Abstract
Background:
One of the most common aesthetic problem encountered in the field of periodontology is gingival recession, which is, perceived by the patients as increase in length of teeth. The treatment of buccal gingival recession is a common requirement due to aesthetic concern or root sensitivity. This study was planned to evaluate the efficacy of PRF membrane compared to that of CTG in Miller's class I gingival recessions.
Materials and Methods:
32 sites with Miller's Class I gingival recessions, out of which 16 sites received PRF (test) and 16 sites received CTG (control). Each patient had undergone an initial periodontal treatment, including oral hygiene instructions, plaque control, and scaling and root planing, followed by re-evaluation. All clinical recordings; recession height, recession width, clinical attachment level, height of keratinized tissue, thickness of keratinized tissue, healing index and pain perception, were performed immediately before surgery (baseline) and after 6 months interval following periodontal surgery.
Results:
In the test group, significant improvement was seen in terms CAL, REC-HT, REC-WD, HKT and TKT from baseline to 6 months. In the control group, only significant improvement seen was in REC-HT and TKT from baseline to 6 months. Comparison of both Healing Index and VAS score was done and it showed no significant difference between test and the control group except VAS at 1 week.
Conclusion:
Though CTG is a gold standard procedure, PRF can be used as an alternative procedure by keeping patient's comfort and recognition in mind.
Keywords: Connective tissue graft, gingival recession, platelet-rich fibrin, recession coverage
Introduction
Esthetic dentistry is defined as “a field of dentistry concerned, especially with the appearance of the dentition and its tooth supporting structures as achieved through its arrangement, form, and color.” One of the most common esthetic problem encountered in the field of periodontology is gingival recession which is perceived by the patients as increasing length of teeth. Gingival recession is defined clinically as “the exposure of the root surface by an apical shift in the position of the gingiva.”
Successful treatment of recession-type defects is based on the use of clinically predictable periodontal plastic surgery procedures.[1] As first proposed by Miller in 1988,[2] periodontal plastic surgery comprises different surgical techniques intended to correct and prevent anatomic, developmental, traumatic, or plaque disease-induced defects of the gingiva, alveolar mucosa, or bone. Coronally advanced flaps (CAFs),[3,4] laterally repositioned flaps,[5,6] free gingival grafts,[7,8] and subepithelial connective tissue grafts (CTGs)[9] appeared as promising and predictable approaches for the treatment of gingival recessions.
The Consensus Report of the Sixth European Workshop on Periodontology affirmed that CAF as a stand-alone procedure is a safe and predictable approach for root coverage in single Miller's Class I and II gingival recession defects. CTG and enamel matrix derivative (EMD) in combination with CAF procedure provided better results than CAF alone.[10]
Use of CTG is a widely accepted procedure for the treatment of isolated and multiple gingival recession defects and is considered as the gold standard procedure.[11] However, it does have certain limitations, such as the requirement of the second surgical site that may cause a certain degree of discomfort, and an increased risk of postoperative complications, such as pain and hemorrhage. Limited availability of the graft material from a single donor site further complicates its use in the treatment of multiple gingival recession defects.
The use of growth factors for periodontal tissue engineering was recently reviewed by Taba et al.[12] A recent innovation in dentistry is the use of second-generation platelet concentrate which is an autologous platelet-rich fibrin (PRF) gel with growth factors and cicatricial properties for root coverage procedures.
Numerous studies have been done on both CTG and PRF for root coverage procedures. A study done by Cortellini et al. in 2009[13] suggested that adjunctive application of a CTG under a CAF increased the probability of achieving complete root coverage (CRC) in maxillary Miller Class I and II defects. Similarly, a study done by Aroca et al. in 2009[14] revealed that the addition of a PRF membrane positioned under a modified CAF provided inferior root coverage but an additional gain in the gingival thickness at 6 months compared to conventional therapy alone.
Looking into the advantages and limitations for both the groups, this study was planned to evaluate the efficacy of PRF membrane for the treatment of gingival recession compared to that of CTG in Miller's class I gingival recessions.
