Abstract
BACKGROUND:
Sedation during flexible bronchoscopy is desirable, but the drugs and the dosage protocols that are used vary.
OBJECTIVE:
To study and compare the effects of midazolam with fentanyl-midazolam combination during flexible bronchoscopy.
MATERIALS AND METHODS:
The study was conducted on 144 patients, from October 2013 to July 2015. They answered Hospital Anxiety and Depression Scale-Anxiety subscale and a prebronchoscopy questionnaire to assess their expectation toward flexible bronchoscopy. The patients were randomized into three groups: placebo, midazolam, and fentanyl-midazolam. Vitals signs including heart rate, respiratory rate, blood pressure, and oxygen saturation (SpO2) were recorded. Furthermore, Ramsay Sedation Scale was assessed during the procedure. Primary outcome measure was the composite score of patient-reported tolerance and satisfaction (assessed after the procedure). Secondary outcome measures were composite score of physician-reported feasibility of the procedure, hemodynamic changes during bronchoscopy, and side effects.
RESULTS:
Patient-reported tolerance and satisfaction composite scores (median, interquartile range) for placebo, midazolam, and fentanyl-midazolam groups were 54 (52, 57), 59 (57, 61.5), 62 (58.5, 66), respectively; P < 0.001. Physician-reported feasibility composite scores (median, interquartile range) for the respective groups were 24.5 (20.5, 28), 25 (21, 27), 26 (25, 29); P = 0.004. There was no significant difference between the groups so far as mean heart rate (P = 0.305), mean systolic blood pressure (P = 0.532), mean diastolic blood pressure (P = 0.516), mean respiratory rate (P = 0.131), and mean SpO2 (P = 0.968) were concerned.
CONCLUSION:
Conscious sedation with fentanyl and midazolam combination can result in better patient and operator satisfaction when compared with midazolam alone.
Key words: Fentanyl, flexible bronchoscopy, midazolam
Introduction
Patient comfort during any invasive procedure is becoming a norm, and hence, sedation during flexible bronchoscopy – a procedure frequently done by pulmonologists – is receiving increasing attention.[1] Moreover, interventional procedures using flexible bronchoscopes have become complex, requiring longer procedure time and necessitating better cough control.[2] These developments have led to recommendations for offering sedation to patients during flexible bronchoscopy, and a variety of sedatives have been used including benzodiazepines, opioids, propofol, and more recently dexmedetomidine.[3,4,5,6] In the United Kingdom, only 11% of pulmonologists did not use sedation, and in a survey from Australia and New Zealand, 94% combined two sedatives, and of these, 96% used fentanyl and midazolam combination.[4,7] In India, a similar data regarding the use of sedation during flexible bronchoscopy are not available. Concerns regarding patient safety may lead to underutilization of sedation or use of inadequate dosage.[8] We are presenting a study aimed at probing the efficacy of sedation in flexible bronchoscopy by comparing midazolam alone with fentanyl-midazolam combination – such studies being few, in India among adults, to the best of our knowledge. We compared the impact of these drugs on patient-reported tolerance and satisfaction and also physician-reported feasibility of the procedure and patient hemodynamics.
Objectives
To study and compare the effects of midazolam with fentanyl-midazolam combination during flexible bronchoscopy.
Materials And Methods
This was a prospective, randomized, placebo-controlled, double-blinded, three-arm, and single-center study. After getting approval from Institutional Ethics Committee (letter no. IEC 509/2013, dated October 9, 2013), the study was conducted on 144 patients from October 2013 to July 2015. Written informed consent was obtained from the patients after they read and understood the patient information sheet. The inclusion criterion was patients in the age group of 18–70 years who were advised flexible bronchoscopy for diagnostic workup. Patients were excluded from the study if they had any of the following: oxygen saturation (SpO2) <95% on room air; hemodynamic instability; renal failure, hepatic insufficiency; chronic obstructive pulmonary disease with FEVI <50%; platelet count <50,000/mm3; body weight was >85 kg; depression of consciousness or cognitive impairment rendering them unable to answer the questionnaires; and history of hypersensitivity or contraindications to the drugs used in the study.
