in vitro hemolytic action of N. naja venom and plant extracts were studied. At 900 rpm, 5 ml of citrated blood was centrifuged for 10 min. The supernatant was discharged off and the pellet was washed twice with physiological salt solution. A volume of 0.5 ml of RBC mixture and 5 ml of physiological saline served as a control. A volume of 0.5 ml of washed RBC with 5 ml of distilled water was used for 100% hemolysis. 5 ml of venom/extract and 0.5 ml of washed RBC served as experimental sample. To measure the optical density using spectrophotometer at a wavelength of 540 nm against water, the supernatant fluid was poured off to separate tubes. The calculation of hemolysis was done by the formula,
Experimental sample − Control sample × 100/100% hemolysis
Indirect hemolysis assay (phospholipase activity)
By the method described by Gutiérrez et al.,[20] phospholipase A2 activity was measured on agarose–erythrocyte–egg yolk gel plate. Increasing concentrations of N. naja venom (μg) was added to 3 mm wells in agarose gels (0.8% in PBS, pH 8.1) containing 1.2% sheep erythrocytes, 1.2% egg yolk as a source of lecithin, and 10 mM CaCl2. Diameters of the hemolytic halos were measured after the incubation of slides at 37°C overnight. Control wells contained 15 μl of saline. The minimum indirect hemolytic dose corresponds to a concentration of venom, which produced hemolytic halo of 11 mm diameter. By mixing constant amount of venom (μg) with different amount of plant extracts (μl) and incubated for 30 min at 37°C, the efficacy of C. peltata root extract in neutralizing the phospholipase activity (PLA2) was estimated. Then, aliquots of 10 μl of to the mixtures were added to wells in agarose–egg yolk–sheep erythrocyte gels. Venom without plant extract was served as control. At 37°C for 20 h, plates were incubated. When compared to the effect by venom alone, counteraction was expressed as the ratio mg plant extract/mg venom which can able to decrease by 50% the diameter of the haemolytic halo.
Procoagulant activity
According to the method described by Laing et al.,[21] procoagulant activity was done.
Various amounts of venom dissolved in 100 μl PBS (pH 7.2) was added to human citrated plasma at 37°C. Coagulation time was recorded, and the minimum coagulant dose (MCD) was determined as the venom concentration, which induced clotting of plasma within 60 s. Plasma incubated with PBS alone served as control. In neutralization assays, constant amount of venom was mixed with various dilutions of plant extract. The mixtures were incubated for 30 min at 37°C. Then, 0.1 ml of mixture was added to 0.3 ml of citrated plasma and the clotting times were recorded. In control tubes, plasma was incubated with either venom alone or plant extract alone. Neutralization was expressed as effective dose (ED50), defined as the ratio μl antivenom (plant extract)/mg venom at which the clotting time increased 3 times when compared with clotting time of plasma incubated with two MCD of venom alone.
In vivo assessment of venom toxicity and antivenom effect of plant extracts
Various doses of venom in 0.2 ml of physiological saline were injected into the tail vein of mice, using groups of 3–5 mice for each venom dose. The LD50 was calculated with the confidence limit at 50% probability by the analysis of deaths occurring within 24 h of venom injection.[22]
The anti-lethal potentials of plant extracts were determined against 2LD50 of N. naja venom. Various amount of plant extracts (μl) were mixed with 2LD50 of venom sample and incubated at 37°C for 30 min and then injected intravenously into mice. A total of 3–5 mice were used at each antivenom dose. Control mice received same amount of venom without antivenom (plant extracts). From the number of deaths within 24h of injection of the venom/antivenom mixture the Effective Dose (ED50) was calculated. ED50 was expressed as μl antivenom/mouse and calculated by probit analysis.[23]
Gas chromatography-mass spectroscopy analysis
Aqueous root extract of C. peltata was evaporated and dissolved in DMSO and was analyzed for the presence of different compounds by GC-MS technique. GC-MS analysis was performed at South Indian Textile Research Association, Coimbatore, South India by Thermo GC - Trace Ultra Version: 5.0, Thermo MS DSQ II equipment in DB 35 - Ms Capillary Standard Non-Polar Column (Dimension: 30 m, ID: 0.25 mm, Film: 0.25 μm). Helium was used as a carrier gas with a flow rate of 1 ml/m and the sample injected was 1 μl. The oven temperature programmed from 70°C to 260°C at the rate of 6°C/min. Total GC running time was 37.53 m. The chromatogram obtained from the GC was then analyzed in the MS to get the mass of all the fractions. Interpretation of mass spectrum of GC–MS was done using the database of National Institute Standard and Technology (NIST). The spectrum of the known component was compared with the spectrum of the known components stored in the inbuilt library.