Materials and Methods
A randomized controlled clinical trial was carried out in the Department of Periodontics, K M Shah Dental College and Hospital, Sumandeep Vidyapeeth. This study was started after Institutional Ethics Committee approval was obtained. A total of 32 sites were taken according to the sample size calculation.
Inclusion and exclusion criteria
Maxillary and mandibular incisors, canines, and premolars of patients aged more than 18 years, who were systemically healthy and who were maintaining good oral hygiene after completion of scaling and root planing with Miller's class I gingival recession were included in the study. Patients who were pregnant or lactating, who underwent any mucogingival procedures at the selected sites within the previous 3 months, had allergy to local anesthesia, chlorhexidine, antibiotic and analgesic, had habit of smoking and tobacco chewing and those who were not willing for participation in the study and further follow-up were excluded from the study.
The patients were explained about both the procedures before commencing the surgery. After fulfilling the inclusion and exclusion criteria, informed consent was obtained.
Each patient had undergone an initial periodontal treatment, including oral hygiene instructions, plaque control, and scaling and root planing, followed by re-evaluation. Before surgery, a customized acrylic stent was fabricated for each patient so that the standard periodontal probe returns to the same position for each successive measurement.
All clinical recordings were performed immediately before surgery (baseline) and after 6 months interval following periodontal surgery. Patients were recalled at 1st week for pain perception, 2nd, 3rd week for healing index and pain perception, end of the 1st month for supragingival debridement, end of the 3rd month for supragingival debridement, and at the end of the 6th month for supragingival debridement and follow-up measurements. No periodontal measurements were recorded at 1st, 2nd, and 3rd week recalls.
The measurements recorded were:
Recession height (REC-HT): Measured from cementoenamel junction to free gingival margin
Recession width (REC-WD): measured mesiodistally at the cementoenamel junction
Clinical attachment level (CAL)
Height of keratinized tissue (HKT): Measured from the most apical point on free gingival margin to mucogingival junction
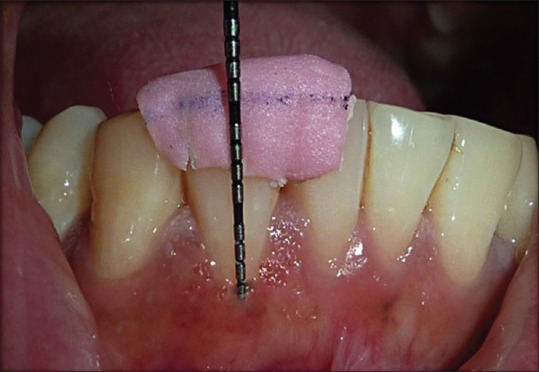
Thickness of keratinized tissue (TKT): Measured at midpoint location between the most apical point of gingival margin and mucogingival junction using image analyzer with stereomicroscope. After topical anesthesia, a 1 1/2” needle with a rubber stopper was pierced perpendicular to the mucosal surface, through the soft tissue, until hard surface was felt. The rubber stopper had marked this level. The distance between the needle tip and the rubber stopper was then measured under a stereomicroscope using image analyzer. These measurements were evaluated at baseline and at 6-month interval
The Landry healing index was performed at the first, second, and 3rd week postsurgery. The healing index rates are healing on the basis of redness, presence of granulation tissue, bleeding and suppuration, and epithelialization. A score of 1–5 is given, where 1 is associated with very poor healing and 5 being excellent
Patient's pain perception was also recorded using visual analog scale (VAS) at the operated sites.
Allotment of the sites
Before surgery, defects were assigned by a coin flip to receive either prf membrane or CTG and informed consent was obtained from the participants. Allotment number was given to the site. Recording of the clinical parameters and presurgical preparation was done by the investigator with reference to the allotment number.
Surgical procedure
Surgical procedures for all patients were carried out by an experienced single periodontist.