All patients who met the inclusion criterion were assessed, and basic data such as indication for flexible bronchoscopy, age, gender, weight, height, body mass index (BMI), and educational status were noted. The patients answered two questionnaires before the procedure: Hospital Anxiety and Depression Scale-Anxiety subscale (HADS-A) to assess the anxiety status of the patient before the procedure and prebronchoscopy questionnaire to assess the expectation of the patient toward flexible bronchoscopy and also their previous experience with flexible bronchoscopy, if any.[9]
After obtaining the above information, the patients were randomly allocated to any of the three groups (Group 1 – placebo, Group 2 – midazolam, and Group 3 – fentanyl-midazolam). Randomization was done by block randomization (chit method). Sealed opaque-envelope method was used for allocation concealment.
Before beginning the procedure, the vital parameters such as pulse rate, noninvasive blood pressure, electrocardiogram, respiratory rate, and SpO2 levels were noted, and continuous monitoring initiated. An intravenous access was established in one of the upper limbs, and the study drugs were loaded in two syringes labeled as A and B by an observer who had no further role in the study, thus ensuring blinding. Syringe A contained either 1 ml (50 μg) of fentanyl or 1 ml of normal saline. Syringe B contained either midazolam 0.035 mg/kg diluted in normal saline to a volume of 5 ml or contained only normal saline 5 ml. Group 1 would receive normal saline in both the syringes, Group 2 would receive normal saline in one and midazolam in another, and Group 3 received fentanyl in one and midazolam in another.
Two ml of 2% lignocaine jelly was administered into one of the patent nostrils of the patient. Initially, 0.5 ml of the drug from syringe A was administered, and then, after 5 min, 2.5 ml of the drug from syringe B was administered. After an additional waiting period of 2 min, the bronchoscope was passed through the patent nostril and then the throat. Two ml of 2% lignocaine spray was sprayed at the throat and additional 2 ml at vocal cords, by spray-as-you-go technique. Patients were administered another 0.5 ml study drug from syringe A and 2.5 ml from syringe B, before crossing the vocal cords. After waiting for a minute, the bronchoscope was advanced below the vocal cords and stationed at the level of carina, where 1 ml 2% lignocaine was sprayed. The bronchoscope was advanced beyond carina where 1 ml of 2% lignocaine was sprayed into each main bronchus. Once the coughing subsided, the bronchoscope was advanced into either bronchi to evaluate the bronchial tree, and the sampling procedure for which the patient was scheduled was completed. Additional 2% lignocaine was sprayed if the patient had recurrence of cough.
Vital parameters such as pulse rate, noninvasive blood pressure, electrocardiogram, respiratory rate, and SpO2 levels were again recorded at 5-min interval from time zero – i.e., from insertion of bronchoscope. If the Ramsay Sedation Scale (RSS) was 1 at any time after the lignocaine application was completed, the rescue medication in the form of Injection Midazolam 0.5 mg IV (open-label syringe) was administered in a stepwise manner, with a 2-min gap between doses. Lidocaine dosage, total number of rescue medication, completeness of the procedure, complications if any, and total duration of procedure were noted. Vital parameters were recorded 10-min after completion of the procedure.
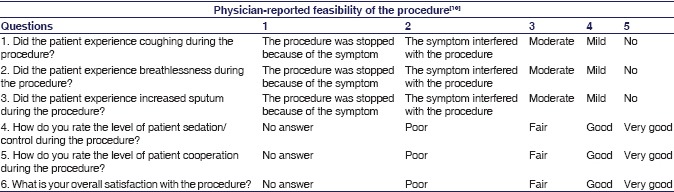
The study involved three different observers. Observer 1 picked the randomization slip and prepared the study drugs ensuring blinding (labeling syringe A and B) and did not have any further involvement with the study individual. Observer 2 noted the patient-related data, recorded vital signs, administered the study drugs, prior to bronchoscopy. Also recorded the relevant data during flexible bronchoscopy and assessed the patients by administering questionnaires, before and after the bronchoscopy. Observer 3 performed the bronchoscopy and filled in the responses in physician-reported feasibility score sheet (on scale of 1–5) [see Appendix 2].[10]
Four hours after completion of flexible bronchoscopy, a questionnaire – adapted from an earlier study – was provided to the patients. This assessed patient's tolerance of the procedure and their satisfaction level (on scale of 1–4) [see Appendix 1].[10]
Primary outcome measure was the composite score of patient-reported tolerance and satisfaction. Secondary outcome measures were composite score of physician-reported feasibility of the procedure, hemodynamic changes during bronchoscopy, and side effects, if any.
