Abstract
Background:
Leukemia inhibitory factor (LIF) has been reported to possess various pharmacological effects, including displaying vascular and neuroprotective properties, during retinal disease. The aim of this study was to investigate the vascular and structural changes in the retina of diabetic mice and to explore whether LIF prevents experimental diabetes-induced retinal injury in the early stages.
Methods:
Diabetes was induced in C57Bl/6J mice with streptozotocin (STZ) injections. Successful diabetic animal models were randomly separated into two groups: the diabetic group (n = 15) and the LIF-treated group (n = 15). Normal C57BL/6 mice served as the normal control group (n = 14). Recombinant human LIF was intravitreally injected 8 weeks after the diabetic model was successfully established. Retinas were collected and evaluated using histological and immunohistochemical techniques, and flat-mounted retinas and Western blotting were performed at 18 weeks after the induction of diabetes and 2 days after the intravitreal injection of LIF. The analysis of variance test were used.
Results:
Histological analysis showed that there were fewer retinal ganglion cells (RGCs) and the inner nuclear layer (INL) became thinner in the diabetic model group (RGC 21.8 ± 4.0 and INL 120.2 ± 4.6 μm) compared with the normal control group (RGC 29.0 ± 6.7, t = −3.02, P = 0.007; INL 150.7 ± 10.6 μm, t = −8.88, P < 0.001, respectively). After LIF treatment, the number of RGCs (26.9 ± 5.3) was significantly increased (t = 3.39, P = 0.030) and the INL (134.5 ± 14.2 μm) was thicker compared to the diabetic group (t = 2.75, P = 0.013). In the anti-Brn-3a-labeled retinas, the number of RGCs in the LIF-treated group (3926.0 ± 143.9) was obviously increased compared to the diabetic group (3507.7 ± 286.1, t = 2.38, P = 0.030), while no significance was found between the LIF-treated group and the control group (4188.3 ± 114.7, t = −2.47, P = 0.069). Flat-mounted retinas demonstrated that a disorganized, dense distribution of the vessel was prominent in the diabetic model group. Vessel distribution in the LIF-treated mouse group was typical and the thickness was uniform. The levels of phosphosignal transducer and activator of transcription 3 activation were obviously higher in the LIF-injected retinas than those in the diabetic control group (t = 3.85, P = 0.019) and the normal control (t = −3.20, P = 0.019).
Conclusion:
The present study provides evidence that LIF treatment protects the integrity of the vasculature and prevents retinal injury in the early stages of diabetic retinopathy in STZ-induced diabetic models.
Keywords: Diabetic Retinopathy, Leukemia Inhibitory Factor, Streptozotocin-induced Diabetic Mice
INTRODUCTION
Diabetic retinopathy (DR) is an important cause of vision loss in working-age adults in developed countries.[1] While the mechanisms underlying the development and progression of DR are not fully understood, it has become increasingly clear that DR not only affects the retinal vasculature, but also induces damage in nonvascular retinal neuronal and glial cells.
In addition to the characteristic vascular changes of DR that have been widely documented in diabetic models,[2,3] recent studies have emphasized the importance of diabetes-induced neuronal damage in the retina.[4,5] Some studies have suggested that changes in the functional molecules and viability of the neurons in the retina occur early after the onset of diabetes and precede the regression of the retinal vasculature.[6,7] Neurodegeneration in the diabetic retina is indicated by the loss of specific cell types and reduced retinal layer thickness.[8,9] Martin et al.[8] reported retinal ganglion cell (RGC) loss and thinning of the retina after 10 weeks in the streptozotocin (STZ)-induced diabetic rat models; this was also reported by Barber et al.[9] in diabetic mouse models. A gradual decrease in the number of RGCs was demonstrated in previous studies with diabetic C57BL/6 mouse models.[10,11] There was a 27% decrease in the thickness of the inner plexiform layer in mice after 5.5 months of STZ-induced diabetes;[12] however, other researchers did not detect ganglion cell loss in diabetic mouse models.[13,14]
Leukemia inhibitory factor (LIF), a glycoprotein belonging to the interleukin 6 family of cytokines, is predominantly expressed in endothelial cells, and LIF receptor (LIFR) is expressed in the surrounding cells, such as retinal astrocytes, during vascular development.[15] In the early 1990s, LIF's neuroprotective potential was recognized in cultured neuronal cells[16] and in a variety of animal models with injury and disease.[17,18,19] LIF's neuroprotective effects on the retina in light damage were also recently reported. For example, the upregulation of LIF in retinas was observed by exposure to excessive levels of light,[20,21] while the lack of LIF signaling led to increased photoreceptor death in the light-induced LIF-/- mice model[22] and in the inherited retinal degeneration model.[23] Thus, Bürgi et al.[22] hypothesized that therapeutic stimulation of the LIF pathway might provide new insights into the potential beneficial effects to prevent or delay photoreceptor degeneration in degenerative retinal diseases;[22] the results were striking. Studies have shown that intravitreal application of recombinant LIF can protect photoreceptors from light damage.[24,25,26] These studies also demonstrated that LIF protection occurred through the activation of the glycoprotein (Gp130) receptor/signal transducer and activator of transcription 3 (STAT3) pathway.