For the platelet-rich fibrin group
Coronally advanced flap
Following measurement recordings and administration of LA, horizontal incision was given at base of interdental papilla, on either side of involved tooth, without involving gingival margin of adjacent tooth. Then, 2 vertical incisions were given, extending apically from the horizontal incisions 1 from each side. Full-thickness mucoperiosteal flap was raised till the mucogingival junction, and beyond mucogingival junction a partial thickness flap was raised to make the flap mobile [Figure 1]. The exposed root was debrided with hand and ultrasonic instruments. Apical to bone exposure, flap elevation continued split thickness and finished when it was possible to move the flap passively in the coronal direction. To permit the coronal advancement of the flap, all muscle insertions present in the thickness of the flap were eliminated. Care was taken to make sure that the flap was stable in its final coronal position, even without the sutures.
Figure 1.
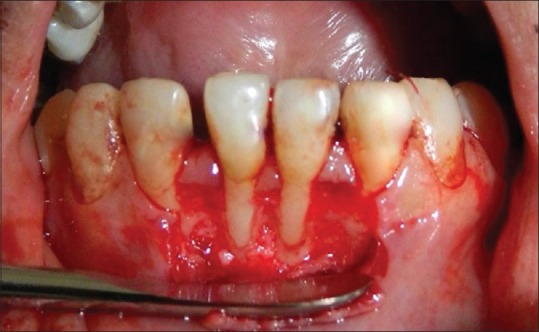
Reflection
Obtaining platelet-rich fibrin
The required quantity of blood was drawn quickly into 10-ml test tubes without an anticoagulant and centrifuged immediately. Blood was centrifuged using a tabletop centrifuge for at least 10 min at 3000 rpm. The resultant product consists of the following three layers; topmost layer consisting of platelet poor plasma, PRF clot in the middle, and red blood cells (RBCs) at the bottom. PRF was available as a fibrin clot. PRF clot was removed from the test tube using sterilized tweezers. After lifting, the RBC layer attached to the PRF clot was carefully removed using a sterilized scissor, in such a way that part of the RBC layer remains attached to the PRF clot. The PRF clots were then compressed between a sterile perforated metal tray and sterilized a nonperforated metal plate to make a separate PRF membrane PRF membrane placement [Figure 2].
Figure 2.
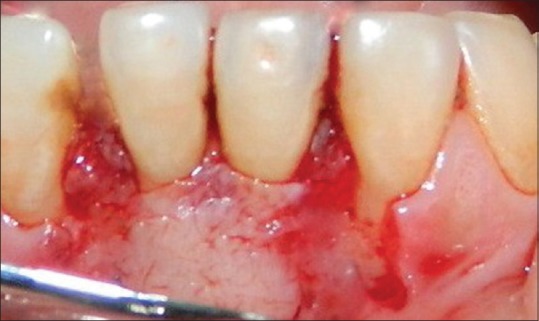
Platelet rich fibrin placement
PRF membrane was then placed under the CAF. The flap was sutured over the membranes such that the membrane always slightly hangs over the edge of gingival margin, thus be positioned over the recession coronal to the cementoenamel junction. The flap was then sutured using 5–0 polyglycolic acid sutures followed by a noneugenol pack.
For the connective tissue graft group
The control group (CTG) [Figure 3] was treated with identical surgical procedure, with the exception of applying the PRF membrane. A CTG with CAF was used as the augmentation material in the control group.
Figure 3.
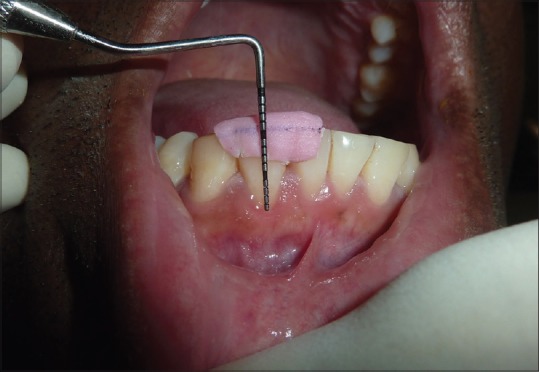
Preoperative picture for connective tissue group
Obtaining connective tissue graft
A 1–2-mm thick CTG was harvested from the palate in the area between the second premolar and the second molar using either single- or double-incision technique. Obtaining Connective Tissue Graft [Figure 4]. The graft was positioned on the instrumented root surface immediately apical or at the level of the CEJ and was stabilized. The wound on the donor site of the palate was also sutured. CAF was sutured using polyglycolic acid sutures followed by noneugenol pack.