Results
Ex vivo assays
All ex vivo evaluation of venom poisonousness and deactivation assesses showed significant results. The inhibitory effect of C. peltata extracts on the acetylcholinesterase activity of venom and protease activity was determined in vitro, and the results were presented in Figures 1 and 2. Significant results for other tests such as direct hemolysis, PLA2, and procoagulant activity were evident during the analysis. Interestingly, C. peltata extracts also showed good neutralization on the toxicity of venom.
Figure 1.
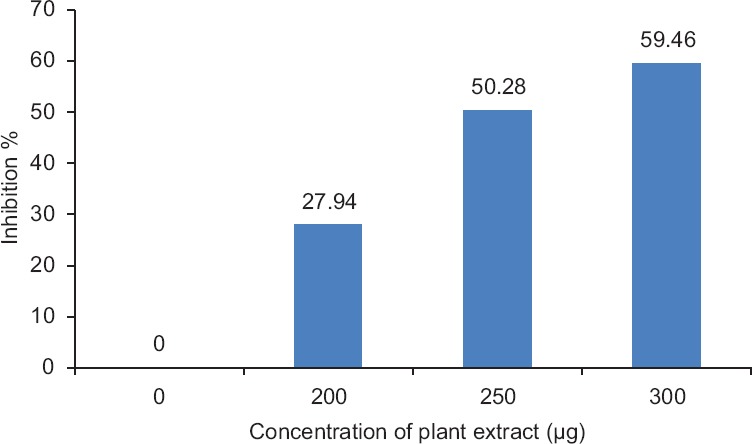
In vitro assessment of neutralization assay: Acetylcholinesterase activity
Figure 2.
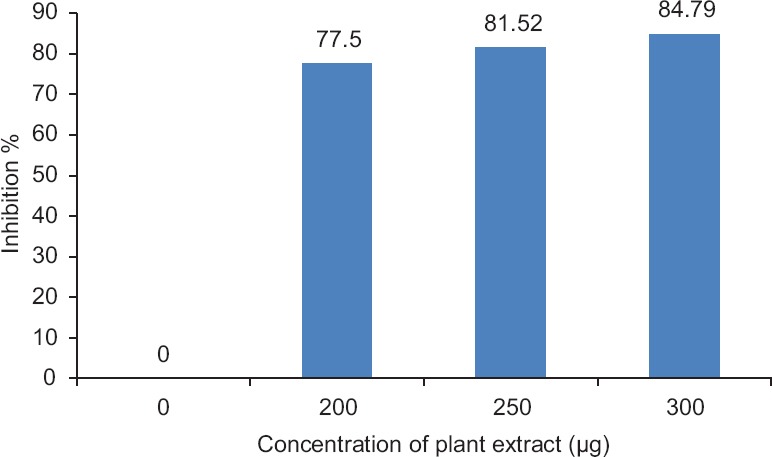
In vitro assessment of neutralization assays: Protease activity
In vivo assay
In vivo evaluation of venom lethality (LD50) of N. naja venom was evaluated by Miller and Tainter method. In vivo assessment of venom toxicity (LD50) of the venom was assessed by LD50 range-finding test and the median lethal dose (LD50) assay using mice (18–20 g). LD50 of N. naja venom was calculated and found to be 0.301 μg/g [Table 1 and Figure 3]. Venom-neutralizing potency test (ED50) using C. peltata extract was carried out by preincubating constant amount of venom (2LD50) with various dilutions of the plant extracts before injection. Calculation of ED50 of C. peltata against 2LD50 of venom was done by Miller and Tainter method and found to be 7.24 mg/2LD50 venom [Table 2 and Figure 4].
Table 1.
Calculation of LD50 of Naja naja venom in mice receiving various doses of Naja naja venom by Miller and Tainter method (n=5)
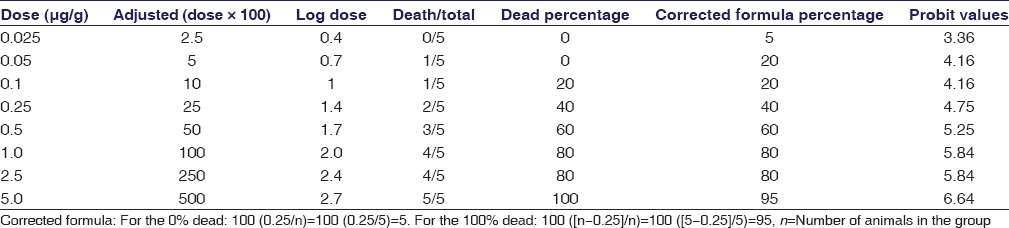
Figure 3.