The effect on the vessels was observed with an examination of LIF expression in animal model studies.[15,27,28] Pepper et al.[28] reported that LIF inhibits angiogenesis in a three-dimensional in vitro model, and this inhibitory effect occurred irrespective of the angiogenic stimulus, including basic fibroblast growth factor and vascular endothelial growth factor (VEGF). Ash et al.[27] showed that LIF reduced the development of the embryonic vasculature in the eye and inhibited retinal vascular development in transgenic mice. In vivo inhibition was independent of VEGF expression. Another study showed that LIF modulates oxygen-dependent VEGF expression, and it is essential for ensuring proper capillary density using LIF-/- mice.[15]
Considering these findings, LIF might prevent the growth and differentiation of vascular endothelial cells during retinal angiogenesis and early neuronal degeneration in ongoing DR; however, the mechanism for this inducible protection is far from fully understood. To our knowledge, the physiological role of LIF in angiogenesis and its neuroprotective effect for DR have not been assessed to date. The study investigated the vascular changes and neuropathy in the retina of C57Bl/6J mice, a commonly used mouse strain. We also explored whether LIF prevents experimental diabetes-induced retinal injury in the early stages.
METHODS
All the procedures were performed according to the Association for Research and Vision in Ophthalmology and China Animal Welfare Legislation statement about the use of animals in ophthalmic and vision research. The protocol used in this study was approved by the Institutional Review Board of Beijing Friendship Hospital and its affiliate, Capital Medicine University (Beijing, China).
Establishment of diabetes model
STZ was purchased from Sigma (St. Louis, MO, USA). Test kits for the following compounds were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, China).
Six-week-old male C57BL/6 mice (No: 2002-5, SCXK [Chuan]) were purchased from the Institute of Laboratory Animals of Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, Sichuan, China. The mice were randomly placed into two groups: the diabetes model and the control. Diabetes was induced by daily intraperitoneal injections of freshly prepared STZ (S0130 Sigma, USA) at a dose of 60 mg/kg (diluted in a 0.1 mol/L citrate buffer, pH 4.2) for 3 days into C57BL/6 mice after they had fasted for 12 h. The blood glucose concentration was measured from a tail vein using a Medisafe Mini (Terumo, Tokyo, Japan) at 1 week after STZ injection. The development of diabetes was defined as having a blood glucose level that was higher than 13.9 mmol/L (2.50 g/L) 7 days after the first injection of STZ. The successful diabetes animal models were randomly separated into two groups: the diabetic group (n = 15) and the LIF-treated group (n = 15). The normal C57BL/6 mice served as the normal control group (n = 14).
Leukemia inhibitory factor injection
Recombinant human LIF was injected intravitreally as described previously.[25] Intravitreal injections were initiated 8 weeks after the diabetic model was successfully established. The mice were injected three times every 2 weeks. Mice were deeply anesthetized with a single intraperitoneal injection of xylazine (7 mg/kg) and ketamine (40 mg/kg). One microliter (0.5 μg) of LIF was injected intravitreally into the right eye using a 36G needle through the temporal limbus of the eye. Any eye that showed signs of damage due to the intravitreal injection, such as bleeding, inflammation, or morphological disruption, was excluded from the analysis.
Histopathological examination and immunohistochemistry
Mice were killed with CO2 asphyxiation. The eyes were enucleated and fixed in 4% paraformaldehyde (PFA) solution, and then sectioned sagittally (4 μm) so that each section that passed through or next to the optic nerve was collected. The sections of eye chips were stained with hematoxylin and eosin (H and E), and then they were observed and photographed under a light microscope. The number of cells in the ganglion cell layer (GCL) was counted for a 500-μm linear distance on each side of the optic nerve (adjacent to the optic nerve; three regions adjacent to each eye measurement, measured nine times). The counts were averaged and reported per unit length of retina.