Figure 4.
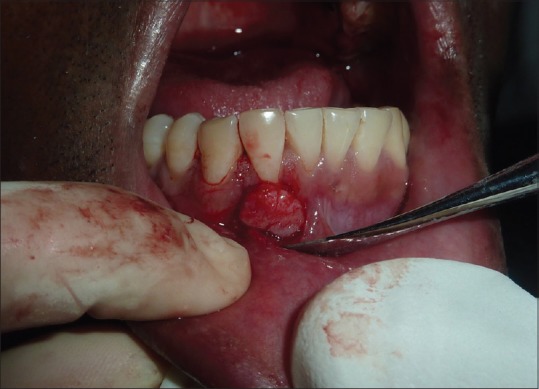
Connective tissue graft placement
Postsurgical instructions and follow-up
All patients were recalled after 1, 2, and 3 weeks to record the healing index and to evaluate the VAS score for pain perception. Patients were given antibiotics and analgesics for 5 days and were instructed not to brush the teeth in the treated area but to rinse with cycloheximide solution (0.2%) twice daily for 1 min. After 3 weeks, the patients were called after 3 months and then at 6 months [Figure 5]. Oral hygiene reinforcement was also done at each visit.
Figure 5.
Six month follow up for connective tissue graft group
Statistical analysis
The statistical tests used were Pearson's Chi-square test, t-test, Wilcoxon signed rank, and Mann–Whitney test. Descriptive data were evaluated using Pearson's Chi-square test. The Wilcoxon test is a nonparametric test which was used to evaluate the difference between two treatments where the samples are correlated. Mann–Whitney test was used to compare baseline characteristics between both the groups and for the intragroup analysis at baseline and 6 months. T-test was used to compare intergroup mean changes in clinical parameters between both the groups at baseline and 6 months.
Results
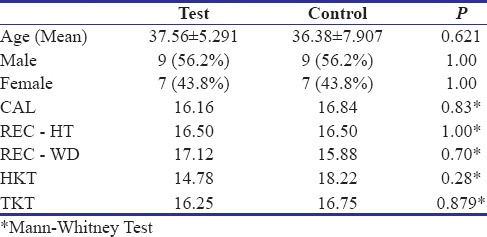
The study included 32 sites and were divided into 2 groups. The demographic data and baseline clinical values in both group were similar at the start of the study as shown in Table 1.
Table 1.
Demographic data and baseline clinical values
In the test group (PRF), significant differences were seen in terms of gain in the CAL, reduction in the REC-HT, decrease in the REC-WD, increase in the HKT and increase in the TKT at 1.25±1.01, 1.07±0.89, 0.5±0.76, 0.38±1.93 and 0.19±0.23 respectively, from baseline to 6 months [Table 2].
Table 2.
Clinical parameters at baseline and 6 months in test group (Mean±SD)

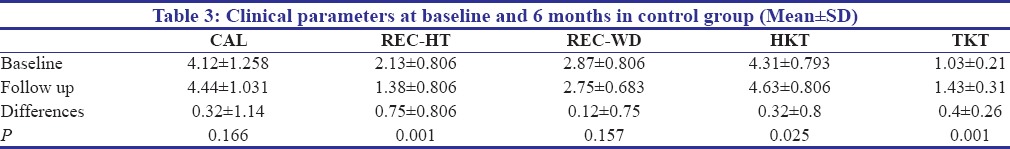
In the control group (CTG), no significant difference was seen in terms of gain in CAL, increase in the REC-WD, increase in the HKT at 0.32±1.14, 0.12±0.75 and 0.32±0.8 respectively, from baseline to 6 months. However, only significant difference seen was in terms of increase in the REC-HT and for the increase in the TKT at 0.75±0.806 and 0.4±0.26 respectively [Table 3].