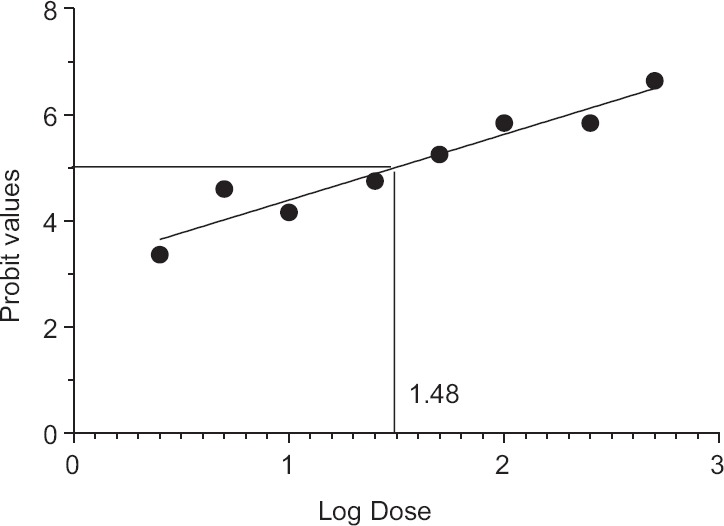
Calculation of lethal dose LD50 of Naja naja venom. LD50 of Naja naja = antilog (log dose)/100 = antilog 1.48/100 = 30.19/100 = 0.301 μg/g
Table 2.
Calculation of ED50 of Cyclea peltata root against Naja naja venom in mice by Miller and Tainter method (n=5)
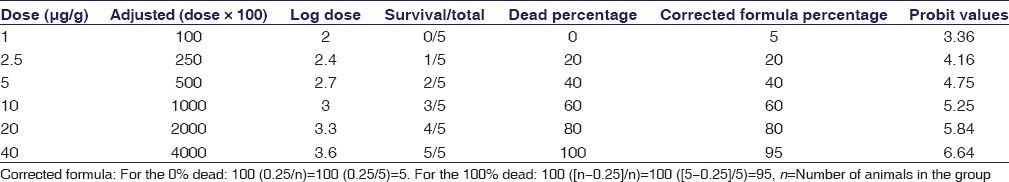
Figure 4.
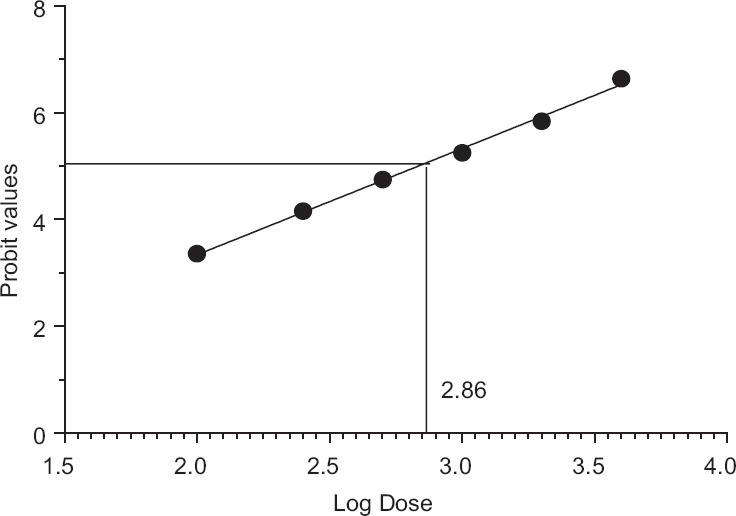
Neutralization of lethality (ED50) of Cyclea peltata root against 3LD50 of Naja naja venom. ED50 of Cyclea peltata root against Naja naja venom = antilog (log dose)/100 = antilog 2.86/100 = 724.4/100 = 7.24 mg
Gas chromatography-mass spectrometry analysis of Cyclea peltata root extract
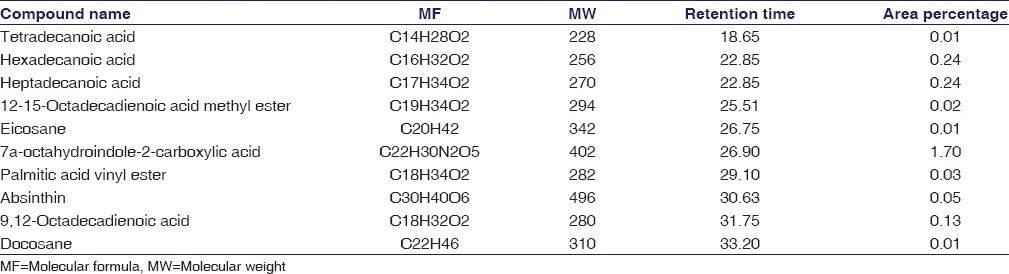
GC-MS investigation led to the recognition of different compounds from the fractions of the C. peltata aqueous extract. These compounds were acknowledged through mass spectrum coupled with gas chromatography. The active principles with their retaining time, molecular formula, and molecular weight (MW) were clearly indicated in Table 3.