Sample Size Estimation: Anticipating the standard deviation of two and minimum clinically important difference of 1.5 in the composite scores of patient-reported tolerance and satisfaction, between any two groups for 80% power and Type I error of 5%, adjusted for multiple comparisons, 48 patients were recruited in each arm of the study.
Statistical analysis
Variables such as age, height, weight, BMI, distribution of the various procedures, duration of flexible bronchoscopy, lignocaine dose, HADS-A score, RSS, rescue medication, and composite scores between groups were compared using Kruskal–Wallis test. A significant Kruskal–Wallis test was followed by a Mann–Whitney U-test with level of significance adjusted for multiple pairwise comparisons (P < 0.017). Whereas categorical data such as gender, educational level, smoking history, and indications were compared between groups using either Fisher's exact test or Chi-square test. Mean heart rate, mean blood pressure, mean respiratory rate, and mean SpO2 levels at different time points were compared using repeated measures of ANOVA. Data were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
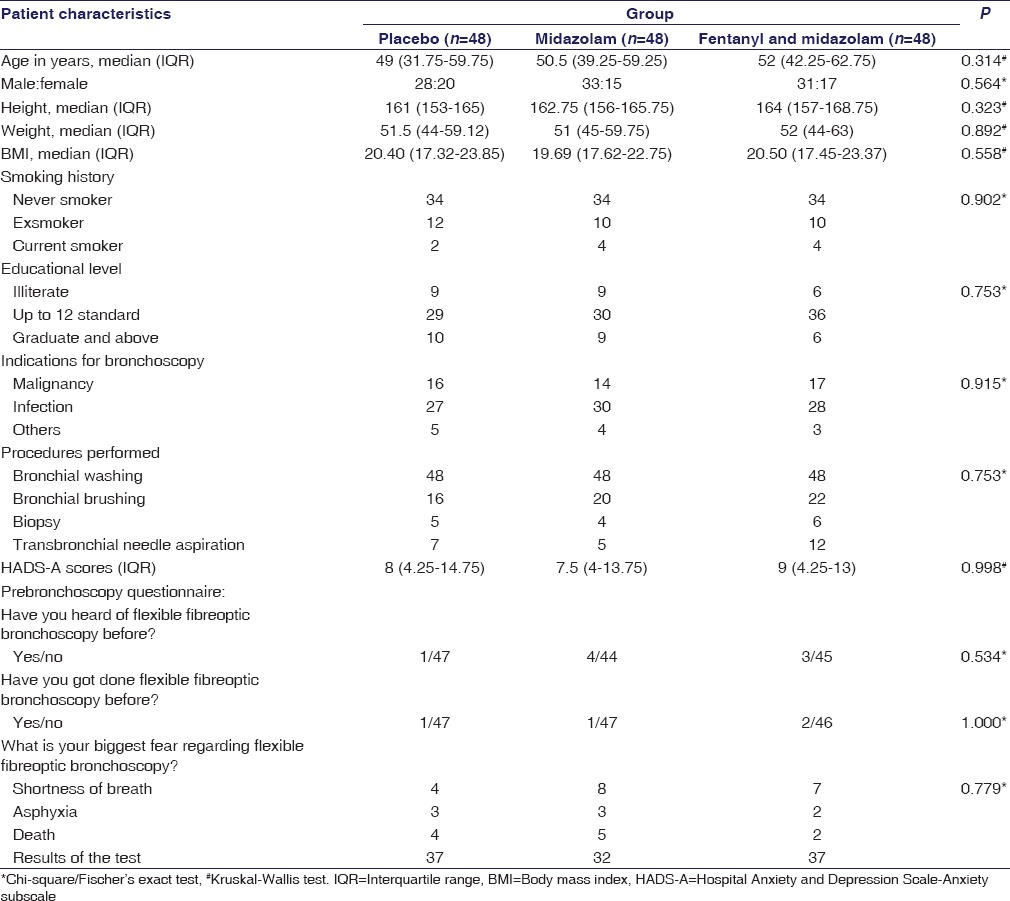
Two hundred and thirteen patients were screened, and out of these, 69 patients were excluded from the study, as per the study methodology. The study was conducted on 144 patients with 48 patients in each of the three arms of the study [Figure 1]. The baseline characteristics of each group are summarized in Table 1.
Figure 1.