Flat-mounted retinas
The enucleated eyes were fixed in 4% PFA for 1 h for whole-mount retinal staining. The anterior segment of the eye and vitreous humor were removed. The retinas were dissected from the sclera and flattened on a glass slide. The retinas were then dissected as flattened whole mounts by making four radial cuts. Retinal sections stained with GSL I-isolectin-B4 (B-1205, Vector Laboratories, USA) or anti-Brn-3a (C-20) antibody (sc-31984, Santa Cruz Biotechnology, USA) were imaged and analyzed with an inverted fluorescent/bright field microscope Nikon Eclipse Ti (Nikon, Tokyo, Japan) with a digital camera CoolSNAP HQ2 (Photometrics, Tucson, AZ, USA) linked to a computer running the NIS-Elements Advanced Research imaging analysis software (Nikon, Tokyo, Japan).
Western blotting
Retinas were harvested immediately after the animals were killed, at day 2 after 3 injections of LIF at a 2-week interval, and homogenized in a lysis buffer (50 mmol/L Tris-HCl [pH 7.5], 150 mmol/L NaCl, 5 mmol/LEDTA, 1% [v/v] NP-40, 5% [v/v] glycerol, and protease inhibitor cocktail, [Calbiochem®, San Diego, CA, USA]). The protein content was measured using a BCA protein assay (Pierce, Rutherford, IL, USA), and the total protein from each sample (15 μg) was electrophoresed on 4–20% gradient SDS-polyacrylamide gels (Invitrogen, Carlsbad, CA, USA) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were incubated in blocking buffer (5% BSA in TBST [20 mmol/L Tris-HCl, pH 7.5, 100 mmol/L NaCl, and 0.1% Tween-20]) for 1 h at room temperature, and then incubated overnight at 4°C with rabbit polyclonal anti-phospho-STAT3 antibody (S2690, Sigma, USA) in blocking buffer, followed by 1 h of incubation at room temperature with anti-rabbit IgG/HRP antibody (SE134, Solarbio, China). Signals were visualized using SuperSignal West Dura Extended Duration Substrate (Pierce, Rutherford, IL, USA) and quantified by conventional digital imaging analysis using an Image Station 4000R (Eastman Kodak, Rochester, NY, USA). Blotting were stripped and reprobed with rabbit polyclonal anti-STAT3 (12640S, Cell Signaling Technology, Beverly, MA, USA) and anti-GAPDH antibody (60004-1-Ig, Proteintech, USA) followed by the appropriate secondary antibodies for quantification of the bands.
Statistical analyses
Results were expressed as mean ± standard deviation (SD). Data analyses were performed using the Statistical Package for the Social Sciences (SPSS version 19.0; SPSS Inc., Chicago, IL, USA). The analysis of variance (ANOVA) test was used to compare the differences between more than two groups. A value of P < 0.05 was deemed to be of statistical significance.
RESULTS
Establishment of diabetes model
Diabetic mice showed a significant decrease in bodyweight and a significant increase in blood glucose compared with the age-matched controls. Diabetic mice had a 14.9% gain in weight from 2 to 14 weeks after the onset of diabetes, whereas age-matched controls had a 36% gain in weight. By 14 weeks after the onset of diabetes, the diabetic mice weighed significantly less than the control mice. Blood glucose levels differed significantly between the diabetic and control mice at all of the ages studied [Table 1].
Table 1.
Average body weight and fasting blood glucose levels of control and diabetic mice at different time periods
| Time (week) | Body weight (g) | t | P | Blood glucose (mmol/L) | t | P | ||
|---|---|---|---|---|---|---|---|---|
| Normal (n = 14) | Diabetic (n = 30) | Normal (n = 14) | Diabetic (n = 30) | |||||
| 1 | 21.95 ± 2.75 | 21.00 ± 1.82 | 1.36 | 0.170 | 9.07 ± 1.31 | 23.47 ± 2.71 | 18.79 | <0.001 |
| 5 | 25.01 ± 2.80 | 21.60 ± 3.68 | 3.97 | 0.004 | 6.86 ± 0.81 | 22.48 ± 7.41 | 7.82 | <0.001 |
| 10 | 26.47 ± 3.14 | 21.94 ± 4.04 | 3.71 | 0.006 | 9.56 ± 1.83 | 19.05 ± 6.09 | 5.68 | <0.001 |
| 14 | 28.90 ± 1.30 | 24.13 ± 4.69 | 3.72 | 0.006 | 7.53 ± 0.57 | 22.89 ± 2.13 | 26.39 | <0.001 |
The data are shown as mean ± standard deviation. All the results were comparisons between the normal group and the diabetic group.