Table 3.
Clinical parameters at baseline and 6 months in control group (Mean±SD)
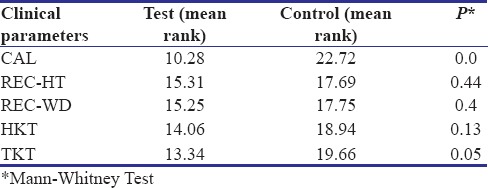
The clinical parameters of both the test and control group were compared at the end of 6 months. When intra-group analysis was done, only CAL and TKT showed significant improvement at 6 months [Table 4].
Table 4.
Clinical parameters of test and control group at 6 months
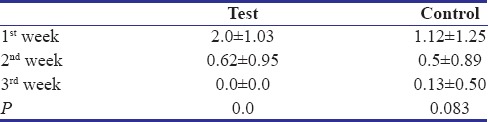
The Landry Healing Index which was taken at 2nd and 3rd week after surgery showed significant differences for both the test and the control group with P = 0.01 and 0.005 respectively [Table 5].
Table 5.
Healing index of test and control groups at 2nd and 3rd week post-surgery (Mean±SD)

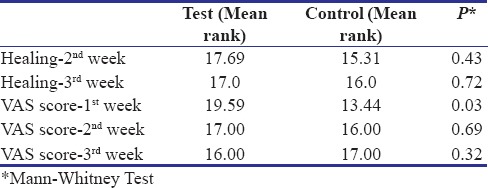
The VAS score evaluated for pain perception at 1st, 2nd and 3rd week showed significant reduction from 1st week to 3rd week in both the groups. However, test group showed significant reduction in pain when compared with the control group (P = 0.0) [Table 6].
Table 6.
Vas score of test and control groups at 1st, 2nd and 3rd week post-surgery (Mean±SD)
Comparison of both Healing Index and VAS score was done and it showed no significant difference between test and the control group except VAS at 1 week [Table 7].
Table 7.
Comparison of healing index and vas score of test and control group
Discussion
The present study aimed to correct the gingival recession using 2 different surgical techniques. The population selected were patients with Miller's Class I gingival recession. Miller's class I recession defects were selected for treatment in this study as esthetic concerns are usually the reasons to perform periodontal plastic procedures, and as Miller's class I recession have highest prevalence (86.16%) among the different class of recession defects.
Choukroun's PRF, a second-generation platelet concentrate, was defined as an autologous leukocyte and PRF biomaterial. PRF was developed in France by Choukroun et al. in 2001.[15] A study done by Ehrenfest et al. in 2010[16] performed a detailed examination of the composition and architecture of the Choukroun's PRF clot (particularly the distribution of the platelets and leukocytes within the fibrin clot) using hematologic counts, photonic microscopy, and scanning electron microscope. This study showed that most of the platelets originating from the whole-blood sample were collected in the PRF membranes. Leukocyte counts confirmed that more than half of the leukocytes were also trapped in PRF membranes. Moreover, the cell composition of PRF implies that this biomaterial is a blood-derived living tissue and must be handled carefully to keep its cellular content alive and stable. The three main platelet cytokines play a fundamental role in initial healing mechanisms owing to their capacity to stimulate cell migration and proliferation (particularly by platelet-derived growth factors [PDGFs]) and induce fibrin matrix remodeling as well as secretion of a cicatricial collagen matrix (particularly by transforming growth factor-beta [TGFb]).[17] With these fundamental considerations, PRF can be considered as a natural fibrin-based biomaterial favorable to the development of a microvascularization and able to guide epithelial cell migration to its surface.
Looking into all the various advantages of PRF, in our study, PRF membrane was used as a test group and was placed under the CAF so that increased initial stability, accelerate healing, and root coverage can be obtained. The main advantage was that it was prepared very easily and a good patient's perception was achieved.