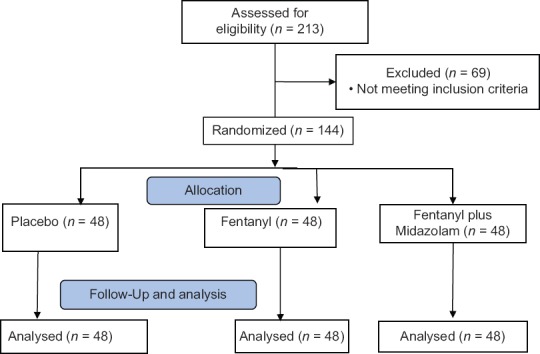
CONSORT flow diagram
Table 1.
Baseline characteristics of patients in the three study groups (n=144)
RSS across the groups is shown in Table 2. No patient had RSS of four or more at any point in the study. On intergroup analysis between midazolam and fentanyl-midazolam group, the difference in RSS score was statistically significant (P = 0.003). Whereas on intergroup comparison, between placebo and midazolam group, the difference in RSS scores was not statistically significant (P = 0.095).
Table 2.
Ramsay sedation score during bronchoscopy across three groups (n=144)

The median duration of flexible bronchoscopy was 10 min (interquartile range 7, 13.75), 10 min (interquartile range 9, 15), and 11 min (interquartile range of 9, 20) for placebo, midazolam, and fentanyl-midazolam group, respectively (P = 0.603). The median dose of lignocaine used was 180 mg (interquartile range of 180,200) in all the groups.
Patient-reported tolerance and satisfaction composite scores (median, interquartile range) for placebo, midazolam, and fentanyl-midazolam groups were 54 (52, 57), 59 (57, 61.5), 62 (58.5, 66), respectively; P < 0.001 [Figure 2]. Physician-reported feasibility composite scores (median, interquartile range) for the respective groups were 24.5 (20.5, 28), 25 (21, 27), 26 (25, 29); P = 0.004 [Figure 3]. On intergroup comparison, of above-mentioned composite scores, the scores in the fentanyl-midazolam combination group showed statistically significant difference from scores in midazolam group with respect to patient-reported tolerance and satisfaction (P = 0.002) and also physician-reported feasibility (P = 0.008). Whereas on intergroup comparison, between placebo and midazolam group, the patient-reported tolerance and satisfaction scores showed statistically significant difference (P < 0.001), but not physician-reported feasibility scores (P = 0.892).
Figure 2.
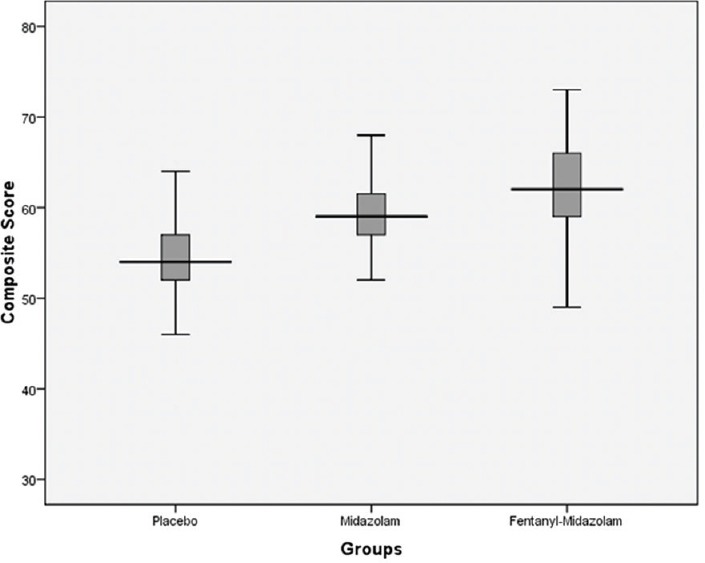
Patient-reported tolerance and satisfaction composite score as per study groups (median, interquartile range)
Figure 3.