Histological analysis showed that the morphology and thickness of the inner nuclear layer were improved in the leukemia inhibitory factor-treated group compared with the diabetic group
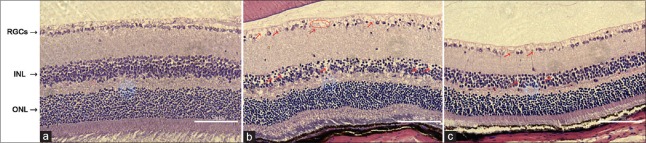
We noted a densely packed structure and a typically uniform distribution of cells in the retina of the normal control group. The thickness of the inner nuclear layer (INL) was significantly reduced by 18 weeks after the onset of diabetes in the diabetic mice group, while there were no obvious changes in the thickness of the outer nuclear layer (ONL). The INL cells were sparse and disordered, and there was a vacuole-like change. A significant reduction in the number of cells within the RGC layer was also noted. While the histological changes of the LIF-treated group were basically the same as that of the model group, the changes in each layer were slightly less than those in the diabetic group. For example, there were more INL cells, they were more ordered, and the vacuole-like changes were better than those in the diabetic group; the same was true for the RGCs [Figure 1].
Figure 1.
Retinal histopathology and morphology results. (a) Densely packed structure and typically uniform distribution of cells were noted in the control retina. (b) Higher levels of vacuole-like change and disordered change were observed in both the ganglion cell layer and the inner nuclear layer in the diabetic group as compared to the control group. (c) Such changes were improved in the LIF-treated group compared to the diabetic group (bar: 100 μm). RGCs: Retinal ganglion cells; INL: Inner nuclear layer; ONL: Outer nuclear layer; LIF: Leukemia inhibitory factor.
The INL in the diabetic group (120.2 ± 4.6 μm) was thinner than that in the normal control group (150.7 ± 10.6 μm, t = −8.88, P < 0.001, Table 2). After the treatment with LIF, the thickness of the INL (134.5 ± 14.2 μm) was obviously increased (t = 2.75, P = 0.013, Table 2), while the thickness of the ONL was 207.6 ± 53.7 μm, 171.1 ± 14.3 μm, and 185.2 ± 27.1 μm in the normal control group, the diabetic group, and the LIF-treated group, respectively. Although there was no significant difference observed in the ONL among the groups, we could see that the ONL in the LIF-treated group was thicker than that in the diabetic group [Table 2]. No significant difference in the thickness of the inner or outer plexiform layers was noted among the groups as well.
Table 2.
Number of ganglion cells and thickness of the retinas from histopathological examination
| Group | n | Ganglion cells, n | Thickness of inner nuclear layer (µm) | Thickness of outer nuclear layer (µm) |
|---|---|---|---|---|
| Control | 5 | 29.0 ± 6.7 | 150.7 ± 10.6 | 207.6 ± 53.7 |
| Diabetic model | 8 | 21.8 ± 4.0* | 120.2 ± 4.6* | 171.1 ± 14.3 |
| LIF-treated mice | 8 | 26.9 ± 5.3† | 134.5 ± 14.2† | 185.2 ± 27.1 |
The data are shown as mean ± SD. The number of cells in the ganglion cell layer adjacent to the optic nerve was counted. *P<0.05 diabetic model versus the control group, t = 2.34 (P = 0.032) and 7.26 (P<0.010), respectively; †P<0.05, LIF-treated group versus the diabetic group, t = 2.17 (P = 0.047) and t = 2.33 (P = 0.030), respectively. LIF: Leukemia inhibitory factor; SD: Standard deviation.
The number of cells in the ganglion cell layer was increased in leukemia inhibitory factor-treated group
Additional measurements were taken of the number of cells in the GCL of the experimental mice using two methods. In the retina chip adjacent to the optic nerve stained with H and E, the number of RGCs in the diabetic model group (21.8 ± 4.0) were less than those in the normal mice (29.0 ± 6.7, t = −3.02, P = 0.007), and the number of RGCs was significantly increased after LIF treatment (26.9 ± 5.3, t = 3.39, P = 0.030, Table 2). Representative retinal sections stained with H and E obtained from each of the experimental groups are shown in Figure 1.
We also counted the number of RGCs in the anti-Brn-3a-labeled flat-mounted retinas. It was significant while comparing among the three groups (F = 9.16, P = 0.010). The number of RGCs in the diabetic group was 3507.7 ± 286.0, which was obviously less compared with the control group, with 4188.3 ± 114.7 (t = −6.35, P < 0.001). In the LIF-treated group, it was 3926.0 ± 143.9, obviously increased compared with the diabetic group (t = 2.38, P = 0.003), but there was no significance compared with the control group (t = −2.47, P = 0.069, Figure 2).