We had also used CAF along with the placement of a PRF membrane. The advantages of CAF include ability to treat multiple areas of root exposure, no need for involvement of adjacent teeth, high degree of success, and even if the procedure does not work, it does not increase the existing problem.[18] The only disadvantage of this technique is that CRC is not obtained in all the sites and the gingival recession tends to relapse in a few sites after some years. The CAF technique used in our study for isolated gingival recession defects was similar to a flap design described by De Sanctis and Zucchelli in 2007.[19]
The results seen in our study were not significant. The height increased from 4.06 ± 1.61 to 4.44 ± 2.25 (P = 0.15). Conversely, the thickness increased significantly in this study with P = 0.001. The healing index and pain perception were also highly significant with the use of PRF with P = 0.001 and P = 0.0, respectively. A study done by Jankovic et al.[20] in 2012 also showed highly significant results in terms of wound healing in the PRF group.
The technique adopted in this study for CTG was similar to the study described by Langer and Langer involved the elevation of a partial-thickness flap in the recipient site; but here, we have used a full-thickness periosteal flap as most of the cases we incorporated had a thin gingival biotype. Moreover, the blood supply to the flap would also have been compromised. Hence, to compensate the blood supply and to enhance healing to the thin biotype of the recipient bed, a full-thickness flap can be justified.
The results of the control group were not as significant as the test group. The only significant difference seen was in the REC-HT and thickness of the keratinized tissue. The REC-HT reduced from 2.13 ± 0.806 to 1.38 ± 0.806 (P = 0.001). The thickness of the tissue also increased significantly from 1.03 ± 0.21 to 1.43 ± 0.31 (P = 0.001). To the best of our knowledge, very few studies have evaluated the thickness of the tissue. Hence, we checked the thickness of the tissue using a stereomicroscope. In this manner, we were able to achieve the exact thickness before and after the surgery. Analogous results were seen in many of the other studies. A study done by Jankovic in 2012 also showed that the thickness of the tissue increased after CTG.[20]
The intergroup analysis of both test and the control group was done. To the best of our knowledge, there has been only one study which has done comparison of PRF and CTG. The results of a study done by Jankovic et al. in 2012[20] were comparable to our study. The results showed significant difference in terms of CAL and thickness of the keratinized tissue with P = 0.01 and P = 0.05, respectively. When the difference between the clinical parameters was done, it showed significant results with the same 2 parameters. However, a study by Jankovic[20] showed significant results in all the parameters except for the healing of the tissue and thickness of the tissue. Healing was better seen in the PRF group, whereas the thickness increased more in the CTG group. The healing index also showed significant improvement in the test group compared to the control group. This can be related to the extremely elevated density of fibrin fibers detected in the PRF membrane. The high-density fibers provide an additional stability of the wounds and promote angiogenesis. Moreover, the concentrated PDGF, vascular endothelial growth factor and TGF which are the main growth factors, enhance soft-tissue healing by angiogenesis and matrix biosynthesis during wound healing.
There are however a few limitations of this study. First of all, a split-mouth design would have been done so as to know the patient's individual objective reactions and healing. Second, the percentage of root coverage was also not established in the study which could have been a contributing factor if taken into consideration. Smoking status has also shown to have adverse effects of healing of the CTG. This could also have been determined if smokers were included in the study.