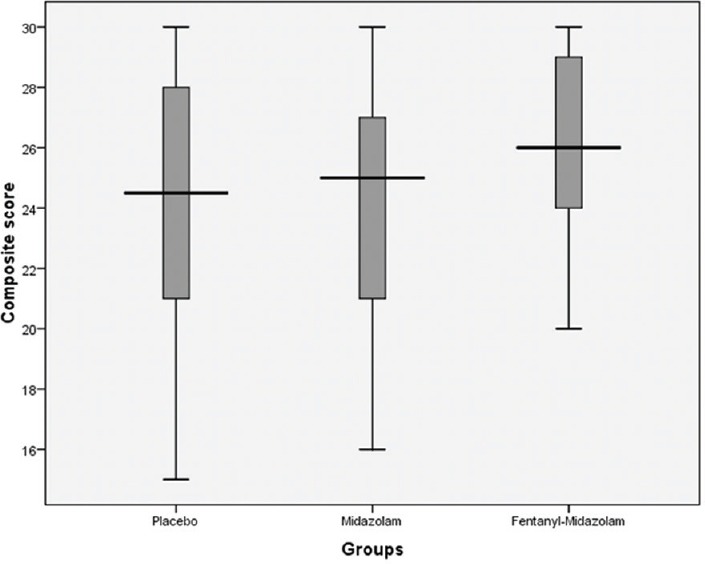
Physician-reported feasibility composite score as per study groups (median, interquartile range)
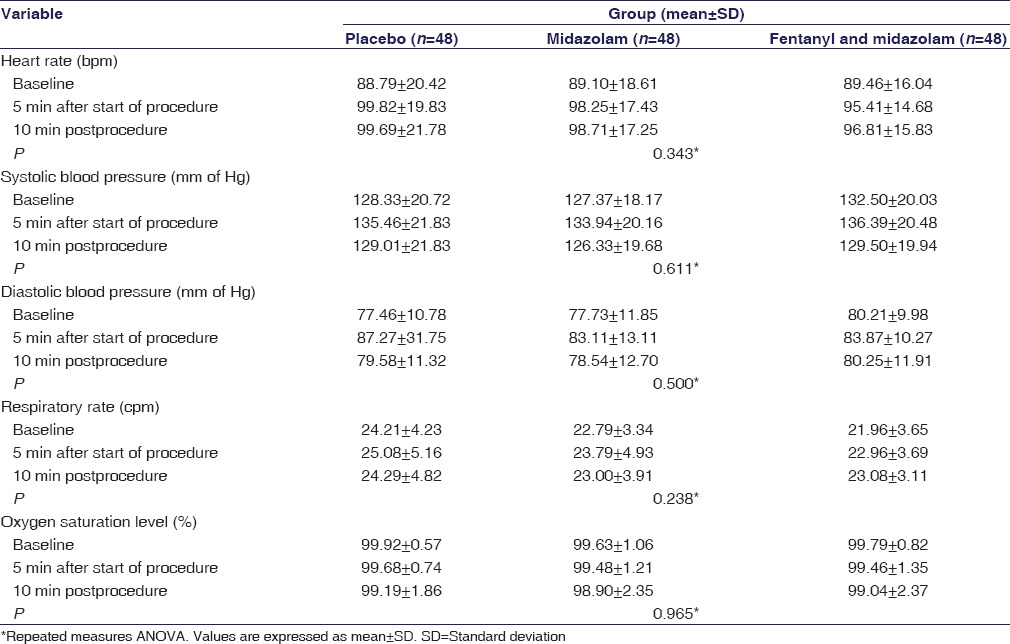
Hemodynamic and respiratory parameters, at various time points (baseline, 5-min after start of procedure, and 10-min postprocedure), across the three groups are shown in Table 3. One patient in the midazolam group developed transient hypotension (blood pressure <90 mmHg) but it recovered without any intervention, and the procedure could be completed. One patient in fentanyl-midazolam group developed significant oxygen desaturation (84%–86%). The procedure was prematurely stopped at the 10th min, and the episode was successfully managed, without any adverse consequences, by administering 60% oxygen and salbutamol nebulization. One patient in placebo group got agitated, restless, and started coughing vigorously; the procedure could not be completed and was stopped at the 10th min. The data regarding hemodynamic and respiratory parameters could be captured at all the required time points (i.e., at baseline, at 5 min from the start of procedure, and also at 10 min after the procedure). No cardiac ischemia or arrhythmia was observed in any of the groups.
Table 3.
Hemodynamic and respiratory parameters across the three groups (n=144)
Comparison of number of rescue doses across the three groups is shown in Table 4.
Table 4.