Figure 2.
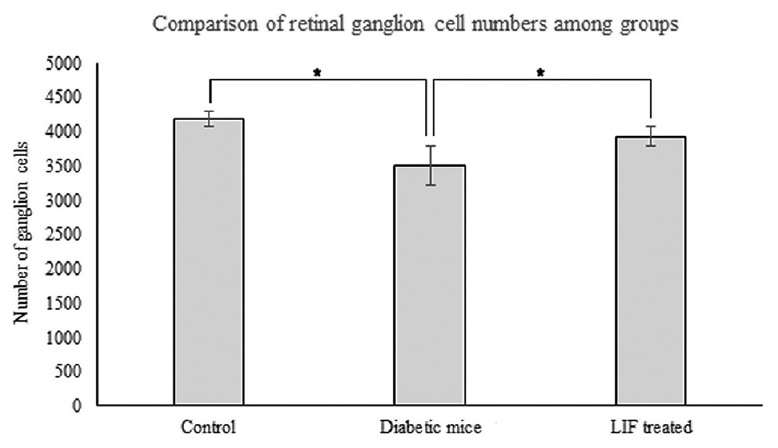
Number of ganglion cells among the groups in anti-Brn-3a-labeled flat-mounted retinas. Comparing with the diabetic group, ganglion cells were higher in the normal control group (t = −6.35, P < 0.001, n = 3) and in the LIF-treated group (t = 2.38, P = 0.030, n = 3). It was significant while comparing among the three groups (F = 9.16, P = 0.010). *P < 0.05. LIF: Leukemia inhibitory factor.
Vessel changes in the leukemia inhibitory factor-treated mice group were better than those in the diabetic group
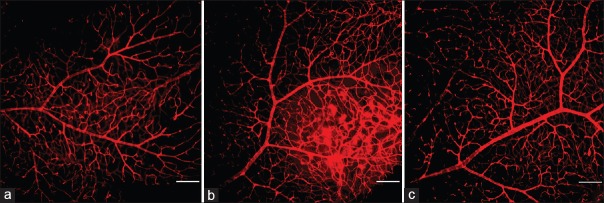
Vascular changes were found in the flat-mounted retinas. A clear organized branching pattern and regular distribution of the blood vessels were found in the control group. The vessels had a straight shape and uniform diameter. In contrast, a disorganized, dense distribution of capillaries was prominent in the retinas as early as 18 weeks after STZ-induced diabetes was established. A plurality of capillaries was convoluted and twisted together in a cluster. The diameter of the capillary lumen was uneven and most of the capillaries showed as a segmental enlargement or twisting into a loop. Compared with the diabetic model group, the retinal microvascular changes in the LIF-treated mice were more subtle. No obvious dilation or tortuous changes in the vessels were found after LIF treatment. The distribution of vessels in the LIF-treated mouse group was regular. Representative retinal sections from each experimental group are shown in Figure 3.
Figure 3.
Characterization of the retinal vasculature in the nondiabetic control and STZ-induced diabetic mice. Freshly dissected retinas were immediately fixed and stained with an endothelial cell marker and visualized. Nondiabetic mice had an organized retinal vascular branching pattern (a). After 18 weeks of diabetes, the vascular distribution in the retinas was dense and disorganized (b), but this improved after LIF treatment (c) (bar: 100 μm). STZ: Streptozotocin; LIF: Leukemia inhibitory factor.
Leukemia inhibitory factor and phosphorylation of signal transducer and activator of transcription 3
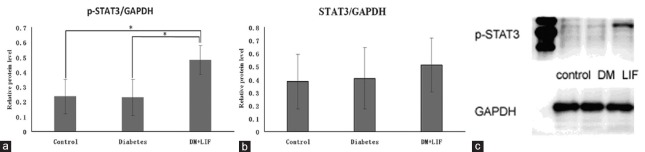
To determine the temporal kinetics of LIF-induced signaling, the phosphorylation of STAT3 (p-STAT3) was quantitatively measured by Western blotting of retinal proteins collected at 2 days following the three injections of LIF at an interval of 2 weeks [Figure 4]. The relative protein level of STAT3/GAPDH was 0.38, 0.40, and 0.50 in the normal control group, the diabetic control group, and the LIF-treated group, respectively. There was no significance among the groups, while the value of p-STAT3/GAPDH was 0.23, 0.22, and 0.48, respectively, for the above-mentioned three groups, and it was significant among the groups (F = 6.40, P = 0.018). The level of p-STAT3/GAPDH was obviously higher in the LIF-injected retinas than that in the diabetic control group (t = 3.85, P = 0.019) and the normal control group (t = −3.20, P = 0.019).