Table 3.
Gas chromatography-mass spectroscopy analysis of Cyclea peltata root extract
Discussion
Snakebite is constantly considered to be as a chief health threat which leads to high humanity rate worldwide. The true global incidence of snakebite and associated mortality is difficult to estimate. Antisnake venom remains the exact remedy to snake venom with different restrictions on its usage. It consumes lots of time for the development and expensive. Since it contains horse immunoglobulins, it leads to complement-mediated side effects, such as serum sickness and anaphylactic shock. Due to these limitations of antivenoms, since last 20 years, more scientific attention on using plants and medically significant herbs against different snakebites was given importance.[24] Antivenom potential of C. peltata root extract against N. naja venom was investigated in the present study by both ex vivo and in vivo experiments.
Ex vivo assay
During this experiment, maximum acetylcholinesterase inhibition was recorded as 59.46% for a known concentration of 300 μg plant extract [Figure 1]. Direct hemolysis of N. naja venom produced 91.66% hemolysis. C. peltata root extract neutralized the hemolysis of RBCs produced by the venom up to 35%. In PLA2, 10 μg of N. naja venom was able to produce 11 mm diameter hemolytic halo, which is considered to be 1 Unit. C. peltata extract is capable of preventing PLA2-dependent hemolysis of sheep RBCs induced by N. naja venom in a dose-dependent manner. In procoagulant activity, 100 μg of N. naja venom was found to clot human-citrated plasma in 60 s. In the neutralization assay, the absence of clot formation shows the neutralizing ability of plant extract. To assess the in vitro antagonism of protease, the venom degrading the substrate (casein) into peptide precipitation was observed at 600 nm. Maximum protease inhibition of 84.79% was observed for 300 μg concentrations of aqueous plant extract [Figure 2]. It was justified that the increased concentration of plant extract could increase the inhibition of protease activity of venom. Different mechanisms involved in the inhibition of these proteases by plant extracts were reported. The phenolic components present in Mangifera indica plant extract form hydrogen bonds with the three histidine residues in the conserved zinc binding motif and could chelate the Zn2+ atom of the snake venom metalloproteinases, which could potentially result in inhibition of the venom enzymatic activities and thereby inhibit tissue necrosis.[25] Similarly, Soares et al. reported that bioactive compounds in the plant extracts will bind to divalent metal ions, required for enzymatic activities. Thus, weaken the protease-metal ion interaction resulting in inhibition of the proteolytic activity.[26]
In vivo assay
In vivo assessment of venom lethality (LD50) and venom-neutralizing potency test (ED50) using C. peltata extract were carried out in the present research. From the obtained results, it was found that C. peltata root extracts could able to neutralize the toxin of venom. LD50 of N. naja venom was calculated and found to be 0.301 μg/g. ED50 of C. peltata against 2LD50 of venom was done by Miller and Tainter method and found to be 7.24 mg/2LD50 venom. In acute oral toxicity, all animals survived and appeared active and healthy throughout the study. Based on the above findings, the LD50 of C. peltata plant extracts was >2000 mg/kg.
The result from the preliminary study designates that C. peltata root extracts own some compounds which can counteract the toxins present in N. naja venom. The presence of these compounds in the plant extracts were further investigated by GC-MS analysis.
Gas chromatography-mass spectrometry analysis of Cyclea peltata root extract
In the current study, the chemical outline of C. peltata using GC-MS was characterized. Totally ten compounds were identified in aqueous extract of cyclea peltata root with retention time and area percentage in [Table 3].