Comparison of rescue doses (intravenous midazolam, 0.5 mg) across three groups (n=144)

Discussion
Flexible bronchoscopy can be well-tolerated, without sedation, by some patients; but a large percentage of bronchoscopists, particularly in developed countries, use sedation – the practices, however, are not uniform with regard to class, dose, and the number of drugs used.[3,4,7,11,12,13] In our study, we had included a placebo group (with provision for rescue medication) to underscore the usefulness of sedation during bronchoscopy, as there are still practitioners who are not using sedation for various reasons. Guidelines, as well, have made sedation preferable but not mandatory – advising to seek patients' preference in this matter.[3,7] Evidence of some level of anxiety, before undergoing flexible bronchoscopy, has been reported in one-third of the patients and this can increase the need for analgesics and may lead to more adverse effects.[9,14] In the current clinical practice, patient comfort and allaying anxiety are desirable, and hence, use of sedation is required.[2] For flexible bronchoscopy, an RSS between two to three is acceptable.[3,6,15] In our study, it is apparent; patients in fentanyl-midazolam group did significantly better than midazolam group in terms of level of sedation, as assessed by RSS.
Patient satisfaction and tolerance of the procedure is an aspect that has been probed and emphasized in many studies on flexible bronchoscopy.[1,6,10] Notwithstanding the opinion which considers overemphasis on patient satisfaction unwarranted, patient satisfaction remains an important part of the postprocedure assessment and has found its place in the bronchoscopy guidelines as well.[3,16] Sedation is one of the important determinants of patient tolerance and satisfaction, and inadequacy of sedation can make the procedure unpleasant in approximately two-thirds of patients, and about one-fourth may be unwilling for a repeat procedure.[17] Hence, the aim of using sedatives is to reduce these to as insignificant a level as possible; and a drug or combination of drugs that can achieve this objective is, obviously, a preferred option. In the present study, patient satisfaction and tolerance of the procedure were significantly better in the fentanyl-midazolam group when compared with the midazolam group. The higher the difference in the composite scores of patient-reported tolerance and satisfaction among the groups, the more is the expected clinical significance.[10] It may be possible to assume, though not assert conclusively, that in the absence of rescue medications (used more often in midazolam group than fentanyl-midazolam group) [Table 4], the difference between these two groups might have been even more pronounced. It is a fact, attested by many studies, that postbronchoscopy, a better satisfaction is achieved among patients receiving sedation when compared to nonsedated patients.[10,17,18] However, the variety of sedatives used – with different pharmacodynamic, pharmacokinetic, and safety profiles – and the nonidentical assessment tools used to quantify patient satisfaction make it difficult, if not impossible, to comment on the best possible sedative protocol.[5,6,10,18,19] Hence, keeping this limitation in mind, fentanyl-midazolam appears, at least, to score over midazolam alone – so far as the results of the present study are concerned.
In fentanyl-midazolam group, the physician felt the patients were significantly more cooperative and better sedated. A significantly less utilization of the physician-directed rescue medication in the fentanyl-midazolam group [Table 4] also alludes to the fact that the patient cooperation and physician's comfort level were significantly better in this group.
Varying results have been obtained regarding the length of the procedure when done under sedation – some reporting shorter duration, while others finding no significant difference between sedation and placebo groups.[9,20] However, a longer postprocedure hospital stay has been observed when sedation is used.[2,21] In our study, the duration of the flexible bronchoscopy was similar in all the groups.
In our study, we used the spray-as-you-go technique for lignocaine application to the airways, which is in accordance with the British Thoracic Society guidelines. The studies on the use of nebulized lignocaine during bronchoscopy have reported conflicting results, and hence, its use is becoming less common.[3] Recently, investigators from India have recommended that 1% lignocaine is as effective as 2% solution and hence exposes the patient to lesser cumulative dose of lignocaine.[22] In our study, we have used 2% lignocaine solution – nonetheless, the total dose of lignocaine used was within the standard prescribed limits.[3] The dose of lignocaine used in individuals, who tolerate flexible bronchoscopy without sedation, has been reported to be within recommended levels.[23] In our study, every patient received, as per study design, a minimum of 180 mg of 2% lignocaine. As a result, if at all in any of the study groups the requirement was <180 mg, it would not become manifest. However, after having used this minimum dose of lignocaine, it seems additional requirement of lignocaine was not different across the groups.