Figure 4.
LIF acts via phosphorylation of STAT3 (p-STAT3). (a) Histogram of the relative protein level of p-STAT3/GAPDH shows that the value of P-STAT3/GAPDH was statistically increased in the LIF-treated group (n = 4), compared with the diabetic control group (t = 3.85, P = 0.019, n = 4) and the normal control group (t = −3.20, P = 0.019, n = 4). It is significant among the groups (F = 6.40, P = 0.018). *P < 0.05. (b) Histogram of STAT3/GAPDH shows that for the value of STAT3/GAPDH, there was no significance among the groups. (c) A representative Western blotting shows obvious STAT3 activation at 2 days after intravitreal injection of LIF. LIF: Leukemia inhibitory factor; DM: Diabetic model; STAT3: Signal transducer and activator of transcription 3; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
The present study investigated both the retinal vascular and structural changes in STZ-induced diabetic C57BL/6 mice and analyzed LIF's possible ability to induce photoreceptor protection following intravitreal LIF injection. The data showed remarkable neuroprotective effects against diabetes-induced RGC degeneration in mice and improved vascular changes. To the best of our knowledge, this was a rare study that intravitreal LIF treatment in a DR animal model was investigated.
Consistent with previous studies,[8,9,10,11,12] reduced RGCs and a thinner INL were found in the diabetic mice compared with the normal control group. We also observed a series of vascular changes after diabetes induction, such as a disorganized, dense distribution of capillaries and dilated capillaries within the retina. Similar phenomena were also reported by Weerasekera et al.[3] The fluctuation of retinal blood flow was an early consequence of diabetes and could bring about capillary dilation.[29]
The number of RGCs was significantly increased and capillary distribution became typical after the intravitreal injection of LIF in the present study, which suggested that LIF probably has neuroprotective and vascular protective effects. The neuroprotective activities of LIF have been documented in a variety of neuronal systems, as well as in the retina.[23,24,25,26,30] Ueki et al.[25] reported the protective effects of photoreceptors against light-induced retinal damage in mice after intravitreal LIF treatments, and signal transduction in the photoreceptors occurred directly by activating the LIFR/gp130 complexes. Gp130 is a common receptor for the IL-6 family of cytokines. Another study showed that photoreceptor-specific Gp130 knockout mice had accelerated photoreceptor degeneration in an animal model of retinitis pigmentosa.[31] Gp130 activation in the photoreceptors had a general protective role independent of whether stress was caused by light or genetic mutations.[26] Similar findings were reported by other studies showing that the photoreceptors with inherited retinal degeneration died faster in the absence of LIF[23] or in the presence of a LIFR antagonist.[21] Joly et al.[23] showed that in the absence of LIF, Muller cells remained quiescent, the molecular pathway was not activated, and retinal degeneration was strongly accelerated. Intravitreal application of recombinant LIF induces the complete molecular pathway, including the activation of Muller cells in wild-type and LIF-/- mice. These studies demonstrated that LIF was essential and sufficient to activate an extensive molecular defense response to photoreceptor injury. It has also been suggested that LIF or its gp130 ligands may be effective in preventing or delaying neurodegeneration in human diseases.[26]
To our knowledge, the effect of LIF on the vessels in vivo was inconsistent. Kubota et al.[15] found that LIF modulates oxygen-dependent VEGF expression, and it is essential to ensure proper capillary density in LIF-/- mice. They also showed that LIF was predominantly expressed in the developing endothelium, and the LIFR was expressed in the surrounding cells, such as retinal astrocytes. Another study examined the expression of LIF in vivo using transgenic mice and suggested that LIF is a potent inhibitor of retinal vascular development.[27] The present study showed that the abnormal vessels were obviously ameliorated in the diabetic mice after they were intravitreally injected with LIF; however, neither the physiological role of LIF in angiogenesis nor the precise underlying mechanisms in the vasoinhibitory effect is known.
STAT3 is viewed as the most important signal transducer following stimulation by LIF, and it is the one that mediates most of the cellular effects.[32] In the LIF-treated group, the p-STAT3 was obviously higher than that in the other groups in the study. Similar as in other studies,[24,25] the present study suggested that this LIF-mediated protection correlates well with the activation of STAT3. Taken together, these studies suggested that LIF might be part of a retinal defense mechanism to increase the survival of ocular cells and improve the vascular changes that accompany diabetes. Although little is known about the mechanism of protection, LIF has been shown to be upregulated in response to different types of retinal stress[24] and it is essential for reducing oxidative stress in the retina.[21] We have suggested a possible effect of LIF on vascular changes in DR, but further studies are warranted.