Conclusion
The present study evaluates PRF versus CTG in combination with CAF to evaluate its clinical efficacy in the treatment of Miller's class I gingival recession. The results of this study permitted the following conclusions to be drawn. In the test group, significant results were obtained in terms of CAL, REC-HT, REC-WD, HKT, TKT, healing index, and VAS scale, whereas in the control group, only REC-HT, TKT, healing index, and VAS scale showed significant improvements. When the intergroup difference was compared, PRF showed to have significant result compared to CTG in all the clinical parameters. Moreover, the healing index and VAS scale showed significant results in the test group. This showed better patient's acceptance and comfort toward the test group. Thus, though CTG is the gold standard procedure, PRF can be used as an alternative procedure by keeping patient's comfort and recognition in mind.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Roccuzzo M, Bunino M, Needleman I, Sanz M. Periodontal plastic surgery for treatment of localized gingival recessions: A systematic review. J Clin Periodontol. 2002;29(Suppl 3):178–94. doi: 10.1034/j.1600-051x.29.s3.11.x. [DOI] [PubMed] [Google Scholar]
- 2.Miller PD., Jr Regenerative and reconstructive periodontal plastic surgery. Mucogingival surgery. Dent Clin North Am. 1988;32:287–306. [PubMed] [Google Scholar]
- 3.Bernimoulin JP, Lüscher B, Mühlemann HR. Coronally repositioned periodontal flap. Clinical evaluation after one year. J Clin Periodontol. 1975;2:1–3. doi: 10.1111/j.1600-051x.1975.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 4.Tenenbaum H, Klewansky P, Roth JJ. Clinical evaluation of gingival recession treated by coronally repositioned flap technique. J Periodontol. 1980;51:686–90. doi: 10.1902/jop.1980.51.12.686. [DOI] [PubMed] [Google Scholar]
- 5.Patur B. The rotation flap for covering denuded root surfaces – A closed wound technique. J Periodontol. 1977;48:41–4. doi: 10.1902/jop.1977.48.1.41. [DOI] [PubMed] [Google Scholar]
- 6.Grupe HE, Warren RF., Jr Repair of gingival defects by a sliding flap operation. J Periodontol. 1956;27:92–5. [Google Scholar]
- 7.Miller PD, Jr, Binkley LH., Jr Root coverage and ridge augmentation in class IV recession using a coronally positioned free gingival graft. J Periodontol. 1986;57:360–3. doi: 10.1902/jop.1986.57.6.360. [DOI] [PubMed] [Google Scholar]
- 8.Miller PD., Jr A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5:8–13. [PubMed] [Google Scholar]
- 9.Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56:715–20. doi: 10.1902/jop.1985.56.12.715. [DOI] [PubMed] [Google Scholar]
- 10.Kuis D, Sciran I, Lajnert V, Snjaric D, Prpic J, Pezelj-Ribaric S, et al. Coronally advanced flap alone or with connective tissue graft in the treatment of single gingival recession defects: A long-term randomized clinical trial. J Periodontol. 2013;84:1576–85. doi: 10.1902/jop.2013.120451. [DOI] [PubMed] [Google Scholar]
- 11.Langer B, Calagna L. The subepithelial connective tissue graft. J Prosthet Dent. 1980;44:363–7. doi: 10.1016/0022-3913(80)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Cortellini P, Tonetti M, Baldi C, Francetti L, Rasperini G, Rotundo R, et al. Does placement of a connective tissue graft improve the outcomes of coronally advanced flap for coverage of single gingival recessions in upper anterior teeth? A multi-centre, randomized, double-blind, clinical trial. J Clin Periodontol. 2009;36:68–79. doi: 10.1111/j.1600-051X.2008.01346.x. [DOI] [PubMed] [Google Scholar]
- 14.Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J Periodontol. 2009;80:244–52. doi: 10.1902/jop.2009.080253. [DOI] [PubMed] [Google Scholar]
- 15.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun's platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546–55. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 17.Cohen ES, editor. Atlas of Cosmetic and Reconstructive Periodontal Surgery. 3rd ed. Hamilton: BC Decker Inc; 2007. [Google Scholar]
- 18.Pini Prato G, Rotundo R, Franceschi D, Cairo F, Cortellini P, Nieri M, et al. Fourteen-year outcomes of coronally advanced flap for root coverage: Follow-up from a randomized trial. J Clin Periodontol. 2011;38:715–20. doi: 10.1111/j.1600-051X.2011.01744.x. [DOI] [PubMed] [Google Scholar]
- 19.Zucchelli G, Mele M, Mazzotti C, Marzadori M, Montebugnoli L, De Sanctis M, et al. Coronally advanced flap with and without vertical releasing incisions for the treatment of multiple gingival recessions: A comparative controlled randomized clinical trial. J Periodontol. 2009;80:1083–94. doi: 10.1902/jop.2009.090041. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, Kenney EB, et al. Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial. Int J Periodontics Restorative Dent. 2012;32:e41–50. [PubMed] [Google Scholar]