In our study, an event of transient hypotension and another one of significant oxygen desaturation occurred in midazolam group and fentanyl-midazolam group, respectively. Both these episodes were managed without any serious consequences. In spite of the above episodes there was no significant difference, among the three study groups, vis-a-vis heart rate, blood pressure, respiratory rate, and SpO2 [Table 3]. Combination of sedatives can cause excessive sedation, but studies have demonstrated no increase in adverse events that are clinically significant.[3,13,19] For obvious reasons, patients undergoing flexible bronchoscopy, particularly under sedation, should be monitored for any evidence of hemodynamic instability and oxygen desaturation. However, adverse events should not be a reason to avoid sedation, as these are manageable. There are many studies which support the fact that patient comfort is enhanced due to sedation, and the risks involved are small and manageable.[24,25,26]
Limitations: The impact of rescue medication doses – midazolam's amnestic effect – on the differences between the patient-reported composite scores across the study groups could not be ascertained. The effect of sedation on postprocedure hospital stay was not assessed.
Conclusion
Conscious sedation with fentanyl-midazolam combination can result in better patient and operator satisfaction when compared with midazolam alone, with manageable adverse event profile.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Appendix
Appendix 1

Appendix 2
References
- 1.Lechtzin N, Rubin HR, White P, Jr, Jenckes M, Diette GB. Patient satisfaction with bronchoscopy. Am J Respir Crit Care Med. 2002;166:1326–31. doi: 10.1164/rccm.200203-231OC. [DOI] [PubMed] [Google Scholar]
- 2.Gasparini S. It is time for patients to undergo bronchoscopy without discomfort. Eur Respir J. 2011;38:507–9. doi: 10.1183/09031936.00047311. [DOI] [PubMed] [Google Scholar]
- 3.Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, et al. British thoracic society guideline for diagnostic flexible bronchoscopy in adults: Accredited by NICE. Thorax. 2013;68(Suppl 1):i1–i44. doi: 10.1136/thoraxjnl-2013-203618. [DOI] [PubMed] [Google Scholar]
- 4.Barnett AM, Jones R, Simpson G. A survey of bronchoscopy practice in Australia and New Zealand. J Bronchology Interv Pulmonol. 2016;23:22–8. doi: 10.1097/LBR.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 5.Hwang J, Jeon Y, Park HP, Lim YJ, Oh YS. Comparison of alfetanil and ketamine in combination with propofol for patient-controlled sedation during fiberoptic bronchoscopy. Acta Anaesthesiol Scand. 2005;49:1334–8. doi: 10.1111/j.1399-6576.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 6.Goneppanavar U, Magazine R, Periyadka Janardhana B, Krishna Achar S. Intravenous dexmedetomidine provides superior patient comfort and tolerance compared to intravenous midazolam in patients undergoing flexible bronchoscopy. Pulm Med. 2015;2015:727530. doi: 10.1155/2015/727530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth CM, Stead RJ. Survey of flexible fibreoptic bronchoscopy in the United Kingdom. Eur Respir J. 2002;19:458–63. doi: 10.1183/09031936.02.00103702. [DOI] [PubMed] [Google Scholar]
- 8.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–17. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Rolo R, Mota PC, Coelho F, Alves D, Fernandes G, Cunha J, et al. Sedation with midazolam in flexible bronchoscopy: A prospective study. Rev Port Pneumol. 2012;18:226–32. doi: 10.1016/j.rppneu.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Contoli M, Gnesini G, Artioli D, Ravenna C, Sferra S, Romanazzi C, et al. Midazolam in flexible bronchoscopy premedication: Effects on patient-related and procedure-related outcomes. J Bronchology Interv Pulmonol. 2013;20:232–40. doi: 10.1097/LBR.0b013e3182a10b7a. [DOI] [PubMed] [Google Scholar]