The main limitation of the study is that the mechanism was not perfect as there might be multiple complicated LIF-mediated signaling pathways and only the STAT3 signaling pathway was tested. Another limitation is the lack of evaluating different times of activation of STAT3. In this study, STAT3 was detected only after 2 days following 3 injections of LIF, but it has been reported that the LIF-induced activation of STAT3 was detectable within 30 min and lasted between 4 and 9 days.[24,25] Third, while the results can serve as a basis for further studies, this article only involved a preclinical animal experiment. Therefore, in order to fully understand the mechanism of LIF, we need to advance the experimental design in the future.
In summary, the present study provides evidence that treatment with LIF, a member of the IL-6 family of cytokines, in STZ-induced DR retinas preserved the integrity of the vasculature and prevented retinal injury in the early stages of DR. This research proposes a possible therapeutic strategy against DR and provides important information to initiate further work.
Financial support and sponsorship
This work was supported by grants from the Scientific Research Common Program of Beijing Municipal Commission of Education (No. KM201410025017), and the High-level Technical Personnel Training Program of Beijing Municipal Health System (No. 2014-3-007).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Stratton IM, Aldington SJ, Taylor DJ, Adler AI, Scanlon PH. A simple risk stratification for time to development of sight-threatening diabetic retinopathy. Diabetes Care. 2013;36:580–5. doi: 10.2337/dc12-0625. doi: 10.2337/dc12-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–12. doi: 10.2337/diabetes.51.10.3107. doi: 10.2337/diabetes. [DOI] [PubMed] [Google Scholar]
- 3.Weerasekera LY, Balmer LA, Ram R, Morahan G. Characterization of retinal vascular and neural damage in a novel model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:3721–30. doi: 10.1167/iovs.14-16289. doi: 10.1167/iovs.14-16289. [DOI] [PubMed] [Google Scholar]
- 4.Oshitari T, Hata N, Yamamoto S. Endoplasmic reticulum stress and diabetic retinopathy. Vasc Health Risk Manag. 2008;4:115–22. doi: 10.2147/vhrm.2008.04.01.115. doi:10.2147/vhrm.2008.04.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586:4401–8. doi: 10.1113/jphysiol.2008.156695. doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber AJ. A new view of diabetic retinopathy: A neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–90. doi: 10.1016/S0278-5846(03)00023-X. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 7.Kern TS. Interrelationships between the retinal neuroglia and vasculature in diabetes. Diabetes Metab J. 2014;38:163–70. doi: 10.4093/dmj.2014.38.3.163. doi: 10.4093/dmj.2014.38.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–6. doi: 10.1167/iovs.04-0247. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- 9.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:1156–63. doi: 10.1167/iovs.10-6293. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Mao D, Chen X, Zhao L, Tian Q, Liu C, et al. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Mol Vis. 2012;18:1411–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan D, Xu Y, Hang H, Liu X, Chen X, Xie P, et al. Edaravone protect against retinal damage in streptozotocin-induced diabetic mice. PLoS One. 2014;9:e99219. doi: 10.1371/journal.pone.0099219. doi: 10.1371/journal.pone.0099219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–8. doi: 10.1167/iovs.04-1340. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 13.Howell SJ, Mekhail MN, Azem R, Ward NL, Kern TS. Degeneration of retinal ganglion cells in diabetic dogs and mice: Relationship to glycemic control and retinal capillary degeneration. Mol Vis. 2013;19:1413–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Feit-Leichman RA, Kinouchi R, Takeda M, Fan Z, Mohr S, Kern TS, et al. Vascular damage in a mouse model of diabetic retinopathy: Relation to neuronal and glial changes. Invest Ophthalmol Vis Sci. 2005;46:4281–7. doi: 10.1167/iovs.04-1361. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- 15.Kubota Y, Hirashima M, Kishi K, Stewart CL, Suda T. Leukemia inhibitory factor regulates microvessel density by modulating oxygen-dependent VEGF expression in mice. J Clin Invest. 2008;118:2393–403. doi: 10.1172/JCI34882. doi: 10.1172/JCI34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy M, Reid K, Hilton DJ, Bartlett PF. Generation of sensory neurons is stimulated by leukemia inhibitory factor. Proc Natl Acad Sci U S A. 1991;88:3498–501. doi: 10.1073/pnas.88.8.3498. doi:10.1073/pnas.88.8.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azari MF, Profyris C, Karnezis T, Bernard CC, Small DH, Cheema SS, et al. Leukemia inhibitory factor arrests oligodendrocyte death and demyelination in spinal cord injury. J Neuropathol Exp Neurol. 2006;65:914–29. doi: 10.1097/01.jnen.0000235855.77716.25. doi: 10.1097/01.jnen.0000235855.77716.25. [DOI] [PubMed] [Google Scholar]