The relative concentration of each component was pointed out with the peak height. The nature and structure of the compounds eluted at different times was analyzed by mass-spectrophotometer. These mass spectra were considered as fingerprint of each obtained compound further identified from the NIST data library.
It was previously reported that tetradecanoic acid binds to catalytic site of (Bothrops neuwiedi) venom PLA2 through hydrogen bonding between amino acid residues and also by hydrophobic interactions with neighboring amino acid residues present in the catalytic site. According to Delatorre et al.,[27] such competitive binding of this molecule was expected to inhibit the PLA2 activity of the toxin. Another significant component, hexadecanoic acid was reported by Vickers et al. in that the compound has anti-inflammatory activity through competitive inhibition of PLA2.[28] In the present study; inhibition of venom by the PLA2 activity of plant extract was previously reported and found to be supportive.
The octadecenoic acid derivatives are strong synthetic inhibitors of neurotoxins; these derivatives aid in inhibiting toxins and its neurotoxicity.[29] The unsaturated fatty acids found in the C. peltata methanolic extract would help in maintaining the cell integrity which in turn prevents the distribution of venom components from the bite site.[30]
In the present study, protease and phospholipase activities were completely inhibited in a dose-dependent manner. This could be due to the strong antivenom activity of the phytosterol (stigmasterol) present in the extract.
Conclusion
In India, snakebite is a main health problem that leads to various demises annually. Traditional therapeutic scheme has a deep-rooted history among rural population in India. The results showed that plant extracts were capable of inhibiting acetylcholinesterase, protease, direct hemolytic, phospholipase, procoagulant activities. The inhibitory activities of plant extract against snake venom were further recognized by ex vivo studies. Thus, this work reveals the scientific validation for the usage of C. peltata root as an antivenom drug. Hence, the presence of multiple bioactive (antisnake venom) compounds in the extract could have contributed for its efficient antivenom activity. We hope these findings may clarify the supposed efficacy of the use of herbal drug as an anti-snake venom agent
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to Principal Dr. Anirudhan, for providing the necessary facilities in the College and sincere thanks to Dr. J. Rathinamala, Head, Department of Microbiology, Nehru Arts and Science College, Coimbatore, for her encouragement and support throughout the study.
References
- 1.Jin H, Varner J. Integrins: Roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–5. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kini RM. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42:827–40. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Gopi K, Kadali R, Gurunathan J. The neutralization effect of methanol extract of Andrographis paniculata on Indian cobra Naja naja snake venom. J Pharm Res. 2011;4:1010–2. [Google Scholar]
- 4.Alam MI, Gomes A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol. 2003;86:75–80. doi: 10.1016/s0378-8741(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 5.Thwin MM, Samy RP, Satyanarayanajois SD, Gopalakrishnakone P. Venom neutralization by purified bioactive molecules: Synthetic peptide derivatives of the endogenous PLA(2) inhibitory protein PIP (a mini-review) Toxicon. 2010;56:1275–83. doi: 10.1016/j.toxicon.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Kumar VS, Navaratnam V. Neem (Azadirachta indica): Prehistory to contemporary medicinal uses to humankind. Asian Pac J Trop Biomed. 2013;3:505–14. doi: 10.1016/S2221-1691(13)60105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunjam SR, Jadhav SK, Tiwari KL. Traditional herbal medicine for the treatment of snake bite and scorpion sting by the tribe of South Surguja, Chhattisgarh, India. Med Aromat Plants. 2013;2:2–1. [Google Scholar]
- 8.Ponna V, Ranjani R, Rao MR, Sundarsanam G. Impact of antidote medicinal plant- Corallocarpus epigaeus extract on lipid peroxidation induced by Naja naja snake venom in albino rat. Int J Med Pharm Sci. 2013;3:23–30. [Google Scholar]
- 9.Yamashita T, Brahmadathan UM, Paramaswaran MK. Traditional poison-healing system in Kerala: An overview. Ejournal Indian Med. 2010;3:101–7. [Google Scholar]