- 11.Morris LG, Zeitler DM, Amin MR. Unsedated flexible fiberoptic bronchoscopy in the resident clinic: Technique and patient satisfaction. Laryngoscope. 2007;117:1159–62. doi: 10.1097/MLG.0b013e31806009e6. [DOI] [PubMed] [Google Scholar]
- 12.Pickles J, Jeffrey M, Datta A, Jeffrey AA. Is preparation for bronchoscopy optimal? Eur Respir J. 2003;22:203–6. doi: 10.1183/09031936.03.00118302. [DOI] [PubMed] [Google Scholar]
- 13.Stolz D, Kurer G, Meyer A, Chhajed PN, Pflimlin E, Strobel W, et al. Propofol versus combined sedation in flexible bronchoscopy: A randomised non-inferiority trial. Eur Respir J. 2009;34:1024–30. doi: 10.1183/09031936.00180808. [DOI] [PubMed] [Google Scholar]
- 14.Uzbeck M, Quinn C, Saleem I, Cotter P, Gilmartin JJ, O'Keeffe ST, et al. Randomised controlled trial of the effect of standard and detailed risk disclosure prior to bronchoscopy on peri-procedure anxiety and satisfaction. Thorax. 2009;64:224–7. doi: 10.1136/thx.2008.101220. [DOI] [PubMed] [Google Scholar]
- 15.Lee K, Orme R, Williams D, Segal R. Prospective pilot trial of dexmedetomidine sedation for awake diagnostic flexible bronchoscopy. J Bronchology Interv Pulmonol. 2010;17:323–8. doi: 10.1097/LBR.0b013e3181f2a002. [DOI] [PubMed] [Google Scholar]
- 16.Mehta AC. Don't lose the forest for the trees: Satisfaction and success in bronchoscopy. Am J Respir Crit Care Med. 2002;166:1306–7. doi: 10.1164/rccm.200208-895IE. [DOI] [PubMed] [Google Scholar]
- 17.Hirose T, Okuda K, Ishida H, Sugiyama T, Kusumoto S, Nakashima M, et al. Patient satisfaction with sedation for flexible bronchoscopy. Respirology. 2008;13:722–7. doi: 10.1111/j.1440-1843.2008.01311.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen XK, Zhou YP, Zhang X, Xia LP, Li AF, Liu H, et al. Conscious sedation with midazolam and dezocine for diagnostic flexible bronchoscopy. Eur Rev Med Pharmacol Sci. 2015;19:3688–92. [PubMed] [Google Scholar]
- 19.Stolz D, Chhajed PN, Leuppi JD, Brutsche M, Pflimlin E, Tamm M, et al. Cough suppression during flexible bronchoscopy using combined sedation with midazolam and hydrocodone: A randomised, double blind, placebo controlled trial. Thorax. 2004;59:773–6. doi: 10.1136/thx.2003.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cases Viedma E, Pérez Pallarés J, Martínez García MA, López Reyes R, Sanchís Moret F, Sanchís Aldás JL, et al. A randomised study of midazolam for sedation in flexible bronchoscopy. Arch Bronconeumol. 2010;46:302–9. doi: 10.1016/j.arbres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Wahidi MM, Jain P, Jantz M, Lee P, Mackensen GB, Barbour SY, et al. American college of chest physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest. 2011;140:1342–50. doi: 10.1378/chest.10-3361. [DOI] [PubMed] [Google Scholar]
- 22.Kaur H, Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R, et al. A randomized trial of 1% vs.2% lignocaine by the spray-as-you-go technique for topical anesthesia during flexible bronchoscopy. Chest. 2015;148:739–45. doi: 10.1378/chest.15-0022. [DOI] [PubMed] [Google Scholar]
- 23.De S. Assessment of patient satisfaction and lidocaine requirement during flexible bronchoscopy without sedation. J Bronchology Interv Pulmonol. 2009;16:176–9. doi: 10.1097/LBR.0b013e3181afca25. [DOI] [PubMed] [Google Scholar]
- 24.Ni YL, Lo YL, Lin TY, Fang YF, Kuo HP. Conscious sedation reduces patient discomfort and improves satisfaction in flexible bronchoscopy. Chang Gung Med J. 2010;33:443–52. [PubMed] [Google Scholar]
- 25.Szczeklik W, Andrychiewicz A, Górka K, Konarska K, Soja J, Sładek K, et al. Flexible bronchoscopy under conscious sedation with midazolam and fentanyl can be safely performed by nonanesthesiologists. Pol Arch Med Wewn. 2015;125:869–71. doi: 10.20452/pamw.3172. [DOI] [PubMed] [Google Scholar]
- 26.Dang D, Robinson PC, Winnicki S, Jersmann HP. The safety of flexible fibre-optic bronchoscopy and proceduralist-administered sedation: A tertiary referral centre experience. Intern Med J. 2012;42:300–5. doi: 10.1111/j.1445-5994.2010.02261.x. [DOI] [PubMed] [Google Scholar]