- 18.Trouillas M, Saucourt C, Guillotin B, Gauthereau X, Taupin JL, Moreau JF, et al. The LIF cytokine: Towards adulthood. Eur Cytokine Netw. 2009;20:51–62. doi: 10.1684/ecn.2009.0148. doi: 10.1684/ecn.2009.0148. [DOI] [PubMed] [Google Scholar]
- 19.Mathieu ME, Saucourt C, Mournetas V, Gauthereau X, Thézé N, Praloran V, et al. LIF-dependent signaling: New pieces in the Lego. Stem Cell Rev. 2012;8:1–5. doi: 10.1007/s12015-011-9261-7. doi: 10.1007/s12015-011-9261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samardzija M, Wenzel A, Aufenberg S, Thiersch M, Remé C, Grimm C, et al. Differential role of Jak-STAT signaling in retinal degenerations. FASEB J. 2006;20:2411–3. doi: 10.1096/fj.06-5895fje. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- 21.Chollangi S, Wang J, Martin A, Quinn J, Ash JD. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol Dis. 2009;34:535–44. doi: 10.1016/j.nbd.2009.03.012. doi: 10.1016/j.nbd.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bürgi S, Samardzija M, Grimm C. Endogenous leukemia inhibitory factor protects photoreceptor cells against light-induced degeneration. Mol Vis. 2009;15:1631–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Joly S, Lange C, Thiersch M, Samardzija M, Grimm C. Leukemia inhibitory factor extends the lifespan of injured photoreceptors in vivo. J Neurosci. 2008;28:13765–74. doi: 10.1523/JNEUROSCI.5114-08.2008. doi: 10.1523/JNEUROSCI.5114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chucair-Elliott AJ, Elliott MH, Wang J, Moiseyev GP, Ma JX, Politi LE, et al. Leukemia inhibitory factor coordinates the down-regulation of the visual cycle in the retina and retinal-pigmented epithelium. J Biol Chem. 2012;287:24092–102. doi: 10.1074/jbc.M112.378240. doi: 10.1074/jbc.M112.378240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueki Y, Wang J, Chollangi S, Ash JD. STAT3 activation in photoreceptors by leukemia inhibitory factor is associated with protection from light damage. J Neurochem. 2008;105:784–96. doi: 10.1111/j.1471-4159.2007.05180.x. doi: 10.1111/j.1471-4159.2007.05180.x. [DOI] [PubMed] [Google Scholar]
- 26.Ueki Y, Le YZ, Chollangi S, Muller W, Ash JD. Preconditioning-induced protection of photoreceptors requires activation of the signal-transducing receptor gp130 in photoreceptors. Proc Natl Acad Sci U S A. 2009;106:21389–94. doi: 10.1073/pnas.0906156106. doi: 10.1073/pnas.0906156106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ash J, McLeod DS, Lutty GA. Transgenic expression of leukemia inhibitory factor (LIF) blocks normal vascular development but not pathological neovascularization in the eye. Mol Vis. 2005;11:298–308. [PubMed] [Google Scholar]
- 28.Pepper MS, Ferrara N, Orci L, Montesano R. Leukemia inhibitory factor (LIF) inhibits angiogenesis in vitro . J Cell Sci. 1995;108(Pt 1):73–83. doi: 10.1242/jcs.108.1.73. [DOI] [PubMed] [Google Scholar]
- 29.Patel V, Rassam S, Newsom R, Wiek J, Kohner E. Retinal blood flow in diabetic retinopathy. BMJ. 1992;305:678–83. doi: 10.1136/bmj.305.6855.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham DR, Overbeek PA, Ash JD. Leukemia inhibitory factor blocks expression of Crx and Nrl transcription factors to inhibit photoreceptor differentiation. Invest Ophthalmol Vis Sci. 2005;46:2601–10. doi: 10.1167/iovs.05-0129. doi: 10.1167/iovs.05-0129. [DOI] [PubMed] [Google Scholar]
- 31.Abu El-Asrar AM, Al-Mezaine HS. Advances in the treatment of diabetic retinopathy. Saudi J Ophthalmol Soc. 2011;25:113–22. doi: 10.1016/j.sjopt.2011.01.005. doi: 10.1016/j.sjopt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raz R, Lee CK, Cannizzaro LA, d’Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 1999;96:2846–51. doi: 10.1073/pnas.96.6.2846. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]