- 10.Gopi K, Kadali R, Gurunathan J. Inhibition of Naja naja venom enzymes by the methanolic extract of Leucas aspera and its chemical profile by GC–MS. Toxicol Rep. 2014;1:667–73. doi: 10.1016/j.toxrep.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes A, Das R, Sarkhel S, Mishra R, Mukherjee S, Bhattacharya S, et al. Herbs and herbal constituents active against snake bite. Indian J Exp Biol. 2010;48:865–78. [PubMed] [Google Scholar]
- 12.Mukherjee AK, Doley R, Saikia D. Isolation of a snake venom phospholipase A2 (PLA2) inhibitor (AIPLAI) from leaves of Azadirachta indica (Neem): Mechanism of PLA2 inhibition by AIPLAI in vitro condition. Toxicon. 2008;51:1548–53. doi: 10.1016/j.toxicon.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Reyes-Chilpa R, Gómez-Garibay F, Quijano L, Magos-Guerrero GA, Ríos T. Preliminary results on the protective effect of (-)-edunol, a pterocarpan from Brongniartia podalyrioides (Leguminosae), against Bothrops atrox venom in mice. J Ethnopharmacol. 1994;42:199–203. doi: 10.1016/0378-8741(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 14.Francischetti IM, Monteiro RQ, Guimarães JA. Identification of glycyrrhizin as a thrombin inhibitor. Biochem Biophys Res Commun. 1997;235:259–63. doi: 10.1006/bbrc.1997.6735. [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Nishteswar K. Review on Cissampelos pareira & Cyclea peltata Patha Dwaya) Phyto-pharmacological perspectives. Int J Ayurvedic Med. 2013;4:282–9. [Google Scholar]
- 16.Kirubha TV, Senthamarai R, Mariya P, Mani P. Pharmacognostical and phytochemical standards of Cyclea peltata (Lam) Hook and Thomson leaves. J Chem Pharm Res. 2012;4:1465–9. [Google Scholar]
- 17.Hullatti KK, Gopikrishna UV, Kuppast IJ. Phytochemical investigation and diuretic activity of Cyclea peltata leaf extracts. J Adv Pharm Technol Res. 2011;2:241–4. doi: 10.4103/2231-4040.90880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 19.Satake M, Murata Y, Suzuki T. Studies on snake venom. XIII. Chromatographic separation and properties of three proteinases from Agkistrodon halys blomhoffii venom. J Biochem. 1963;53:438–47. doi: 10.1093/oxfordjournals.jbchem.a127720. [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez JM, Avila C, Rojas E, Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26:411–3. doi: 10.1016/0041-0101(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 21.Laing GD, Theakston RD, Leite RP, da Silva WD, Warrell DA. Comparison of the potency of three Brazilian Bothrops antivenoms using in vivo rodent and in vitro assays. BIASG (Butantan Institute Antivenom Study Group) Toxicon. 1992;30:1219–25. doi: 10.1016/0041-0101(92)90438-b. [DOI] [PubMed] [Google Scholar]
- 22.Randhawa MA. Calculation of LD50 values from the method of Miller and Tainter, 1944. J Ayub Med Coll Abbottabad. 2009;21:184–5. [PubMed] [Google Scholar]
- 23.Miller LC, Tainter ML. Estimationof LD50 and its error by means of log-probit graph paper. Proc Soc Exp Biol Med. 1944;57:261. [Google Scholar]
- 24.Fattepur SR, Gawade SP. Preliminary screening of herbal plant extracts for anti-venom activity against common sea snake (Enhydrina schistosa) poisoning. Pharmacogn Mag. 2007;3:56–60. [Google Scholar]
- 25.Pithayanukul P, Leanpolchareanchai J, Saparpakorn P. Molecular docking studies and anti-snake venom metalloproteinase activity of Thai mango seed kernel extract. Molecules. 2009;14:3198–213. doi: 10.3390/molecules14093198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares AM, Ticli FK, Marcussi S, Lourenço MV, Januário AH, Sampaio SV, et al. Medicinal plants with inhibitory properties against snake venoms. Curr Med Chem. 2005;12:2625–41. doi: 10.2174/092986705774370655. [DOI] [PubMed] [Google Scholar]
- 27.Delatorre P, Rocha BA, Santi-Gadelha T, Gadelha CA, Toyama MH, Cavada BS. Crystal structure of Bn IV in complex with myristic acid: A Lysmyotoxic phospholipase A2 from Bothrops neuwiedi venom. Biochimie. 2011;93:513–8. doi: 10.1016/j.biochi.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, et al. Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ. 2009;32:520–31. doi: 10.1111/j.1365-3040.2009.01946.x. [DOI] [PubMed] [Google Scholar]
- 29.Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M, et al. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem Biol Drug Des. 2012;80:434–9. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 30.Gill I, Valivety R. Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends Biotechnol. 1997;15:401–9. doi: 10.1016/S0167-7799(97)01076-7. [DOI] [PubMed] [Google Scholar]


